Apoptotic Cell Death in Bicuspid-Aortic-Valve-Associated Aortopathy
Abstract
:1. Introduction
1.1. Apoptosis
1.2. Autophagic Cell Death
2. Results
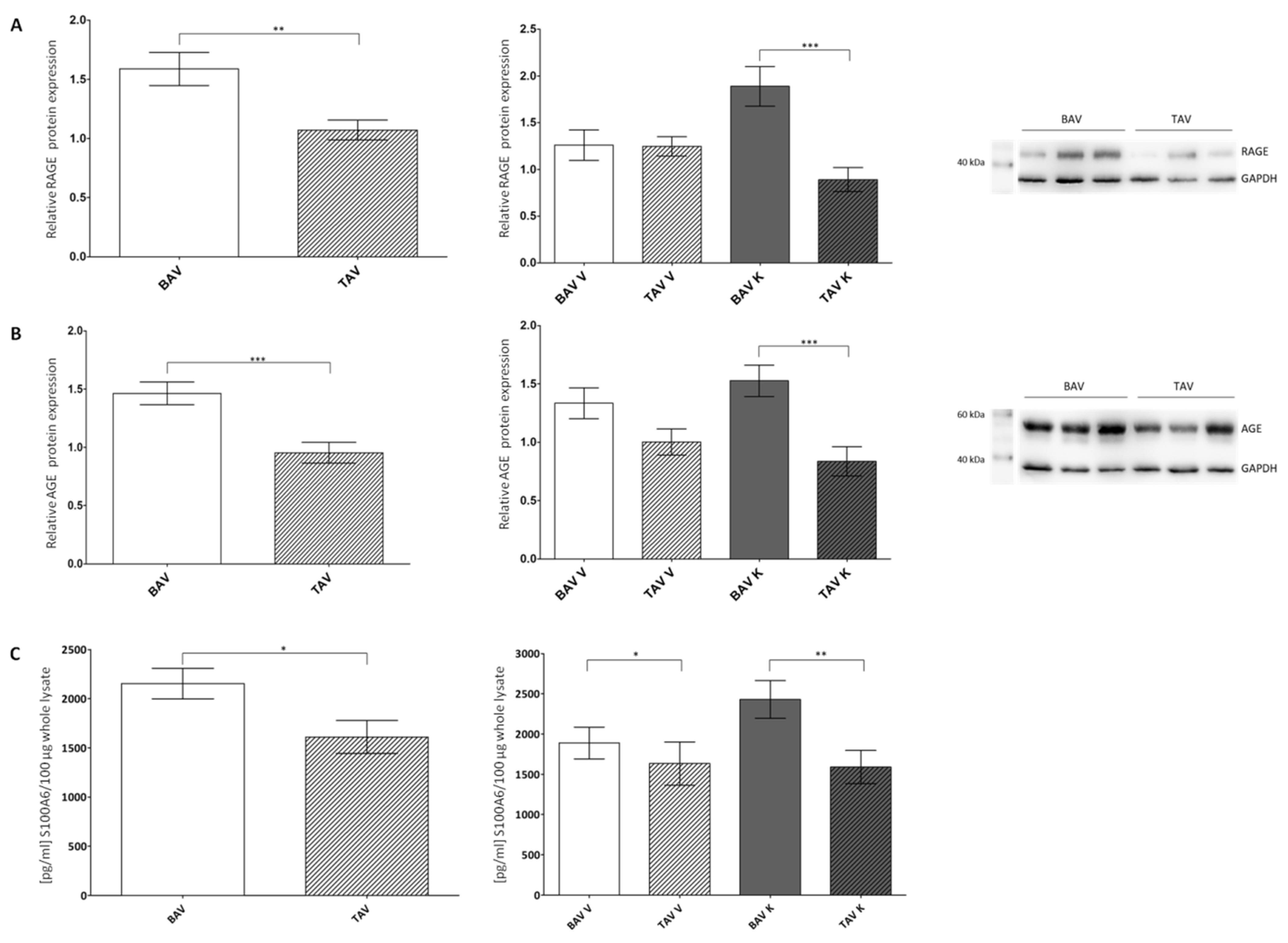
2.1. Expression of AGE, RAGE and S100A6
2.2. Protein Expression of Pro-Apoptotic Proteins
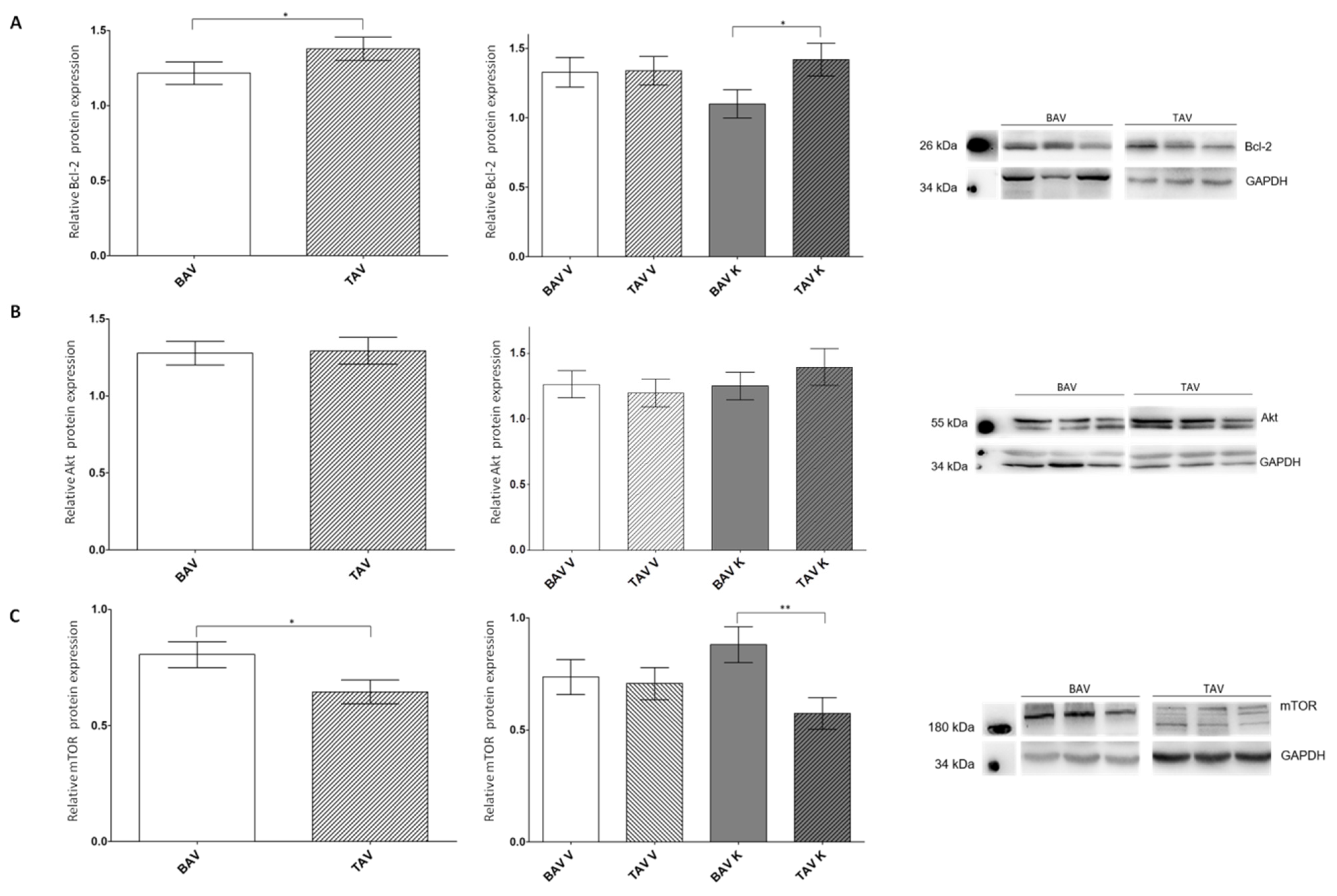
2.3. Protein Expression Levels of Anti-Apoptotic Proteins
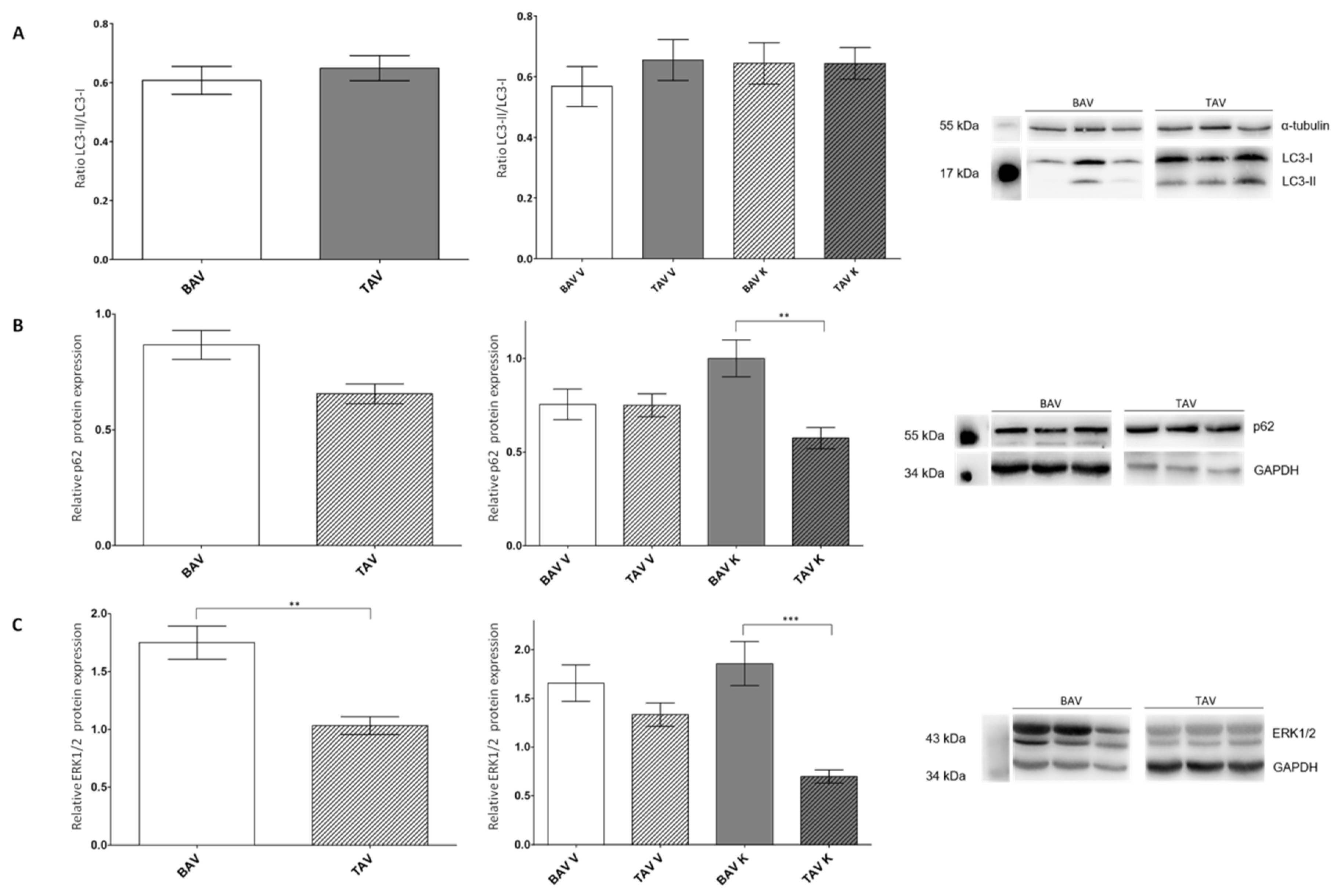
2.4. Investigation of Autophagy-Linked Proteins LC3 and p62
3. Discussion
3.1. Increased Expression of RAGE and Its Ligands AGE and S100A6
3.2. Protein Expression of Pro-Apoptotic Proteins Suggesting a Higher Affinity for Apoptosis in the Tissues of Patients with BAV
3.3. Protein Expression Levels of Anti-Apoptotic Proteins Imply a Protection from Apoptosis in the Tissue of Patients with TAV
3.4. Autophagic Cell Death Is More Likely to Occur in Patients with BAV
4. Materials and Methods
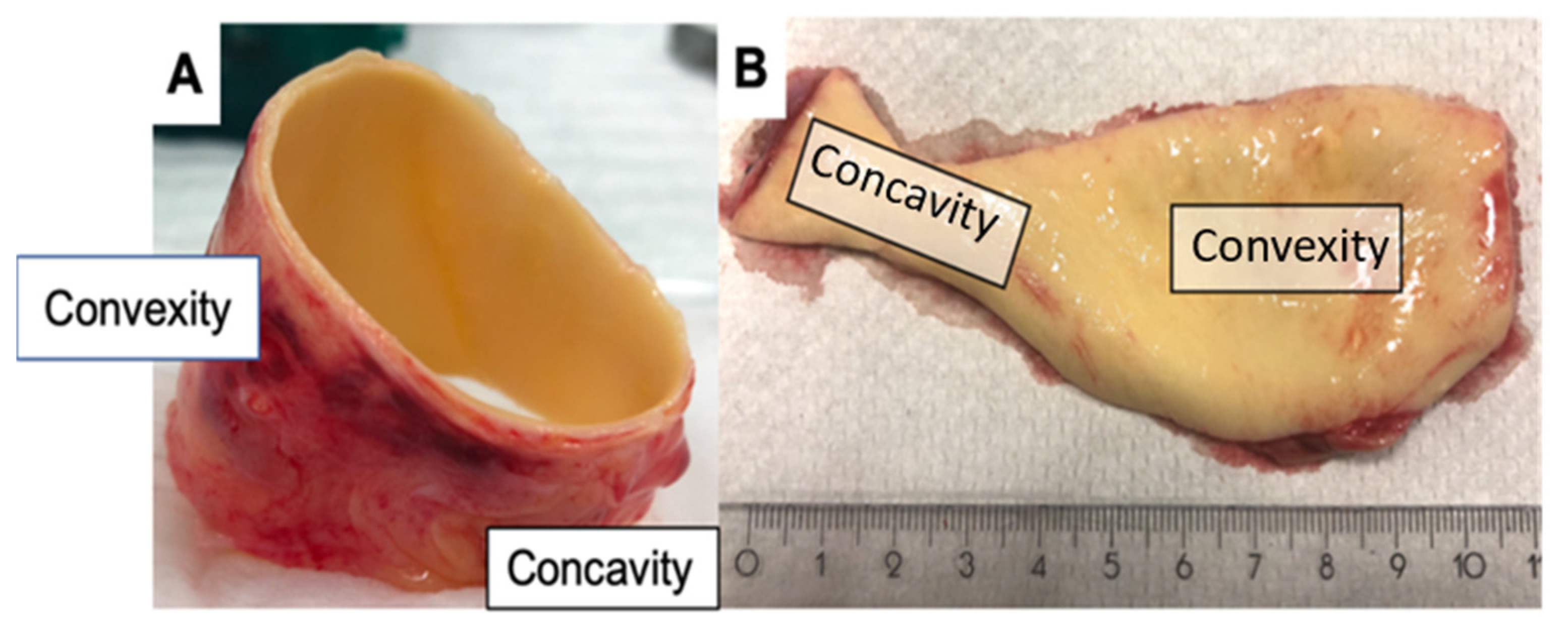
4.1. Patient Population and Sample Collection
4.2. Preparation of Protein Lysates
4.3. Western Blot Analysis
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4E-BP1 | eukaryotic translation initiation factor 4E-binding protein 1 |
| AGE | advanced glycation end products |
| Atg | autophagy-related protein |
| BAV | bicuspid aortic valve |
| Bcl-2 | B-cell lymphoma 2 |
| ELISA | enzyme-linked immunosorbent assay |
| ERK | extracellular signal-regulated kinases |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HRP | horseradish peroxidase |
| K | concave |
| LC3 | microtubule-associated protein 1 light chain 3 |
| MAPK | mitogen-activated protein kinase |
| MMP-2 | matrix metalloproteinase 2 |
| mTOR | mechanistic target of rapamycin |
| mTORC | mTOR complex |
| p62 | nucleoporin 62 |
| p70S6K | p70 ribosomal protein S6 kinase |
| PI3K | phosphoinositide 3-kinase |
| RAGE | receptor for advanced glycation products |
| S100A6 | S100 calcium-binding protein A6 |
| sRAGE | soluble RAGE |
| TAV | tricuspid aortic valve |
| TSC | tuberous sclerosis complex subunit |
| V | convex |
| VSMC | vascular smooth muscle cells |
