Neuropilin 1 (NRP1) Positively Regulates Adipogenic Differentiation in C3H10T1/2 Cells
Abstract
:1. Introduction
2. Results
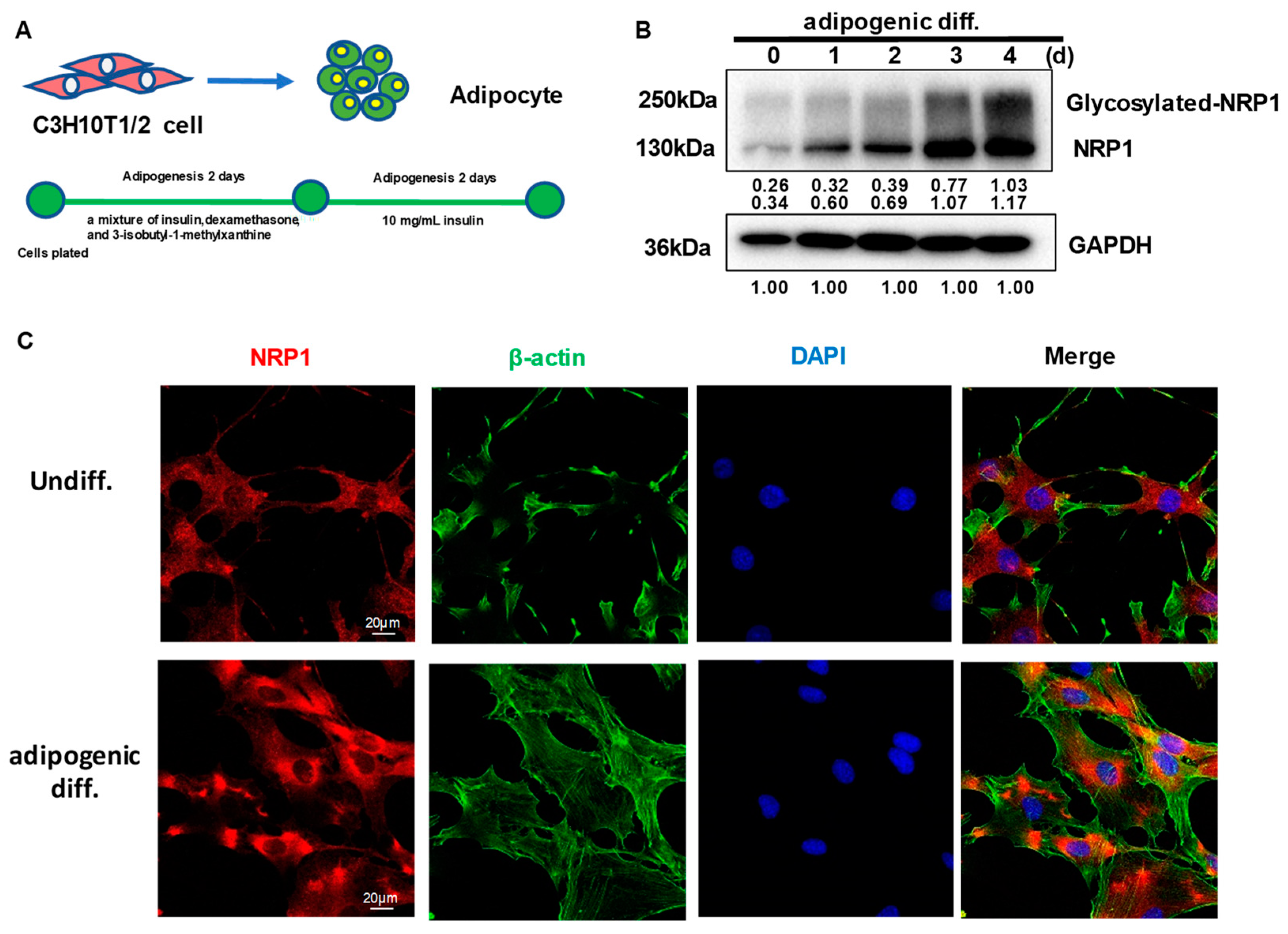
2.1. NRP1 Protein Expression Increases and Its Subcellular Localization Slightly Changes during Adipogenesis in C3H10T1/2 Cells
2.2. Knockdown of NRP1 Represses Adipogenesis of C3H10T1/2 Cells
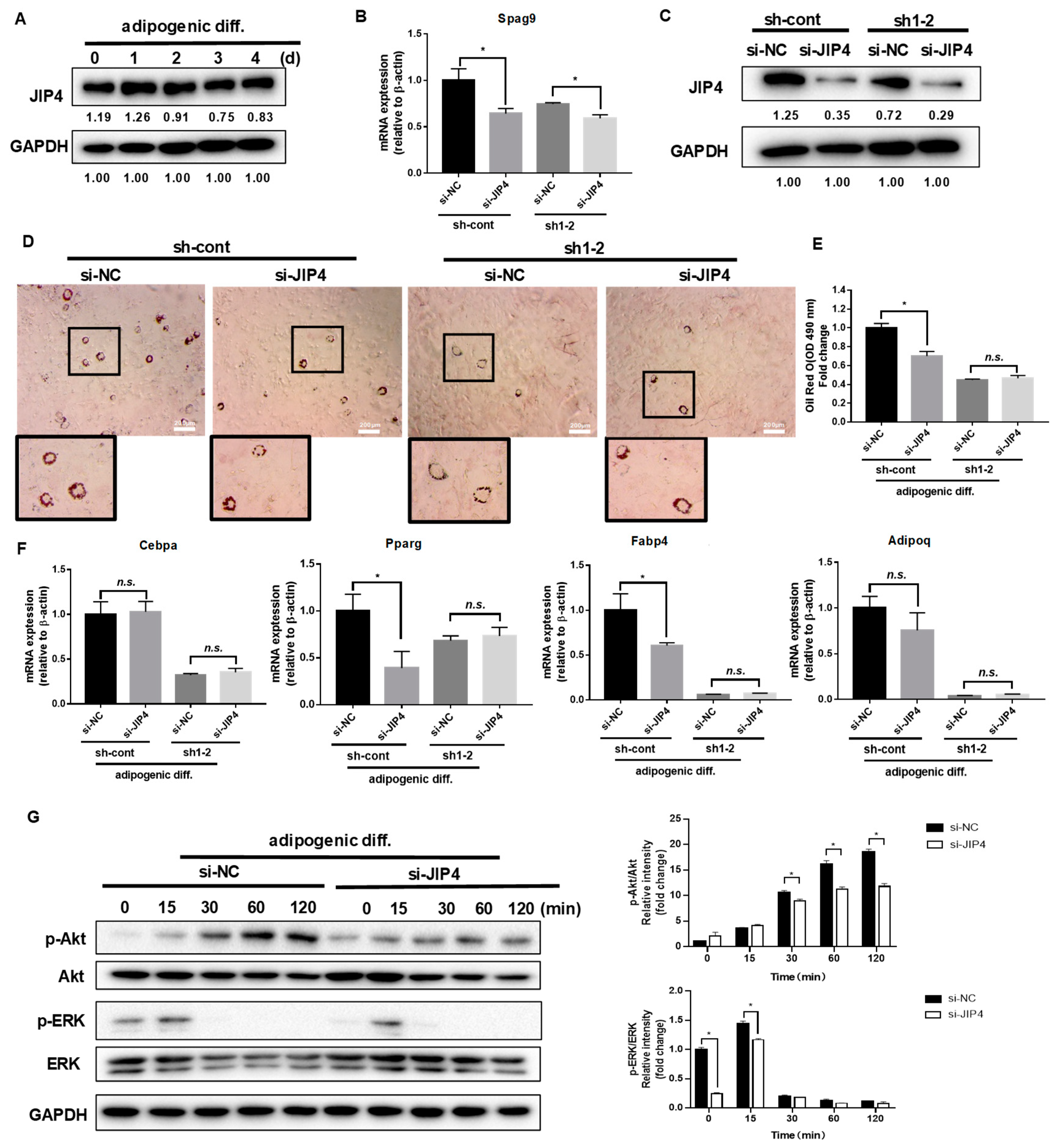
2.3. Scaffold Protein JIP4 Silencing Represses Adipogenic Differentiation
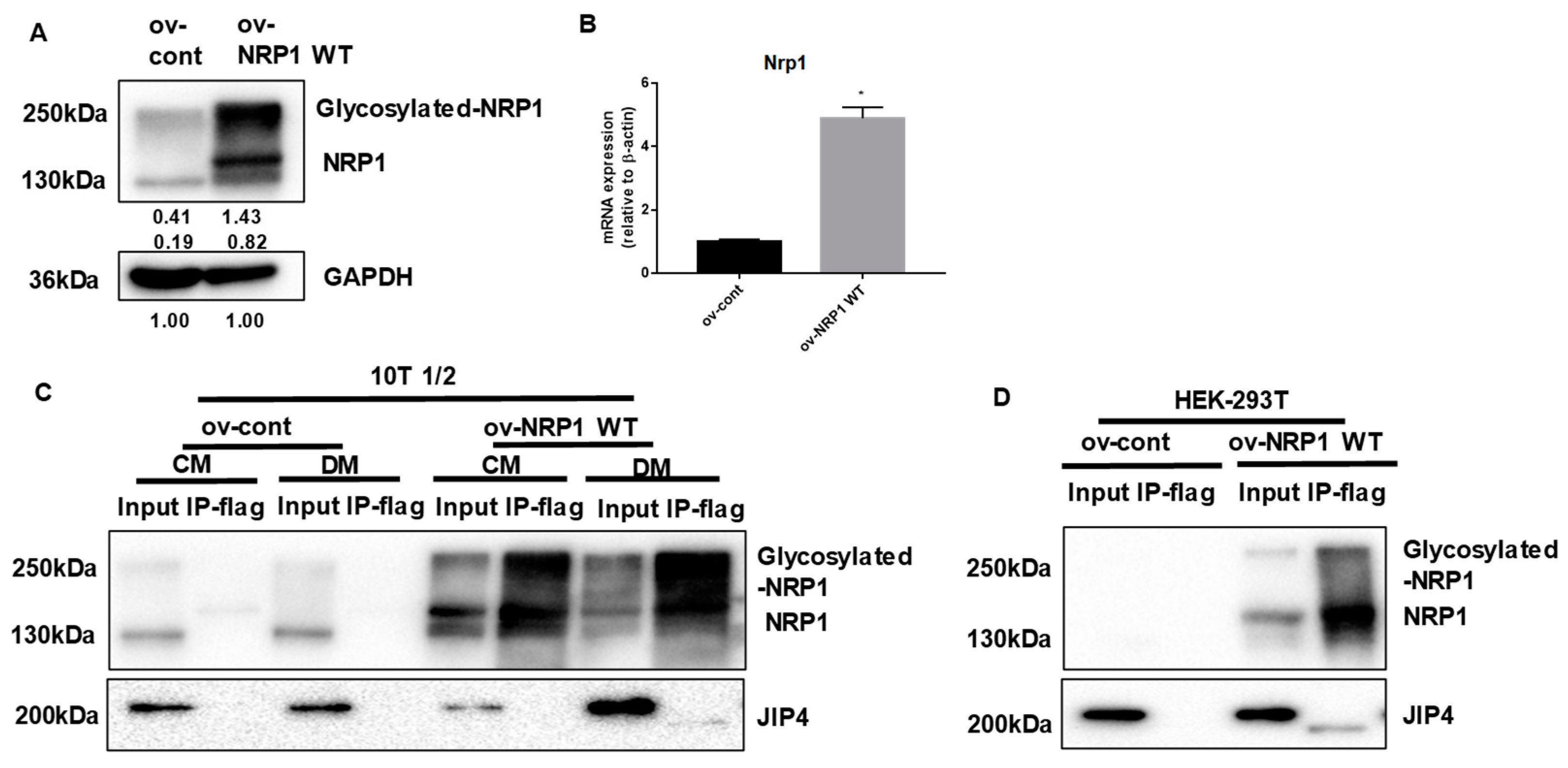
2.4. NRP1 Interacts with JIP4 in Adipogenic Differentiation
2.5. Nonmodifiable NRP1 Mutant (S612A) Promotes Adipogenic Differentiation
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Differentiation
4.2. Oil Red O Staining and Quantification
4.3. SDS-PAGE and Western Blotting
4.4. Immunocytochemistry
4.5. Reverse-Transcription and Quantitative Real-Time PCR (qPCR)
4.6. NRP1 shRNA Knockdown
4.7. JIP4 siRNA Transfection
4.8. Lentiviral and Retroviral Vector Production and Infection
4.9. Co-Immunoprecipitation (Co-IP)
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal stem cells |
| NRP1 | Neuropilin 1 |
| GAG | Glycosaminoglycan |
| JIP4 | C-Jun N-terminal kinase (JNK)–interacting protein 4 |
| MAPK | Mitogen-activated protein kinase |
| ERK | Ras-extracellular signal-regulated kinase |
| JNK | C-Jun N-terminal kinase |
| Co-IP | Co-immunoprecipitation |
| C/EBPα | CCAAT/enhancer binding protein α |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| FABP4 | Fatty acid-binding protein 4 |
References
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef] [PubMed]
