Ursolic Acid against Prostate and Urogenital Cancers: A Review of In Vitro and In Vivo Studies
Abstract
1. Introduction
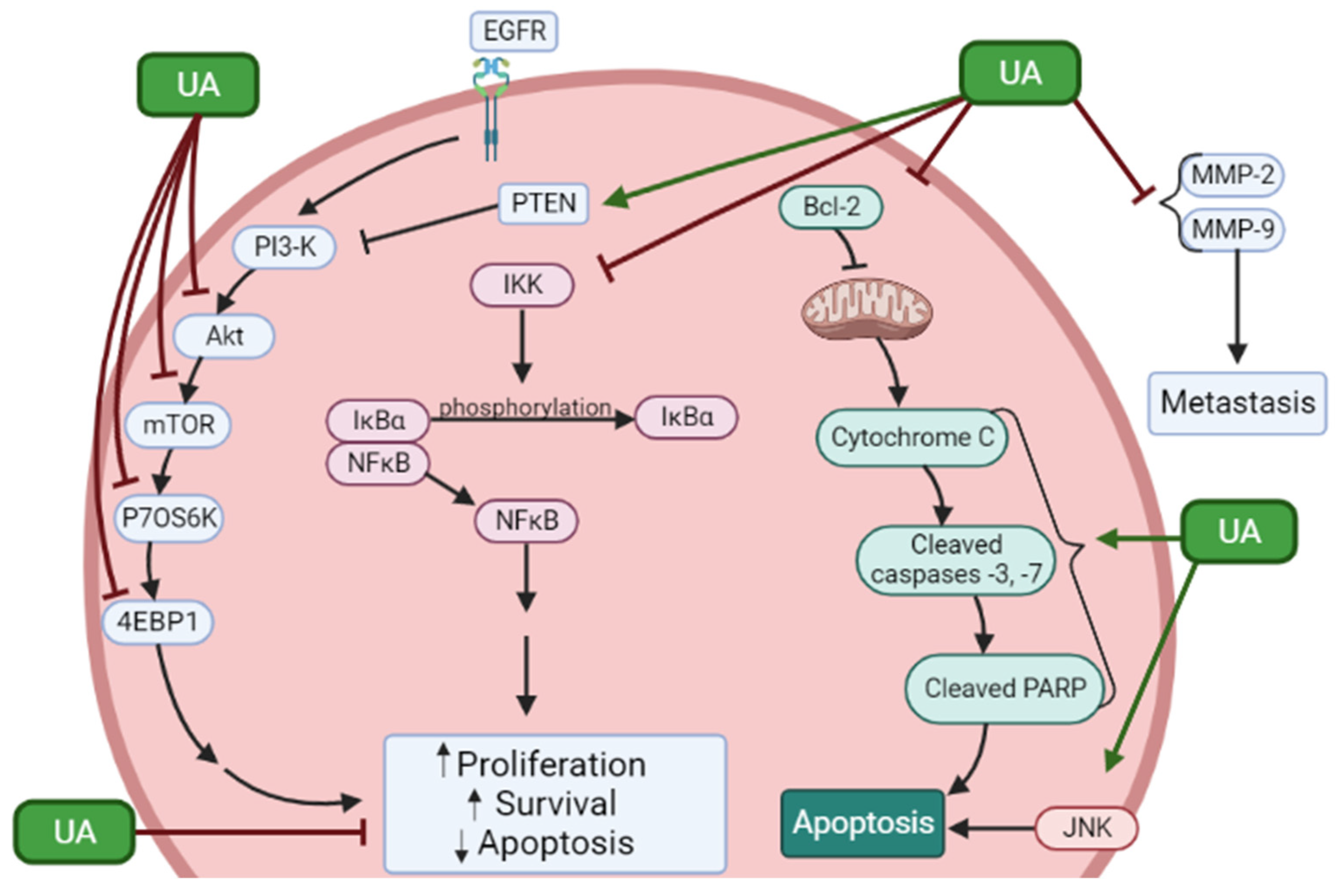
2. Effects of Ursolic Acid against Prostate Cancer
2.1. Effects of Ursolic Acid against Prostate Cancer: Evidence from In Vitro Studies
| Cell Type | Dose | Findings | Mechanism | Reference |
|---|---|---|---|---|
| PC-3 LNCaP | UA 55 µM (PC-3) 45 µM (LNCaP) | ↓ Cell survival ↑ Apoptosis | ↓ Bcl-2 protein | [38] |
| LNCaP LNCaP-A1 | UA 10, 20, 50, 80, 100 µM | ↓ Cell viability ↑ Apoptosis | ↑ p-JNK ↑ p-c-Jun ↑ caspase-3 activity ↑ caspase-9 activity ↑ p-Bcl-2 protein | [39] |
| LNCaP | UA 40 µM | ↓ Cell proliferation ↑ Apoptosis | ↑ cleaved ROCK1 ↑ p-PTEN protein ↑ cofilin-1 ↑ cytochrome c ↑ caspase-3 activity ↑ caspase-9 activity | [40] |
| RC-58T/h/SA#4 | UA 40 µM | ↓ Cell survival ↓ Cell density ↑ Apoptosis ↑ SubG1 cell population | ↑ DNA fragmentation ↑ caspase-3,-8, and -9 activity ↑ PARP cleavage ↑ Bax protein ↓ Bcl-2 protein ↓ Bid protein ↑ AIF protein ↑ AIF nuclear translocation | [41] |
| PC-3 | UA 80 µM | ↓ Cell viability ↑ Apoptosis | ↑ caspase-3, -8, and -9 activity ↑ caspase-8 and -9 cleavage ↑ p-JNK protein ↓ total-Bcl-2 protein ↑ p-Bcl-2 protein ↑ FasL mRNA and protein ↓ p-Akt ↓ MMP-9 levels | [42] |
| PC-3 | UA 40 µM | ↓ Cell viability G1 phase arrest | ↑ PARP cleavage ↓ cyclin D1 and D3 protein ↓ CDK4 protein ↑ p21 protein ↑ LC3-II protein ↓ p-Akt protein ↓ p-mTOR protein ↓ p-p70S6K ↓ p-4EBP1 | [43] |
| PC-3 LNCaP | UA 0–80 µM | ↓ Proliferation ↑ Apoptosis | ↓ Bcl-2 ↓ Bcl-xl ↓ survivin ↓ PI3K ↓ p-Akt protein ↓ p-mTOR protein ↑ cleaved caspase-3 | [44] |
| DU145 LNCaP | UA 50 µM | ↓ Cell proliferation ↑ Apoptosis | ↓ p-AKT protein ↓ p-IkBα protein ↓ p65 protein and nuclear translocation ↓ p-IKKα/β protein ↓ NK-kB DNA binding ↓ p-STAT3 protein ↓ p-Src protein ↓ p-JAK2 protein | [45] |
| DU145 | UA 50 µM | ↓ Cell viability ↑ Apoptosis | ↑ p-JNK protein ↑ p-c-Jun protein ↑ caspase-3, -9 activity ↑ p-Bcl-2, ↓ Bcl-2 protein | [46] |
| DU145 | UA 25 µM | ↑ Apoptosis | ↑ ATP in cytosol ↑ P2Y2 mRNA ↑ COX-2 protein ↑ DNA fragmentation ↑ p-p38 ↑ p-Src protein | [47] |
| DU145 | UA 10–40 µM | ↓ Cell viability ↑ Apoptosis | ↑ caspase-3 activity ↑ caspase-9 activity ↑ cyt-cytochrome c ↓ Mit-cytochrome c ↓ ROCK protein expression ↑ PTEN protein expression ↓ Cofilin-1 | [48] |
| PC-3 LNCaP DU145 | UA 35 µM 47 µM 80 µM | ↓ Cell viability ↑ Cytotoxicity ↑ Apoptosis | ↑cleaved PARP ↑ cleaved caspase-9 ↑ cleaved caspase-3 ↓ Wnt5α/β protein ↑ p-GS3β protein ↓ β-catenin | [49] |
| PC-3 LNCaP DU145 | UA (30 µM) UA + TRAIL | ↓ Cell viability ↑ Apoptosis | ↑ cleaved PARP ↑ cleaved caspase-9 ↑ cleaved caspase-3 ↑ CHOP | [50] |
| HMVP2 DU145 PC-3 C4-2B | UA 20 µM | ↓ Cell viability ↑ ROS | ↓ ATP bioluminescence | [51] |
| PC-3 DU145 LNCaP | UA 50 µM | ↓ Cell viability ↓ Cell Migration | ↓ CXCR4 protein ↓ CXCR4 mRNA ↓ CXCL12 | [52] |
| DU145 | UA 2.2–21.9 µM | ↓ MMP-2 activity ↓ MMP-9 activity | [53] | |
| LNCaP PC-3 | UA 20 µM | ↓ Colony formation ↑ ROS | [54] | |
| DU145 Exposed to radiation | UA 30 µM | ↓ Cell survival ↑ Apoptosis ↑ DNA fragmentation | ↑ cleaved PARP ↓ Bid | [55] |
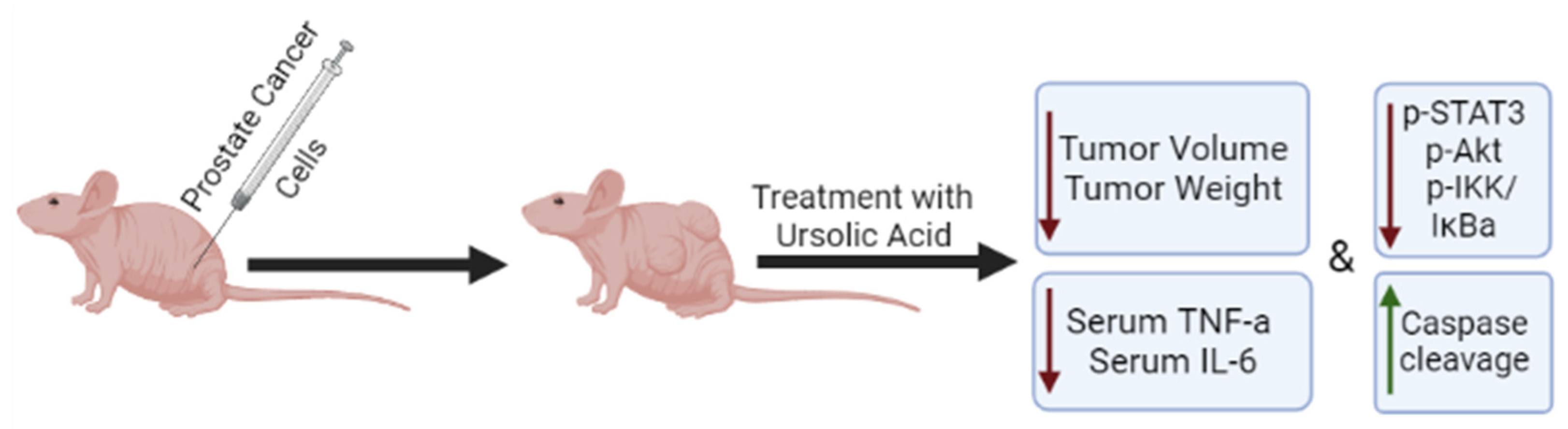
2.2. Effects of Ursolic Acid against Prostate Cancer: Evidence from In Vivo Studies
| Model | Dose/Duration | Findings | Mechanism | Reference |
|---|---|---|---|---|
| Allograft mouse HMVP2 cells | UA UA + CUR UA + RES | ↓ Tumor volume ↓ Tumor weight | Not investigated | [51] |