References
- Hoffman, J.I. The global burden of congenital heart disease. Cardiovasc. J. Afr. 2013, 24, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.W.; Edwards, W.D. Risk factors for aortic dissection: A necropsy study of 161 cases. Am. J. Cardiol. 1984, 53, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Fedak, P.W.; de Sa, M.P.; Verma, S.; Nili, N.; Kazemian, P.; Butany, J.; Strauss, B.H.; Weisel, R.D.; David, T.E. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: Implications for aortic dilatation. J. Thorac. Cardiovasc. Surg. 2003, 126, 797–805. [Google Scholar] [CrossRef]
- LeMaire, S.A.; Wang, X.; Wilks, J.A.; Carter, S.A.; Wen, S.; Won, T.; Leonardelli, D.; Anand, G.; Conklin, L.D.; Wang, X.L.; et al. Matrix metalloproteinases in ascending aortic aneurysms: Bicuspid versus trileaflet aortic valves. J. Surg. Res. 2005, 123, 40–48. [Google Scholar] [CrossRef]
- Haunschild, J.; Schellinger, I.N.; Barnard, S.J.; Aspern, K.; von Davierwala, P.; Misfeld, M.; Petroff, D.; Borger, M.A.; Etz, C.D. Bicuspid aortic valve patients show specific epigenetic tissue signature increasing extracellular matrix destruction. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Yamagishi, S.-I.; Coughlan, M.T.; Harcourt, B.E.; Kantharidis, P.; Thallas-Bonke, V.; Okuda, S.; Cooper, M.E.; Forbes, J.M. Ramipril inhibits AGE-RAGE-induced matrix metalloproteinase-2 activation in experimental diabetic nephropathy. Diabetol. Metab. Syndr. 2014, 6, 86. [Google Scholar] [CrossRef]
- Malherbe, P.; Richards, J.; Gaillard, H.; Thompson, A.; Diener, C.; Schuler, A.; Huber, G. cDNA cloning of a novel secreted isoform of the human receptor for advanced glycation end products and characterization of cells co-expressing cell-surface scavenger receptors and Swedish mutant amyloid precursor protein. Mol. Brain Res. 1999, 71, 159–170. [Google Scholar] [CrossRef]
- Brett, J.; Schmidt, A.M.; Du Yan, S.; Zou, Y.S.; Weidman, E.; Pinsky, D.; Nowygrod, R.; Neeper, M.; Przysiecki, C.; Shaw, A.; et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am. J. Pathol. 1993, 143, 1699–1712. [Google Scholar]
