Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Questionnaires
4.3. Blood Sample Collection and Biochemical Assays
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Deer, R.R.; Rock, M.A.; Vasilevsky, N.; Carmody, L.; Rando, H.; Anzalone, A.J.; Basson, M.D.; Bennett, T.D.; Bergquist, T.; Boudreau, E.A.; et al. Characterizing Long COVID: Deep Phenotype of a Complex Condition. EBioMedicine 2021, 74, 103722. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2021. Available online: WHO/2019-nCoV/Post_COVID-19_condition/Clinical_case_definition/2021 (accessed on 2 January 2022).
- National Institute for Health and Care Excellence (NICE); Scottish Intercollegiate Guidelines Network (SIGN); Royal College of General Practitioners (RCGP). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; NICE: Manchester, UK, 2022; Available online: https://www.nice.org.uk/guidance/ng188/ (accessed on 7 February 2022).
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open. 2021, 4, e2128568. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, N.H.; Kohn, J.N.; Ogan, W.S.; Sitapati, A.; Longhurst, C.A.; Wang, A.; Lee, S.; Hong, S.; Horton, L.E. Deep Dive into the Long Haul: Analysis of Symptom Clusters and Risk Factors for Post-Acute Sequelae of COVID-19 to Inform Clinical Care. Int. J. Environ. Res. Public Health 2022, 15, 16841. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. COVERSCAN study investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.D. Innate immunity, cytokine storm, and inflammatory cell death in COVID-19. J. Transl. Med. 2022, 20, 542. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- van Eijk, L.E.; Tami, A.; Hillebrands, J.L.; den Dunnen, W.F.A.; de Borst, M.H.; van der Voort, P.H.J.; Bulthuis, M.L.C.; Veloo, A.C.M.; Wold, K.I.; Vincenti González, M.F.; et al. Mild Coronavirus Disease 2019 (COVID-19) Is Marked by Systemic Oxidative Stress: A Pilot Study. Antioxidants 2021, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Suhail, S.; Zajac, J.; Fossum, C.; Lowater, H.; McCracken, C.; Severson, N.; Laatsch, B.; Narkiewicz-Jodko, A.; Johnson, B.; Liebau, J.; et al. Role of Oxidative Stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) Infection: A Review. Protein J. 2020, 39, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Komaravelli, N.; Casola, A. Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses. J. Pharm. Pharm. 2014, 30, 1000141. [Google Scholar]
- Medvedev, R.; Ploen, D.; Hildt, E. HCV and Oxidative Stress: Implications for HCV Life Cycle and HCV-Associated Pathogenesis. Oxid. Med. Cell. Longev. 2016, 2016, 9012580. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell. Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef]
- Ledur, P.F.; Karmirian, K.; Pedrosa, C.D.S.G.; Souza, L.R.Q.; Assis-de-Lemos, G.; Martins, T.M.; Ferreira, J.C.C.G.; de Azevedo Reis, G.F.; Silva, E.S.; Silva, D.; et al. Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human iPSC-derived astrocytes. Sci. Rep. 2020, 10, 1218. [Google Scholar] [CrossRef]
- Lim, J.Y.; Oh, E.; Kim, Y.; Jung, W.W.; Kim, H.S.; Lee, J.; Sul, D. Enhanced oxidative damage to DNA, lipids, and proteins and levels of some antioxidant enzymes, cytokines, and heat shock proteins in patients infected with influenza H1N1 virus. Acta Virol. 2014, 58, 253–260. [Google Scholar] [CrossRef]