| Male NCr immunodeficient mice VcaP cells injected subcutaneously | UA 0.1% (w/w) Orally 8 weeks | ↓ Tumor growth | ↑ SAM | [56] |
| 6-week nude mice DU145 cells injected subcutaneously | UA 200 mg/kg Orally Twice a week, 6 weeks | ↓ Tumor volume | ↓ VEGF ↑ caspase-3 | [45] |
| TRAMP mice 4 weeks 12 weeks 24 weeks | UA 1% (w/w) 4–12 weeks 12–18 weeks 24–36 weeks | ↓ PIN ↓ Tumor volume ↑ Overall survival | ↓ p-STAT3 ↓ p-AKT ↓ p-IKKα/β ↓ serum TNF-α ↓ serum IL-6 | [57] |
| TRAMP mice | UA 1% w/w 12 weeks | ↓ Metastasis | ↓ CXCR4 | [52] |
| Prostate-specific PTEN KO male mice | UA 0.1% (w/w) Orally 6 and 14 weeks | ∆ Methylation ∆ Gene expression | ↓ Has3 mRNA ↓Cfh mRNA ↓ Msx1 mRNA ↑BDH2 ↓ Ephas ↓ Isg15 ↓ Nos2 | [58] |
| Female athymic nude mice | UA 20–40 mg/kg 3 weeks | ↓ Tumor volume ↓ Tumor weight | ↓ p-Akt ↓ p-mTOR ↓ Ki67 | [44] |
3. Effects of Ursolic Acid against Other Urogenital Cancers
3.1. Effects of Ursolic Acid against Renal and Bladder Cancer
3.2. Effects of Ursolic Acid Derivatives/Nanoformulations against Renal and Bladder Cancers
3.3. Effects of Ursolic Acid against Pheochromocytoma and Testicular Cancer
4. Bioavailability and Pharmacokinetics of Ursolic Acid
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Tan, M.H.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.-L. Androgen Receptor: Structure, Role in Prostate Cancer and Drug Discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Karantanos, T.; Corn, P.G.; Thompson, T.C. Prostate Cancer Progression after Androgen Deprivation Therapy: Mechanisms of Castrate Resistance and Novel Therapeutic Approaches. Oncogene 2013, 32, 5501–5511. [Google Scholar] [CrossRef]
- Sun, M.; Wang, G.; Paciga, J.E.; Feldman, R.I.; Yuan, Z.-Q.; Ma, X.-L.; Shelley, S.A.; Jove, R.; Tsichlis, P.N.; Nicosia, S.V.; et al. AKT1/PKBα Kinase Is Frequently Elevated in Human Cancers and Its Constitutive Activation Is Required for Oncogenic Transformation in NIH3T3 Cells. Am. J. Pathol. 2001, 159, 431–437. [Google Scholar] [CrossRef]
- Drake, J.M.; Graham, N.A.; Lee, J.K.; Stoyanova, T.; Faltermeier, C.M.; Sud, S.; Titz, B.; Huang, J.; Pienta, K.J.; Graeber, T.G.; et al. Metastatic Castration-Resistant Prostate Cancer Reveals Intrapatient Similarity and Interpatient Heterogeneity of Therapeutic Kinase Targets. Proc. Natl. Acad. Sci. USA 2013, 110, E4762–E4769. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative Genomic Profiling of Human Prostate Cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal Feedback Regulation of PI3K and Androgen Receptor Signaling in PTEN-Deficient Prostate Cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Ursolic Acid in Cancer Prevention and Treatment: Molecular Targets, Pharmacokinetics and Clinical Studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef]
- Guo, X.E.; Ngo, B.; Modrek, A.S.; Lee, W.-H. Targeting Tumor Suppressor Networks for Cancer Therapeutics. Curr. Drug Targets 2014, 15, 2–16. [Google Scholar] [CrossRef]
- Sever, R.; Brugge, J.S. Signal Transduction in Cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef]
- Cordon-Cardo, C.; Koff, A.; Drobnjak, M.; Capodieci, P.; Osman, I.; Millard, S.S.; Gaudin, P.B.; Fazzari, M.; Zhang, Z.-F.; Massague, J.; et al. Distinct Altered Patterns of p27KIP1 Gene Expression in Benign Prostatic Hyperplasia and Prostatic Carcinoma. JNCI J. Natl. Cancer Inst. 1998, 90, 1284–1291. [Google Scholar] [CrossRef]