- Konige, M.; Wang, H.; Sztalryd, C. Role of adipose specific lipid droplet proteins in maintaining whole body energy homeostasis. Biochim. Biophys. Acta 2014, 1842, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, B.; Wang, R.; Gong, S.; Chen, G.; Xu, W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res. Ther. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Lee, Y.J.; Shin, M.K.; Sung, J.S. Regulation of CXCR6 Expression on Adipocytes and Osteoblasts Differentiated from Human Adipose Tissue-Derived Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, 8870133. [Google Scholar] [CrossRef]
- Lu, D.; Shang, G.; He, X.; Bai, X.C.; Zhang, X. Architecture of the Sema3A/PlexinA4/Neuropilin tripartite complex. Nat. Commun. 2021, 12, 3172. [Google Scholar] [CrossRef]
- Shintani, Y.; Takashima, S.; Asano, Y.; Kato, H.; Liao, Y.; Yamazaki, S.; Tsukamoto, O.; Seguchi, O.; Yamamoto, H.; Fukushima, T.; et al. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. EMBO J. 2006, 25, 3045–3055. [Google Scholar] [CrossRef]
- Muhl, L.; Folestad, E.B.; Gladh, H.; Wang, Y.; Moessinger, C.; Jakobsson, L.; Eriksson, U. Neuropilin 1 binds PDGF-D and is a co-receptor in PDGF-D-PDGFRβ signaling. J. Cell Sci. 2017, 130, 1365–1378. [Google Scholar]
- Ceccarelli, S.; Nodale, C.; Vescarelli, E.; Pontecorvi, P.; Manganelli, V.; Casella, G.; Onesti, M.G.; Sorice, M.; Romano, F.; Angeloni, A.; et al. Neuropilin 1 Mediates Keratinocyte Growth Factor Signaling in Adipose-Derived Stem Cells: Potential Involvement in Adipogenesis. Stem Cells Int. 2018, 2018, 1075156. [Google Scholar] [CrossRef]
- Pellet-Many, C.; Frankel, P.; Evans, I.M.; Herzog, B.; Jünemann-Ramírez, M.; Zachary, I.C. Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochem. J. 2011, 435, 609–618. [Google Scholar] [CrossRef]