- Palmer, C.S. Innate metabolic responses against viral infections. Nat. Metab. 2022, 10, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Medini, H.; Zirman, A.; Mishmar, D. Immune system cells from COVID-19 patients display compromised mitochondrial-nuclear expression co-regulation and rewiring toward glycolysis. iScience 2021, 24, 103471. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J. Med. Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Kosanovic, T.; Sagic, D.; Djukic, V.; Pljesa-Ercegovac, M.; Savic-Radojevic, A.; Bukumiric, Z.; Lalosevic, M.; Djordjevic, M.; Coric, V.; Simic, T. Time Course of Redox Biomarkers in COVID-19 Pneumonia: Relation with Inflammatory, Multiorgan Impairment Biomarkers and CT Findings. Antioxidants 2021, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Gadotti, A.C.; Lipinski, A.L.; Vasconcellos, F.T.; Marqueze, L.F.; Cunha, E.B.; Campos, A.C.; Oliveira, C.F.; Amaral, A.N.; Baena, C.P.; Telles, J.P.; et al. Susceptibility of the patients infected with Sars-Cov2 to oxidative stress and possible interplay with severity of the disease. Free Radic. Biol. Med. 2021, 165, 184–190. [Google Scholar] [CrossRef]
- Mehri, F.; Rahbar, A.H.; Ghane, E.T.; Souri, B.; Esfahani, M. Changes in oxidative markers in COVID-19 patients. Arch. Med. Res. 2022, 52, 843–849. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Chang, C.H.; Chen, H.X.; Yü, G.; Peng, C.C.; Peng, R.Y. Curcumin-Protected PC12 Cells Against Glutamate-Induced Oxidative Toxicity. Food Technol. Biotechnol. 2014, 52, 468–478. [Google Scholar] [CrossRef]
- Gonzalo, H.; Brieva, L.; Tatzber, F.; Jové, M.; Cacabelos, D.; Cassanyé, A.; Lanau-Angulo, L.; Boada, J.; Serrano, J.C.; González, C.; et al. Lipidome analysis in multiple sclerosis reveals protein lipoxidative damage as a potential pathogenic mechanism. J. Neurochem. 2012, 123, 622–634. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Fois, A.G.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Carru, C.; Zinellu, A. Circulating malondialdehyde concentrations in patients with stable chronic obstructive pulmonary disease: A systematic review and meta-analysis. Biomark. Med. 2018, 12, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 71, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Qin, D.; Zhao, C.; Chai, L.; Yu, Z.; Wang, W.; Tong, L.; Lv, L.; Wang, Y.; Rehwinkel, J.; et al. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat. Immunol. 2020, 21, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2022, 24, 1–15. [Google Scholar] [CrossRef]
- Avila-Nava, A.; Pech-Aguilar, A.G.; Lugo, R.; Medina-Vera, I.; Guevara-Cruz, M.; Gutiérrez-Solis, A.L. Oxidative Stress Biomarkers and Their Association with Mortality among Patients Infected with SARS-CoV-2 in Mexico. Oxid. Med. Cell. Longev. 2022, 17, 1058813. [Google Scholar] [CrossRef]
- Martín-Fernández, M.; Aller, R.; Heredia-Rodríguez, M.; Gómez-Sánchez, E.; Martínez-Paz, P.; Gonzalo-Benito, H.; Sánchez-de Prada, L.; Gorgojo, Ó.; Carnicero-Frutos, I.; Tamayo, E.; et al. Lipid peroxidation as a hallmark of severity in COVID-19 patients. Redox Biol. 2021, 48, 102181. [Google Scholar] [CrossRef]
- PHOSP-COVID Collaborative Group. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: A prospective observational study. Lancet Respir. Med. 2022, 10, 761–775. [Google Scholar] [CrossRef]