- Branchetti, E.; Bavaria, J.E.; Grau, J.B.; Shaw, R.E.; Poggio, P.; Lai, E.K.; Desai, N.D.; Gorman, J.H.; Gorman, R.C.; Ferrari, G. Circulating soluble receptor for advanced glycation end product identifies patients with bicuspid aortic valve and associated aortopathies. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2349–2357. [Google Scholar] [CrossRef]
- Kuźnicki, J.; Filipek, A.; Heimann, P.; Kaczmarek, L.; Kamińska, B. Tissue specific distribution of calcyclin—10.5 kDa Ca2+—Binding protein. FEBS Lett. 1989, 254, 141–144. [Google Scholar] [CrossRef]
- Engelkamp, D.; Schäfer, B.W.; Erne, P.; Heizmann, C.W. S100α, CAPL, and CACY: Molecular cloning and expression analysis of three calcium-binding proteins from human heart. Biochemistry 1992, 31, 10258–10264. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Fritz, G.; Weibel, M.; Heizmann, C.W.; Galichet, A. S100B and S100A6 differentially modulate cell survival by interacting with distinct RAGE (receptor for advanced glycation end products) immunoglobulin domains. J. Biol. Chem. 2007, 282, 31317–31331. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.J.; Hickson, I.D. Cellular responses to DNA damage. Annu. Rev. Pharmacol Toxicol. 2001, 41, 367–401. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Majtnerová, P.; Roušar, T. An overview of apoptosis assays detecting DNA fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef]
- Nakagawa, T.; Zhu, H.; Morishima, N.; Li, E.; Xu, J.; Yankner, B.A.; Yuan, J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 2000, 403, 98–103. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- van Cruchten, S.; van den Broeck, W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat. Histol. Embryol. 2002, 31, 214–223. [Google Scholar] [CrossRef]
- Byun, Y.; Chen, F.; Chang, R.; Trivedi, M.; Green, K.J.; Cryns, V.L. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001, 8, 443–450. [Google Scholar] [CrossRef]