- Whang, Y.E.; Wu, X.; Suzuki, H.; Reiter, R.E.; Tran, C.; Vessella, R.L.; Said, J.W.; Isaacs, W.B.; Sawyers, C.L. Inactivation of the Tumor Suppressor PTEN/MMAC1 in Advanced Human Prostate Cancer through Loss of Ex-pression. Proc. Natl. Acad. Sci. USA 1998, 95, 5246–5250. [Google Scholar] [CrossRef] [PubMed]
- Osman, I.; Drobnjak, M.; Fazzari, M.; Ferrara, J.; Scher, H.I.; Cordon-Cardo, C. Inactivation of the P53 Pathway in Prostate Cancer: Impact on Tumor Progression. Clin. Cancer Res. 1999, 5, 2082–2088. [Google Scholar]
- Lomonosova, E.; Chinnadurai, G. BH3-Only Proteins in Apoptosis and beyond: An Overview. Oncogene 2008, 27, S2–S19. [Google Scholar] [CrossRef]
- Oliver, F.J.; de la Rubia, G.; Rolli, V.; Ruiz-Ruiz, M.C.; de Murcia, G.; Murcia, J.M.-D. Importance of Poly(ADP-Ribose) Polymerase and Its Cleavage in Apoptosis: Lesson from an Uncleavable Mutant. J. Biol. Chem. 1998, 273, 33533–33539. [Google Scholar] [CrossRef] [PubMed]
- Pinkawa, M. External Beam Radiotherapy for Prostate Cancer. Panminerva Med. 2010, 52, 195–207. [Google Scholar] [PubMed]
- Stish, B.J.; Davis, B.J.; Mynderse, L.A.; Deufel, C.L.; Choo, R. Brachytherapy in the Management of Prostate Cancer. Surg. Oncol. Clin. N. Am. 2017, 26, 491–513. [Google Scholar] [CrossRef]
- Mohile, S.G.; Mustian, K.; Bylow, K.; Hall, W.; Dale, W. Management of Complications of Androgen Deprivation Therapy in the Older Man. Crit. Rev. Oncol. 2009, 70, 235–255. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Van Oosterom, A.T.; Schriivers, D. Docetaxel (Taxotere), a Review of Preclinical and Clinical Experience. Part II: Clinical Experience. Anti-Cancer Drugs 1995, 6, 356–368. [Google Scholar] [CrossRef]
- Hashemi, M.; Zandieh, M.A.; Talebi, Y.; Rahmanian, P.; Shafiee, S.S.; Nejad, M.M.; Babaei, R.; Sadi, F.H.; Rajabi, R.; Abkenar, Z.O.; et al. Paclitaxel and Docetaxel Resistance in Prostate Cancer: Molecular Mechanisms and Possible Therapeutic Strate-gies. Biomed. Pharmacother. 2023, 160, 114392. [Google Scholar] [CrossRef]
- Brizuela, L.; Dayon, A.; Doumerc, N.; Ader, I.; Golzio, M.; Izard, J.-C.; Hara, Y.; Malavaud, B.; Cuvillier, O. The Sphingosine Kinase-1 Survival Pathway Is a Molecular Target for the Tumor-Suppressive Tea and Wine Polyphe-nols in Prostate Cancer. FASEB J. 2010, 24, 3882–3894. [Google Scholar] [CrossRef] [PubMed]
- Tabasinezhad, M.; Samadi, N.; Ghanbari, P.; Mohseni, M.; Saei, A.A.; Sharifi, S.; Saeedi, N.; Pourhassan, A. Sphingosin 1-Phosphate Contributes in Tumor Progression. J. Cancer Res. Ther. 2013, 9, 556. [Google Scholar] [CrossRef]
- Zou, H.; Li, Y.; Liu, X.; Wu, Z.; Li, J.; Ma, Z. Roles of Plant-Derived Bioactive Compounds and Related MicroRNAs in Cancer Therapy. Phytotherapy Res. 2021, 35, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tao, S.; Zhang, S. Characterization and Quantification of Polyphenols and Triterpenoids in Thinned Young Fruits of Ten Pear Varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef]
- Kowalski, R. Studies of Selected Plant Raw Materials as Alternative Sources of Triterpenes of Oleanolic and Ursolic Acid Types. J. Agric. Food Chem. 2007, 55, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants–Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef]