- Munblit, D.; O’Hara, M.E.; Akrami, A.; Perego, E.; Olliaro, P.; Needham, D.M. Long COVID: Aiming for a consensus. Lancet Respir. Med. 2022, 10, 632–634. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Shen, Y.; Zhang, X.; Cen, Y.; Wang, B.; Zhao, S.; Zhou, Y.; Hu, B.; Wang, M.; et al. Symptoms and Health Outcomes Among Survivors of COVID-19 Infection 1 Year After Discharge from Hospitals in Wuhan, China. JAMA Netw. Open. 2021, 4, e2127403. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Molnar, T.; Varnai, R.; Schranz, D.; Zavori, L.; Peterfi, Z.; Sipos, D.; Tőkés-Füzesi, M.; Illes, Z.; Buki, A.; Csecsei, P. Severe Fatigue and Memory Impairment Are Associated with Lower Serum Level of Anti-SARS-CoV-2 Antibodies in Patients with Post-COVID Symptoms. J. Clin. Med. 2021, 10, 4337. [Google Scholar] [CrossRef] [PubMed]
- Fogh., K.; Larsen, T.G.; Hansen, C.B.; Hasselbalch, R.B.; Eriksen, A.R.R.; Bundgaard, H.; Frikke-Schmidt, R.; Hilsted, L.M.; Østergaard, L.; Johansen, I.S.; et al. Self-Reported Long COVID and Its Association with the Presence of SARS-CoV-2 Antibodies in a Danish Cohort up to 12 Months after Infection. Microbiol. Spectr. 2022, 10, e0253722. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Jové, M.; Mota-Martorell, N.; Pradas, I.; Martín-Gari, M.; Ayala, V.; Pamplona, R. The Advanced Lipoxidation End-Product Malondialdehyde-Lysine in Aging and Longevity. Antioxidants 2020, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 23, 172. [Google Scholar] [CrossRef]
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; Jayaseelan, D.L.; Kumar, G.; Raftopoulos, R.E.; Zambreanu, L.; et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020, 143, 3104–3120. [Google Scholar] [CrossRef]
- Nersesjan, V.; Amiri, M.; Lebech, A.M.; Roed, C.; Mens, H.; Russell, L.; Fonsmark, L.; Berntsen, M.; Sigurdsson, S.T.; Carlsen, J.; et al. Central and peripheral nervous system complications of COVID-19: A prospective tertiary center cohort with 3-month follow-up. J. Neurol. 2021, 268, 3086–3104. [Google Scholar] [CrossRef]
- Lucchese, G.; Vogelgesang, A.; Boesl, F.; Raafat, D.; Holtfreter, S.; Bröker, B.M.; Stufano, A.; Fleischmann, R.; Prüss, H.; Franke, C.; et al. Anti-neuronal antibodies against brainstem antigens are associated with COVID-19. EBioMedicine 2022, 83, 104211. [Google Scholar] [CrossRef]
- Plantone, D.; Locci, S.; Bergantini, L.; Manco, C.; Cortese, R.; Meocci, M.; Cavallaro, D.; d’Alessandro, M.; Bargagli, E.; De Stefano, N. Brain neuronal and glialdamageduring acute COVID-19 infection in absence of clinical neurologicalmanifestations. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1343–1348. [Google Scholar]
- Paul, B.D.; Lemle, M.D.; Komaroff, A.L.; Snyder, S.H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2021, 118, e2024358118. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Centrone, F.; Morcavallo, C.; Campanella, S.; Sallustio, A.; Accogli, M.; Fortunato, F.; Parisi, A.; Chironna, M. Rapid Spread of the SARS-CoV-2 Variant of Concern 202012/01 in Southern Italy (December 2020–March 2021). Int. J. Environ. Res. Public Health 2021, 18, 4766. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2021. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 2 April 2022).