- Nakazono-Kusaba, A.; Takahashi-Yanaga, F.; Morimoto, S.; Furue, M.; Sasaguri, T. Staurosporine-induced cleavage of α-smooth muscle actin during myofibroblast apoptosis. J. Investig. Dermatol. 2002, 119, 1008–1013. [Google Scholar] [CrossRef]
- García-Sáez, A.J. The secrets of the Bcl-2 family. Cell Death Differ. 2012, 19, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C. PI3K: Downstream AKTion blocks apoptosis. Cell 1997, 88, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. United at last: The tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem. Soc. Trans. 2003, 31, 573–578. [Google Scholar] [CrossRef] [PubMed]
- McManus, E.J.; Alessi, D.R. TSC1-TSC2: A complex tale of PKB-mediated S6K regulation. Nat. Cell Biol. 2002, 4, E214–E216. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, H.; Gao, J.; Liu, Y.; Li, J.; Wang, J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J. Mol. Cell. Cardiol. 2019, 136, 27–41. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Giricz, Z.; Mentzer, R.M.; Gottlieb, R.A. Autophagy, myocardial protection, and the metabolic syndrome. J. Cardiovasc. Pharmacol. 2012, 60, 125–132. [Google Scholar] [CrossRef]
- Jiang, P.; Mizushima, N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015, 75, 13–18. [Google Scholar] [CrossRef]
- Komatsu, M.; Kageyama, S.; Ichimura, Y. p62/SQSTM1/A170: Physiology and pathology. Pharmacol. Res. 2012, 66, 457–462. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell. Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Athonvarangkul, D.; Mishall, P.; Sahu, S.; Singh, R. Autophagy proteins regulate ERK phosphorylation. Nat. Commun. 2013, 4, 2799. [Google Scholar] [CrossRef] [PubMed]
- Mofid, A.; Newman, N.S.; Lee, P.J.H.; Abbasi, C.; Matkar, P.N.; Rudenko, D.; Kuliszewski, M.A.; Chen, H.H.; Afrasiabi, K.; Tsoporis, J.N.; et al. Cardiac overexpression of S100A6 Attenuates cardiomyocyte apoptosis and reduces infarct size after myocardial ischemia-reperfusion. J. Am. Heart Assoc. 2017, 6, e004738. [Google Scholar] [CrossRef] [PubMed]