- Ivanov, I.; Petkova, N.; Tumbarski, Y.; Vrancheva, R.; Stoyanova, M. Lavender Waste-Promising Source of Triterpenoids and Polypneols with Antioxidant and Antimicrobial Activity. Ind. Technol. 2018, 5, 26–32. [Google Scholar]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; Sharma, A.K. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Patents Inflamm. Allergy Drug Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, H.; Sui, Z.; Jing, F.; Quan, X.; Zhao, W.; Liu, G. Ursolic Acid Exhibits Anti-Inflammatory Effects through Blocking TLR4-MyD88 Pathway Mediated by Autophagy. Cytokine 2019, 123, 154726. [Google Scholar] [CrossRef]
- Habtemariam, S. Antioxidant and Anti-inflammatory Mechanisms of Neuroprotection by Ursolic Acid: Addressing Brain Injury, Cerebral Ischemia, Cognition Deficit, Anxiety, and Depression. Oxidative Med. Cell. Longev. 2019, 2019, 8512048. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef]
- Camer, D.; Yu, Y.; Szabo, A.; Huang, X.-F. The Molecular Mechanisms Underpinning the Therapeutic Properties of Oleanolic Acid, Its Isomer and Deriva-tives for Type 2 Diabetes and Associated Complications. Mol. Nutr. Food Res. 2014, 58, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Li, T.; Tian, J.X.; Xi, P.; Liu, R.H. Ursolic Acid, a Potential Anticancer Compound for Breast Cancer Therapy. Crit. Rev. Food Sci. Nutr. 2018, 58, 568–574. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Soon, C.Y.; Tan, J.B.L.; Wong, S.K.; Hui, Y.W. Ursolic Acid: An Overview on Its Cytotoxic Activities against Breast and Colorectal Cancer Cells. J. Integr. Med. 2019, 17, 155–160. [Google Scholar] [CrossRef]
- Kornel, A.; Nadile, M.; Tsiani, E. Evidence of the Beneficial Effects of Ursolic Acid against Lung Cancer. Molecules 2022, 27, 7466. [Google Scholar] [CrossRef]
- Kassi, E.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Manoussakis, M.; Moutsatsou, P. Ursolic Acid, a Naturally Occurring Triterpenoid, Demonstrates Anticancer Activity on Human Prostate Cancer Cells. J. Cancer Res. Clin. Oncol. 2007, 133, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-X.; Kong, C.-Z.; Wang, L.-H.; Li, J.-Y.; Liu, X.-K.; Xu, B.; Xu, C.-L.; Sun, Y.-H. Ursolic Acid Overcomes Bcl-2-Mediated Resistance to Apoptosis in Prostate Cancer Cells Involving Activation of JNK-Induced Bcl-2 Phosphorylation and Degradation. J. Cell. Biochem. 2009, 109, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Zhou, G.; Li, J.; Su, B.; Guo, H. Ursolic Acid Activates the Apoptosis of Prostate Cancer via ROCK/PTEN Mediated Mitochondrial Translocation of Cofilin-1. Oncol. Lett. 2017, 15, 3202–3206. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Park, H.-Y.; Kim, J.-Y.; Jeong, I.-Y.; Lee, M.-K.; Seo, K.-I. Apoptotic Action of Ursolic Acid Isolated from Corni Fructus in RC-58T/h/SA#4 Primary Human Prostate Cancer Cells. Bioorg. Med. Chem. Lett. 2010, 20, 6435–6438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, C.; Zeng, Y.; Wang, L.; Li, Z.; Wang, H.; Xu, C.; Sun, Y. Ursolic Acid Induces PC-3 Cell Apoptosis via Activation of JNK and Inhibition of Akt Pathways in Vitro. Mol. Carcinog. 2010, 49, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Kim, S.Y.; Park, J.-W. Autophagy Inhibition Enhances Ursolic Acid-Induced Apoptosis in PC3 Cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lin, Z.-M.; Ge, N.; Zhang, D.-L.; Huang, J.; Kong, F. Ursolic Acid Induces Apoptosis of Prostate Cancer Cells via the PI3K/Akt/mTOR Pathway. Am. J. Chin. Med. 2015, 43, 1471–1486. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; Ho, P.C.; et al. Ursolic Acid Inhibits Multiple Cell Survival Pathways Leading to Suppression of Growth of Prostate Cancer Xen-ograft in Nude Mice. J. Mol. Med. 2011, 89, 713–727. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Kong, C.-Z.; Wang, H.-Q.; Wang, L.-H.; Xu, C.-L.; Sun, Y.-H. Phosphorylation of Bcl-2 and Activation of Caspase-3 via the c-Jun N-Terminal Kinase Pathway in Ursolic Ac-id-Induced DU145 Cells Apoptosis. Biochimie 2009, 91, 1173–1179. [Google Scholar] [CrossRef]