- Kelkar, N.; Standen, C.L.; Davis, R.J. Role of the JIP4 scaffold protein in the regulation of mitogen-activated protein kinase signaling pathways. Mol. Cell. Biol. 2005, 25, 2733–2743. [Google Scholar] [CrossRef]
- Eke, I.; Schneider, L.; Förster, C.; Zips, D.; Kunz-Schughart, L.A.; Cordes, N. EGFR/JIP-4/JNK2 signaling attenuates cetuximab-mediated radiosensitization of squamous cell carcinoma cells. Cancer Res. 2013, 73, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Chan, D.; Wolozin, B. LRRK2 and the stress response: Interaction with MKKs and JNK-interacting proteins. Neurodegener. Dis. 2010, 7, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Marchesin, V.; Castro-Castro, A.; Lodillinsky, C.; Castagnino, A.; Cyrta, J.; Bonsang-Kitzis, H.; Fuhrmann, L.; Irondelle, M.; Infante, E.; Montagnac, G.; et al. ARF6-JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion. J. Cell Biol. 2015, 211, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Willett, R.; Martina, J.A.; Zewe, J.P.; Wills, R.; Hammond, G.; Puertollano, R. TFEB regulates lysosomal positioning by modulating TMEM55B expression and JIP4 recruitment to lysosomes. Nat. Commun. 2017, 8, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, W.; Zhang, J.; Ji, S.; Jing, Z.; Chen, Y.Q. Slc25a5 regulates adipogenesis by modulating ERK signaling in OP9 cells. Cell. Mol. Biol. Lett. 2022, 27, 11. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, C.E.; Falkevall, A.; Wang, X.; Larsson, E.; Huusko, J.; Nilsson, I.; van Meeteren, L.A.; Samen, E.; Lu, L.; Vanwildemeersch, M.; et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 2010, 464, 917–921. [Google Scholar] [CrossRef]