- Camillo, C.; Abramov, A.; Allen, P.; Castillero, E.; Roberts, E.; Xue, Y.; Frasca, A.; Moreno, V.; Kurade, M.; Robinson, K.; et al. RAGE antagonist peptide mitigates AGE-mediated endothelial hyperpermeability and accumulation of glycoxidation products in human ascending aortas and in a murine model of aortic aneurysm. bioRxiv 2021. [Google Scholar] [CrossRef]
- Joo, J.H.; Yoon, S.Y.; Kim, J.H.; Paik, S.-G.; Min, S.R.; Lim, J.-S.; Choe, I.S.; Choi, I.; Kim, J.W. S100A6 (calcyclin) enhances the sensitivity to apoptosis via the upregulation of caspase-3 activity in Hep3B cells. J. Cell. Biochem. 2008, 103, 1183–1197. [Google Scholar] [CrossRef]
- Słomnicki, Ł.P.; Nawrot, B.; Leśniak, W. S100A6 binds p53 and affects its activity. Int. J. Biochem. Cell Biol. 2009, 41, 784–790. [Google Scholar] [CrossRef]
- Tsoporis, J.N.; Izhar, S.; Parker, T.G. Expression of S100A6 in cardiac myocytes limits apoptosis induced by tumor necrosis factor-α. J. Biol. Chem. 2008, 283, 30174–30183. [Google Scholar] [CrossRef]
- van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; de Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Misfeld, M.; Hanke, T.; Charitos, E.I.; Bullerdiek, J.; Belge, G.; Kuehnel, W.; Sievers, H.H. Inhibition of caspase-3 differentially affects vascular smooth muscle cell apoptosis in the concave versus convex aortic sites in ascending aneurysms with a bicuspid aortic valve. Ann. Anat. 2010, 192, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Hakem, R.; Soengas, M.S.; Duncan, G.S.; Shahinian, A.; Kägi, D.; Hakem, A.; McCurrach, M.; Khoo, W.; Kaufman, S.A.; et al. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998, 12, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Hakem, R.; Furlonger, C.; Hakem, A.; Duncan, G.S.; Sasaki, T.; Bouchard, D.; Lu, L.; Wu, G.E.; Paige, C.J.; et al. Caspase-3 regulates cell cycle in B cells: A consequence of substrate specificity. Nat. Immunol. 2003, 4, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Hope, M.D.; Hope, T.A.; Crook, S.E.S.; Ordovas, K.G.; Urbania, T.H.; Alley, M.T.; Higgins, C.B. 4D flow CMR in assessment of valve-related ascending aortic disease. JACC Cardiovasc. Imaging 2011, 4, 781–787. [Google Scholar] [CrossRef]