- Limami, Y.; Pinon, A.; Leger, D.Y.; Pinault, E.; Delage, C.; Beneytout, J.-L.; Simon, A.; Liagre, B. The P2Y2/Src/P38/COX-2 Pathway Is Involved in the Resistance to Ursolic Acid-Induced Apoptosis in Colorectal and Prostate Cancer Cells. Biochimie 2012, 94, 1754–1763. [Google Scholar] [CrossRef]
- Gai, W.-T.; Yu, D.-P.; Wang, X.-S.; Wang, P.-T. Anti-Cancer Effect of Ursolic Acid Activates Apoptosis through ROCK/PTEN Mediated Mitochondrial Transloca-tion of Cofilin-1 in Prostate Cancer. Oncol. Lett. 2016, 12, 2880–2885. [Google Scholar] [CrossRef]
- Park, J.-H.; Kwon, H.-Y.; Sohn, E.J.; Kim, K.A.; Kim, B.; Jeong, S.-J.; Song, J.H.; Koo, J.S.; Kim, S.-H. Inhibition of Wnt/β-Catenin Signaling Mediates Ursolic Acid-Induced Apoptosis in PC-3 Prostate Cancer Cells. Pharmacol. Rep. 2013, 65, 1366–1374. [Google Scholar] [CrossRef]
- Shin, S.W.; Park, J.-W. Ursolic Acid Sensitizes Prostate Cancer Cells to TRAIL-Mediated Apoptosis. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 723–730. [Google Scholar] [CrossRef]
- Lodi, A.; Saha, A.; Lu, X.; Wang, B.; Sentandreu, E.; Collins, M.; Kolonin, M.G.; DiGiovanni, J.; Tiziani, S. Combinatorial Treatment with Natural Compounds in Prostate Cancer Inhibits Prostate Tumor Growth and Leads to Key Modulations of Cancer Cell Metabolism. NPJ Precis. Oncol. 2017, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Manu, K.A.; Ong, T.H.; Ramachandran, L.; Surana, R.; Bist, P.; Lim, L.H.K.; Prem Kumar, A.; Hui, K.M.; Sethi, G. Inhibition of CXCR4/CXCL12 Signaling Axis by Ursolic Acid Leads to Suppression of Metastasis in Transgenic Adenocarcinoma of Mouse Prostate Model. Int. J. Cancer 2011, 129, 1552–1563. [Google Scholar] [CrossRef]
- Kondo, M.; MacKinnon, S.L.; Craft, C.C.; Matchett, M.D.; Hurta, R.A.R.; Neto, C.C. Ursolic Acid and Its Esters: Occurrence in Cranberries and Other Vaccinium Fruit and Effects on Matrix Metallo-proteinase Activity in DU145 Prostate Tumor Cells: Anti-Tumor Activity and Content of Ursolic Acid from Vac-cinium Fruit. J. Sci. Food Agric. 2011, 91, 789–796. [Google Scholar] [CrossRef]
- Wang, C.; Shu, L.; Zhang, C.; Li, W.; Wu, R.; Guo, Y.; Yang, Y.; Kong, A.-N. Histone Methyltransferase Setd7 Regulates Nrf2 Signaling Pathway by Phenethyl Isothiocyanate and Ursolic Acid in Human Prostate Cancer Cells. Mol. Nutr. Food Res. 2018, 62, e1700840. [Google Scholar] [CrossRef]
- Koh, S.J.; Tak, J.K.; Kim, S.T.; Nam, W.S.; Kim, S.Y.; Park, K.M.; Park, J.-W. Sensitization of Ionizing Radiation-Induced Apoptosis by Ursolic Acid. Free. Radic. Res. 2012, 46, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, R.; Wang, L.; Kuo, H.D.; Sargsyan, D.; Zheng, X.; Wang, Y.; Su, X.; Kong, A. Triterpenoid Ursolic Acid Drives Metabolic Rewiring and Epigenetic Reprogramming in Treatment/Prevention of Human Prostate Cancer. Mol. Carcinog. 2022, 61, 111–121. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ong, T.H.; Kumar, A.P.; Lun, C.K.; Ho, P.C.; Wong, P.T.H.; Hui, K.M.; Sethi, G. Ursolic Acid Inhibits the Initiation, Progression of Prostate Cancer and Prolongs the Survival of TRAMP Mice by Modulating Pro-Inflammatory Pathways. PLoS ONE 2012, 7, e32476. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Sarwar, M.S.; Chou, P.; Wang, Y.; Su, X.; Kong, A.