- Wilson, A.M.; Shao, Z.; Grenier, V.; Mawambo, G.; Daudelin, J.F.; Dejda, A.; Pilon, F.; Popovic, N.; Boulet, S.; Parinot, C.; et al. Neuropilin-1 expression in adipose tissue macrophages protects against obesity and metabolic syndrome. Sci. Immunol. 2018, 3, eaan4626. [Google Scholar] [CrossRef]
- Ghode, S.S.; Bajaj, M.S.; Kulkarni, R.S.; Limaye, L.S.; Shouche, Y.S.; Kale, V.P. Neuropilin-1 Is an Important Niche Component and Exerts Context-Dependent Effects on Hematopoietic Stem Cells. Stem Cells Dev. 2017, 26, 35–48. [Google Scholar] [CrossRef]
- Lumb, R.; Wiszniak, S.; Kabbara, S.; Scherer, M.; Harvey, N.; Schwarz, Q. Neuropilins define distinct populations of neural crest cells. Neural Dev. 2014, 9, 24. [Google Scholar] [CrossRef]
- Issitt, T.; Bosseboeuf, E.; De Winter, N.; Dufton, N.; Gestri, G.; Senatore, V.; Chikh, A.; Randi, A.M.; Raimondi, C. Neuropilin-1 Controls Endothelial Homeostasis by Regulating Mitochondrial Function and Iron-Dependent Oxidative Stress. iScience 2019, 11, 205–223. [Google Scholar] [CrossRef]
- Pinskey, J.M.; Franks, N.E.; McMellen, A.N.; Giger, R.J.; Allen, B.L. Neuropilin-1 promotes Hedgehog signaling through a novel cytoplasmic motif. J. Biol. Chem. 2017, 292, 15192–15204. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shen, N.; Zhang, M.L.; Pan, F.Y.; Wang, C.; Jia, W.P.; Liu, C.; Gao, Q.; Gao, X.; Xue, B.; et al. Egr-1 decreases adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal balance in mice. EMBO J. 2011, 30, 3754–3765. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Grønborg, M.; Huang, H.; Kim, J.W.; Otto, T.C.; Pandey, A.; Lane, M.D. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9766–9771. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liao, S.; Nelson, E.R.; Schmalzigaug, R.; Spurney, R.F.; Guilak, F.; Premont, R.T.; Gesty-Palmer, D. The cytoskeletal regulatory scaffold protein GIT2 modulates mesenchymal stem cell differentiation and osteoblastogenesis. Biochem. Biophys. Res. Commun. 2012, 425, 407–412. [Google Scholar] [CrossRef]
- Li, Z.; Yan, M.; Yu, Y.; Wang, Y.; Lei, G.; Pan, Y.; Li, N.; Gobin, R.; Yu, J. LncRNA H19 promotes the committed differentiation of stem cells from apical papilla via miR-141/SPAG9 pathway. Cell Death Dis. 2019, 10, 130. [Google Scholar] [CrossRef]
- Yoshida, A.; Shimizu, A.; Asano, H.; Kadonosono, T.; Kondoh, S.K.; Geretti, E.; Mammoto, A.; Klagsbrun, M.; Seo, M.K. VEGF-A/NRP1 stimulates GIPC1 and Syx complex formation to promote RhoA activation and proliferation in skin cancer cells. Biol. Open 2015, 4, 1063–1076. [Google Scholar] [CrossRef]
- Huang, X.; Ye, Q.; Chen, M.; Li, A.; Mi, W.; Fang, Y.; Zaytseva, Y.Y.; O’Connor, K.L.; Vander Kooi, C.W.; Liu, S.; et al. N-glycosylation-defective splice variants of neuropilin-1 promote metastasis by activating endosomal signals. Nat. Commun. 2019, 10, 3708. [Google Scholar] [CrossRef]
- Frankel, P.; Pellet-Many, C.; Lehtolainen, P.; D’Abaco, G.M.; Tickner, M.L.; Cheng, L.; Zachary, I.C. Chondroitin sulphate-modified neuropilin 1 is expressed in human tumour cells and modulates 3D invasion in the U87MG human glioblastoma cell line through a p130Cas-mediated pathway. EMBO Rep. 2008, 9, 983–989. [Google Scholar] [CrossRef]
- Li, C.W.; Lim, S.O.; Xia, W.; Lee, H.H.; Chan, L.C.; Kuo, C.W.; Khoo, K.H.; Chang, S.S.; Cha, J.H.; Kim, T.; et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 2016, 7, 12632. [Google Scholar] [CrossRef]