- van Engeland, N.C.A.; Suarez Rodriguez, F.; Rivero-Müller, A.; Ristori, T.; Duran, C.L.; Stassen, O.M.J.A.; Antfolk, D.; Driessen, R.C.H.; Ruohonen, S.; Ruohonen, S.T.; et al. Vimentin regulates Notch signaling strength and arterial remodeling in response to hemodynamic stress. Sci. Rep. 2019, 9, 12415. [Google Scholar] [CrossRef]
- Desmoulière, A.; Redard, M.; Darby, I.; Gabbiani, G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 1995, 146, 56–66. [Google Scholar]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell. 2001, 12, 2730–2741. [Google Scholar] [CrossRef]
- Hardikar, A.; Harle, R.; Marwick, T.H. Aortic thickness: A forgotten paradigm in risk stratification of aortic disease. Aorta 2020, 8, 132–140. [Google Scholar] [CrossRef]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell. Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Yu, H.; Littlewood, T.; Bennett, M. Akt isoforms in vascular disease. Vascul. Pharmacol. 2015, 71, 57–64. [Google Scholar] [CrossRef]
- Gao, G.; Chen, W.; Yan, M.; Liu, J.; Luo, H.; Wang, C.; Yang, P. Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling. Int. J. Mol. Med. 2020, 45, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-X.; Hu, Y.-W.; Zheng, L.; Wang, Q. Shear stress in autophagy and its possible mechanisms in the process of atherosclerosis. DNA Cell Biol. 2017, 36, 335–346. [Google Scholar] [CrossRef]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Myeku, N.; Figueiredo-Pereira, M.E. Dynamics of the degradation of ubiquitinated proteins by proteasomes and autophagy: Association with sequestosome 1/p62. J. Biol. Chem. 2011, 286, 22426–22440. [Google Scholar] [CrossRef] [PubMed]
- Paine, M.G.; Babu, J.R.; Seibenhener, M.L.; Wooten, M.W. Evidence for p62 aggregate formation: Role in cell survival. FEBS Lett. 2005, 579, 5029–5034. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qiu, F.; Tashiro, S.; Onodera, S.; Ikejima, T. ERK and JNK mediate TNFα-induced p53 activation in apoptotic and autophagic L929 cell death. Biochem. Biophys. Res. Commun. 2008, 376, 483–488. [Google Scholar] [CrossRef]