-N.T. PTEN-Knockout Regulates Metabolic Rewiring and Epigenetic Reprogramming in Prostate Cancer and Chemoprevention by Triterpenoid Ursolic Acid. FASEB J. 2022, 36, e22626. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Nie, M.; Tian, Y.; Chen, X.; Chen, C.; Chen, H.; Liu, R. Ursolic Acid Derivative FZU-03,010 Inhibits STAT3 and Induces Cell Cycle Arrest and Apoptosis in Renal and Breast Cancer Cells. Acta Biochim. Biophys. Sin. 2017, 49, 367–373. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Tang, B.-X.; Chen, W.-Y.; Zhao, M.-S. Ursolic Acid Inhibits the Invasiveness of A498 Cells via NLRP3 Inflammasome Activation. Oncol. Lett. 2020, 20, 170. [Google Scholar] [CrossRef]
- Gai, L.; Cai, N.; Wang, L.; Xu, X.; Kong, X. Ursolic Acid Induces Apoptosis via Akt/NF-κB Signaling Suppression in T24 Human Bladder Cancer Cells. Mol. Med. Rep. 2013, 7, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Agrawal, S.S.; Alhaider, A.A. Ursolic Acid Inhibits Tumor Angiogenesis and Induces Apoptosis through Mitochondrial-Dependent Pathway in Ehrlich Ascites Carcinoma Tumor. Chem. Interactions 2013, 206, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-H.; Zhang, Z.-H.; Li, M.-Y.; Wei, Z.-Y.; Jin, X.-J.; Piao, H.-R. Synthesis and Evaluation of the HIF-1α Inhibitory Activities of Novel Ursolic Acid Tetrazole Derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Mennitto, A.; Huber, V.; Ratta, R.; Sepe, P.; de Braud, F.; Procopio, G.; Guadalupi, V.; Claps, M.; Stellato, M.; Daveri, E.; et al. Angiogenesis and Immunity in Renal Carcinoma: Can We Turn an Unhappy Relationship into a Happy Marriage? J. Clin. Med. 2020, 9, 930. [Google Scholar] [CrossRef]
- Chadalapaka, G.; Jutooru, I.; McAlees, A.; Stefanac, T.; Safe, S. Structure-Dependent Inhibition of Bladder and Pancreatic Cancer Cell Growth by 2-Substituted Glycyrrhetinic and Ursolic Acid Derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 2633–2639. [Google Scholar] [CrossRef]
- Tu, H.-Y.; Huang, A.-M.; Wei, B.-L.; Gan, K.-H.; Hour, T.-C.; Yang, S.-C.; Pu, Y.-S.; Lin, C.-N. Ursolic Acid Derivatives Induce Cell Cycle Arrest and Apoptosis in NTUB1 Cells Associated with Reactive Oxy-gen Species. Bioorg. Med. Chem. 2009, 17, 7265–7274. [Google Scholar] [CrossRef]
- Zheng, Q.-Y.; Jin, F.-S.; Yao, C.; Zhang, T.; Zhang, G.-H.; Ai, X. Ursolic Acid-Induced AMP-Activated Protein Kinase (AMPK) Activation Contributes to Growth Inhibition and Apoptosis in Human Bladder Cancer T24 Cells. Biochem. Biophys. Res. Commun. 2012, 419, 741–747. [Google Scholar] [CrossRef]
- Zheng, Q.-Y.; Li, P.-P.; Jin, F.-S.; Yao, C.; Zhang, G.-H.; Zang, T.; Ai, X. Ursolic Acid Induces ER Stress Response to Activate ASK1-JNK Signaling and Induce Apoptosis in Human Blad-der Cancer T24 Cells. Cell. Signal. 2013, 25, 206–213. [Google Scholar] [CrossRef]
- Lin, K.-W.; Huang, A.-M.; Lin, C.-C.; Chang, C.-C.; Hsu, W.-C.; Hour, T.-C.; Pu, Y.-S.; Lin, C.-N. Anti-Cancer Effects of Ursane Triterpenoid as a Single Agent and in Combination with Cisplatin in Bladder Can-cer. Eur. J. Pharmacol. 2014, 740, 742–751. [Google Scholar] [CrossRef]
- Huang, X.; Sun, Y.; Zhu, J.; Tong, H.; Wen, P.; He, W. Ursolic Acid Enhances Gemcitabine—Induced Apoptosis in Bladder Cancer via the PI3K/AKT and JNK Signaling Pathways, In Review. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Chapter 5—Chemosensitization by Ursolic Acid: A New Avenue for Cancer Therapy. In Role of Nutraceuticals in Cancer Chemosensitization; Bharti, A.C., Aggarwal, B.B., Eds.; Cancer Sensitizing Agents for Chemotherapy; Academic Press: Cambridge, MA, USA, 2018; Volume 2, pp. 99–109. [Google Scholar]