- Zheng, S.; Song, Q.; Zhang, P. Metabolic Modifications, Inflammation, and Cancer Immunotherapy. Front. Oncol. 2021, 11, 703681. [Google Scholar] [CrossRef] [PubMed]
- Corredor, M.; Bonet, R.; Moure, A.; Domingo, C.; Bujons, J.; Alfonso, I.; Pérez, Y.; Messeguer, À. Cationic Peptides and Peptidomimetics Bind Glycosaminoglycans as Potential Sema3A Pathway Inhibitors. Biophys. J. 2016, 110, 1291–1303. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Uchida-Fukuhara, Y.; Weng, Y.; He, Y.; Ikegame, M.; Wang, Z.; Yoshida, K.; Okamura, H.; Qiu, L. Neuropilin 1 (NRP1) Positively Regulates Adipogenic Differentiation in C3H10T1/2 Cells. Int. J. Mol. Sci. 2023, 24, 7394. https://doi.org/10.3390/ijms24087394
Yu Y, Uchida-Fukuhara Y, Weng Y, He Y, Ikegame M, Wang Z, Yoshida K, Okamura H, Qiu L. Neuropilin 1 (NRP1) Positively Regulates Adipogenic Differentiation in C3H10T1/2 Cells. International Journal of Molecular Sciences. 2023; 24(8):7394. https://doi.org/10.3390/ijms24087394
Chicago/Turabian StyleYu, Yaqiong, Yoko Uchida-Fukuhara, Yao Weng, Yuhan He, Mika Ikegame, Ziyi Wang, Kaya Yoshida, Hirohiko Okamura, and Lihong Qiu. 2023. "Neuropilin 1 (NRP1) Positively Regulates Adipogenic Differentiation in C3H10T1/2 Cells" International Journal of Molecular Sciences 24, no. 8: 7394. https://doi.org/10.3390/ijms24087394
APA StyleYu, Y., Uchida-Fukuhara, Y., Weng, Y., He, Y., Ikegame, M., Wang, Z., Yoshida, K., Okamura, H., & Qiu, L. (2023). Neuropilin 1 (NRP1) Positively Regulates Adipogenic Differentiation in C3H10T1/2 Cells. International Journal of Molecular Sciences, 24(8), 7394. https://doi.org/10.3390/ijms24087394