- Oh, S.-H.; Lim, S.-C. Endoplasmic reticulum stress-mediated autophagy/apoptosis induced by capsaicin (8-methyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin is regulated by the extent of c-Jun NH2-terminal kinase/extracellular signal-regulated kinase activation in WI38 lung epithelial fibroblast cells. J. Pharmacol. Exp. Ther. 2009, 329, 112–122. [Google Scholar] [CrossRef]
- Sivaprasad, U.; Basu, A. Inhibition of ERK attenuates autophagy and potentiates tumour necrosis factor-α-induced cell death in MCF-7 cells. J. Cell. Mol. Med. 2008, 12, 1265–1271. [Google Scholar] [CrossRef]
- Cagnol, S.; Chambard, J.-C. ERK and cell death: Mechanisms of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]

| BAV V + K | TAV V + K | BAV V | TAV V | BAV K | TAV K | |
|---|---|---|---|---|---|---|
| RAGE n | 1.59 ± 0.14 69 | 1.07 ± 0.08 59 | 1.26 ± 0.16 33 | 1.25 ± 0.10 30 | 1.89 ± 0.21 36 | 0.89 ± 0.13 29 |
| AGE n | 1.46 ± 0.10 69 | 0.95 ± 0.09 59 | 1.34 ± 0.13 33 | 1.00 ± 0.11 30 | 1.53 ± 0.13 36 | 0.84 ± 0.12 29 |
| S100A6 n | 2154 ± 155 57 | 1610 ± 167 89 | 1889 ± 197 29 | 1633 ± 270 42 | 2429 ± 235 28 | 1589 ± 207 47 |
| BAV V + K | TAV V + K | BAV V | TAV V | BAV K | TAV K | |
|---|---|---|---|---|---|---|
| active/mature caspase-3 n | 1.21 ± 0.09 97 | 1.56 ± 0.12 87 | 1.36 ± 0.17 50 | 1.40 ± 0.14 42 | 1.25 ± 0.13 47 | 1.83 ± 0.22 45 |
| vimentin 57 kDa n | 2.13 ± 0.26 99 | 2.18 ± 0.25 94 | 2.38 ± 0.46 49 | 2.13 ± 0.39 48 | 1.89 ± 0.25 50 | 2.23 ± 0.30 46 |
| vimentin 48 kDa n | 3.20 ± 0.34 96 | 2.73 ± 0.27 93 | 3.21 ± 0.49 48 | 2.33 ± 0.32 46 | 3.19 ± 0.49 48 | 3.11 ± 0.44 47 |
| α-smooth muscle actin n | 1.51 ± 0.10 104 | 1.07 ± 0.09 86 | 1.44 ± 0.14 52 | 1.10 ± 0.11 44 | 1.59 ± 0.14 52 | 1.04 ± 0.14 42 |
| BAV V + K | TAV V + K | BAV V | TAV V | BAV K | TAV K | |
|---|---|---|---|---|---|---|
| Bcl-2 n | 1.22 ± 0.07 98 | 1.38 ± 0.08 85 | 1.36 ± 0.17 50 | 1.33 ± 0.1 43 | 1.10 ± 0.10 48 | 1.42 ± 0.12 42 |
| Akt n | 1.28 ± 0.08 103 | 1.29 ± 0.09 84 | 1.26 ± 0.10 53 | 1.20 ± 0.11 43 | 1.25 ± 0.11 50 | 1.40 ± 0.14 41 |