- Jung, J.; Seo, J.; Kim, J.; Kim, J.H. Ursolic Acid Causes Cell Death in PC-12 Cells by Inducing Apoptosis and Impairing Autophagy. Anticancer. Res. 2018, 38, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-H.; Youn, K.; Ho, C.-T.; Karwe, M.V.; Jeong, W.-S.; Jun, M. p-Coumaric Acid and Ursolic Acid from Corni fructus Attenuated β-Amyloid25–35-Induced Toxicity through Regulation of the NF-κB Signaling Pathway in PC12 Cells. J. Agric. Food Chem. 2014, 62, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-J.; Yin, M.-C. Antioxidative and Anti-Inflammatory Protection of Oleanolic Acid and Ursolic Acid in PC12 Cells. J. Food Sci. 2008, 73, H174–H178. [Google Scholar] [CrossRef] [PubMed]
- Giannatempo, P.; Greco, T.; Mariani, L.; Nicolai, N.; Tana, S.; Farè, E.; Raggi, D.; Piva, L.; Catanzaro, M.; Biasoni, D.; et al. Radiotherapy or Chemotherapy for Clinical Stage IIA and IIB Seminoma: A Systematic Review and Meta-Analysis of Patient Outcomes. Ann. Oncol. 2015, 26, 657–668. [Google Scholar] [CrossRef]
- Sun, Q.; He, M.; Zhang, M.; Zeng, S.; Chen, L.; Zhou, L.; Xu, H. Ursolic Acid: A Systematic Review of Its Pharmacology, Toxicity and Rethink on Its Pharmacokinetics Based on PK-PD Model. Fitoterapia 2020, 147, 104735. [Google Scholar] [CrossRef]
- Hua, W.J.; Fang, H.J.; Hua, W.X. Transepithelial Transport of Rosuvastatin and Effect of Ursolic Acid on Its Transport in Caco-2 Monolayers. Eur. J. Drug Metab. Pharmacokinet. 2012, 37, 225–231. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, S.; Zhang, Y.; Chen, Z. Development of a Liquid Chromatography-Mass Spectrometry Method for the Determination of Ursolic Acid in Rat Plasma and Tissue: Application to the Pharmacokinetic and Tissue Distribution Study. Anal. Bioanal. Chem. 2011, 399, 2877–2884. [Google Scholar] [CrossRef]
- Liao, Q.; Yang, W.; Jia, Y.; Chen, X.; Gao, Q.; Bi, K. LC-MS Determination and Pharmacokinetic Studies of Ursolic Acid in Rat Plasma after Administration of the Traditional Chinese Medicinal Preparation Lu-Ying Extract. Yakugaku Zasshi 2005, 125, 509–515. [Google Scholar] [CrossRef]
- Yin, M.-C.; Lin, M.-C.; Mong, M.-C.; Lin, C.-Y. Bioavailability, Distribution, and Antioxidative Effects of Selected Triterpenes in Mice. J. Agric. Food Chem. 2012, 60, 7697–7701. [Google Scholar] [CrossRef]
- Hirsh, S.; Huber, L.; Zhang, P.; Stein, R.; Joyal, S. A Single Ascending Dose, Initial Clinical Pharmacokinetic and Safety Study of Ursolic Acid in Healthy Adult Volunteers (1044.6). FASEB J. 2014, 28, 1044.6. [Google Scholar] [CrossRef]
- Miatmoko, A.; Mianing, E.A.; Sari, R.; Hendradi, E. Nanoparticles Use for Delivering Ursolic Acid in Cancer Therapy: A Scoping Review. Front. Pharmacol. 2021, 12, 787226. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wei, G.; Si, D.; Liu, C. Quantitation of Ursolic Acid in Human Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry and Its Pharmacokinetic Study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhu, Z.; Qian, Z.; Zhao, C.; Wang, H.; Ying, G. A Phase I Pharmacokinetic Study of Ursolic Acid Nanoliposomes in Healthy Volunteers and Patients with Ad-vanced Solid Tumors. Int. J. Nanomed. 2013, 8, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Zhou, S.-Y.; Qian, Z.-Z.; Zhang, H.-L.; Qiu, L.-H.; Song, Z.; Zhao, J.; Wang, P.; Hao, X.-S.; Wang, H.