| mTOR n | 0.81 ± 0.06 94 | 0.64 ± 0.05 95 | 0.74 ± 0.08 49 | 0.71 ± 0.07 50 | 0.88 ± 0.08 45 | 0.58 ± 0.07 45 |
| BAV V + K | TAV V + K | BAV V | TAV V | BAV K | TAV K | |
|---|---|---|---|---|---|---|
| LC3-II/LC3-I n | 0.61 ± 0.05 56 | 0.65 ± 0.04 67 | 0.57 ± 0.07 27 | 0.64 ± 0.07 34 | 0.65 ± 0.07 29 | 0.64 ± 0.05 32 |
| p62 n | 0.87 ± 0.06 93 | 0.66 ± 0.04 94 | 0.75 ± 0.08 49 | 0.75 ± 0.06 49 | 1.00 ± 0.10 44 | 0.58 ± 0.06 46 |
| ERK1/2 n | 1.75 ± 0.14 93 | 1.03 ± 0.08 99 | 1.66 ± 0.19 50 | 1.33 ± 0.12 52 | 1.86 ± 0.23 43 | 0.70 ± 0.07 47 |
| BAV (n = 57) | TAV (n = 49) | |
|---|---|---|
| age (year) | 61.8 ± 11.8 | 65.5 ± 11.6 |
| male | 50 (88%) | 36 (73%) |
| diameter (mm) | 49.4 ± 3.9 | 51.1 ± 10.6 |
| aortic regurgitation | 19 (33%) | 40 (82%) |
| aortic stenosis | 33 (58%) | 5 (10%) |
| diabetes | 3 (5%) | 3 (6%) |
| coronary heart diseases | 6 (11%) | 9 (18%) |
| arterial hypertension | 48 (84%) | 43 (88%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnard, S.J.; Haunschild, J.; Heiser, L.; Dieterlen, M.T.; Klaeske, K.; Borger, M.A.; Etz, C.D. Apoptotic Cell Death in Bicuspid-Aortic-Valve-Associated Aortopathy. Int. J. Mol. Sci. 2023, 24, 7429. https://doi.org/10.3390/ijms24087429
Barnard SJ, Haunschild J, Heiser L, Dieterlen MT, Klaeske K, Borger MA, Etz CD. Apoptotic Cell Death in Bicuspid-Aortic-Valve-Associated Aortopathy. International Journal of Molecular Sciences. 2023; 24(8):7429. https://doi.org/10.3390/ijms24087429
Chicago/Turabian StyleBarnard, Sarah J., Josephina Haunschild, Linda Heiser, Maja T. Dieterlen, Kristin Klaeske, Michael A. Borger, and Christian D. Etz. 2023. "Apoptotic Cell Death in Bicuspid-Aortic-Valve-Associated Aortopathy" International Journal of Molecular Sciences 24, no. 8: 7429. https://doi.org/10.3390/ijms24087429
APA StyleBarnard, S. J., Haunschild, J., Heiser, L., Dieterlen, M. T., Klaeske, K., Borger, M. A., & Etz, C. D. (2023). Apoptotic Cell Death in Bicuspid-Aortic-Valve-Associated Aortopathy. International Journal of Molecular Sciences, 24(8), 7429. https://doi.org/10.3390/ijms24087429