-Q. Evaluation of Toxicity and Single-Dose Pharmacokinetics of Intravenous Ursolic Acid Liposomes in Healthy Adult Volunteers and Patients with Advanced Solid Tumors. Expert Opin. Drug Metab. Toxicol. 2013, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Wang, X.; Song, Z.; Zhang, H.; Zhou, S.; Zhao, J.; Wang, H. A Phase I Trial to Evaluate the Multiple-Dose Safety and Antitumor Activity of Ursolic Acid Liposomes in Subjects with Advanced Solid Tumors. BioMed Res. Int. 2015, 2015, 809714. [Google Scholar] [CrossRef] [PubMed]

| Cell Type | Dose/Duration | Findings | Mechanism | Reference |
|---|---|---|---|---|
| Renal Cancer | ||||
| 786-O | UA 5 and 10 µM 48 h | ↓ Cell viability ↑ Apoptosis | Cell cycle arrest G0/G1 phase | [59] |
| A498 | UA 0.5 and 5 µM 12 h | ↓ Cell viability ↓ Invasiveness | ↑ NLRP3 ↑ caspase-1 ↑ IL-1β ↓ MMP-2 | [60] |
| Bladder Cancer | ||||
| T24 | UA 12.5, 25, 50 µmol/L 48 h | ↓ Proliferation ↑ Apoptosis | ↓ p-Akt protein ↓ p-IkBα protein ↓ NF-κBp65 protein and mRNA ↓ Bcl-2 protein and mRNA ↑ caspase-3 protein and mRNA | [61] |
| Cell Type | Dose/Duration | Findings | Mechanism | Reference |
|---|---|---|---|---|
| Renal Cancer | ||||
| 786-O | FZU-03,010 48 h | ↓ Cell viability ↑ Apoptosis | Cell cycle arrest G0/G1 phase ↓ p-STAT3 ↑ p21 ↑ p27 ↑ Cleaved PARP ↑ Cleaved caspase-3 ↑ Cleaved caspase-7 | [59] |
| Bladder Cancer | ||||
| KU7, 253JB-V | UA derivatives | ↓ Proliferation | Not investigated | [65] |
| NTUB1 | UA derivatives | ↓ Proliferation ↑ Apoptosis | ↑ G2/M phase ↑ G1 phase ↑ROS ↓ Tubulin polymerization | [66] |
| T24 | UA derivatives | ↓ Proliferation ↑ Apoptosis | ↑ AMPK activation ↑ JNK activation ↓ mTORC1 activation ↓ Survivin | [67] |
| NTUB1 | UA derivatives | ↓ Proliferation ↑ G2/M phase ↑ G1 phase ↑ Apoptosis ↓ Tubulin polymerization | ↑ROS | [68] |
| NTUB1 | UA derivative (UA17) | ↓ Proliferation ↑ Apoptosis | ↑ROS ↑ p53 ↑ p38 MAPK activation | [69] |
| MBT-2 murine bladder cells injected to mice | UA derivative (UA17) 50, 100 mg/kg/day, intratumorally | ↓ Tumor size ↑ Survival | ↑ ROS | [69] |
| NTUB1 | UA derivatives | ↓ Proliferation ↑ G2/M phase | ↑ ROS | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornel, A.; Nadile, M.; Retsidou, M.I.; Sakellakis, M.; Gioti, K.; Beloukas, A.; Sze, N.S.K.; Klentrou, P.; Tsiani, E. Ursolic Acid against Prostate and Urogenital Cancers: A Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2023, 24, 7414. https://doi.org/10.3390/ijms24087414
Kornel A, Nadile M, Retsidou MI, Sakellakis M, Gioti K, Beloukas A, Sze NSK, Klentrou P, Tsiani E. Ursolic Acid against Prostate and Urogenital Cancers: A Review of In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 2023; 24(8):7414. https://doi.org/10.3390/ijms24087414
Chicago/Turabian StyleKornel, Amanda, Matteo Nadile, Maria Ilektra Retsidou, Minas Sakellakis, Katerina Gioti, Apostolos Beloukas, Newman Siu Kwan Sze, Panagiota Klentrou, and Evangelia Tsiani. 2023. "Ursolic Acid against Prostate and Urogenital Cancers: A Review of In Vitro and In Vivo Studies" International Journal of Molecular Sciences 24, no. 8: 7414. https://doi.org/10.3390/ijms24087414
APA StyleKornel, A., Nadile, M., Retsidou, M. I., Sakellakis, M., Gioti, K., Beloukas, A., Sze, N. S. K., Klentrou, P., & Tsiani, E. (2023). Ursolic Acid against Prostate and Urogenital Cancers: A Review of In Vitro and In Vivo Studies. International Journal of Molecular Sciences, 24(8), 7414. https://doi.org/10.3390/ijms24087414