- Stufano, A.; Lucchese, G.; Stahl, B.; Grattagliano, I.; Dassisti, L.; Lovreglio, P.; Flöel, A.; Iavicoli, I. Impact of COVID-19 emergency on the psychological well-being of susceptible individuals. Sci. Rep. 2022, 12, 11152. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, G.; Perrone, S.; Longini, M.; Terzuoli, L.; Bracci, R. Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies. Pediatr. Res. 2000, 47, 221–2244. [Google Scholar] [CrossRef] [PubMed]
- Isgrò, C.; Spagnuolo, L.; Pannucci, E.; Mondello, L.; Santi, L.; Dugo, L.; Sardanelli, A.M. Rhus Coriaria L. Extract: Antioxidant Effect and Modulation of Bioenergetic Capacity in Fibroblasts from Parkinson’s Disease Patients and THP-1 Macrophages. Int. J. Mol. Sci. 2022, 23, 12774. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, S.T.; Tang, Q.; Wu, J.; Zhu, X. A Novel Consistent Random Forest Framework: Bernoulli Random Forests. IEEE Trans. Neural Netw. Learn. Syst. 2018, 29, 3510–3523. [Google Scholar]
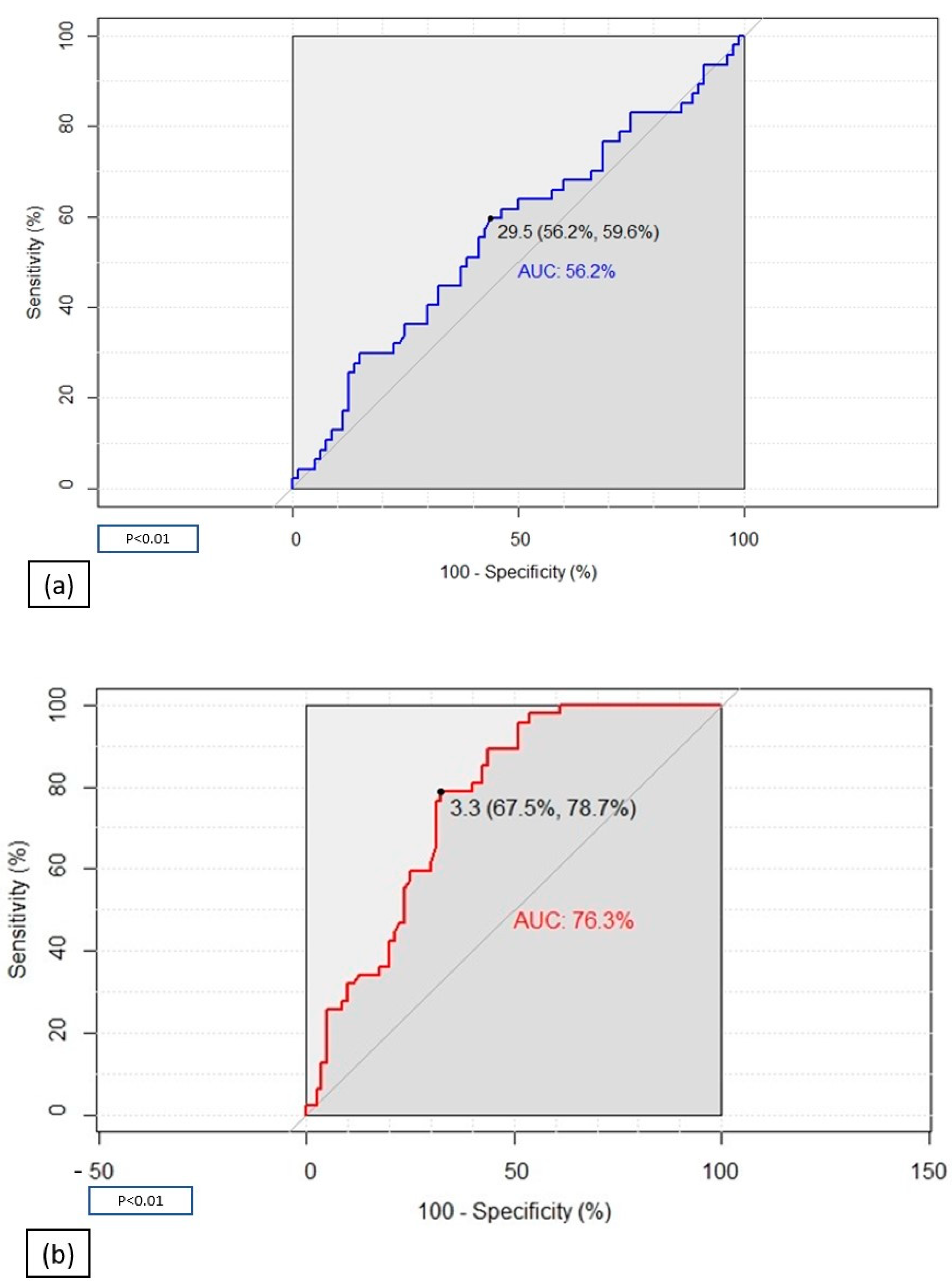

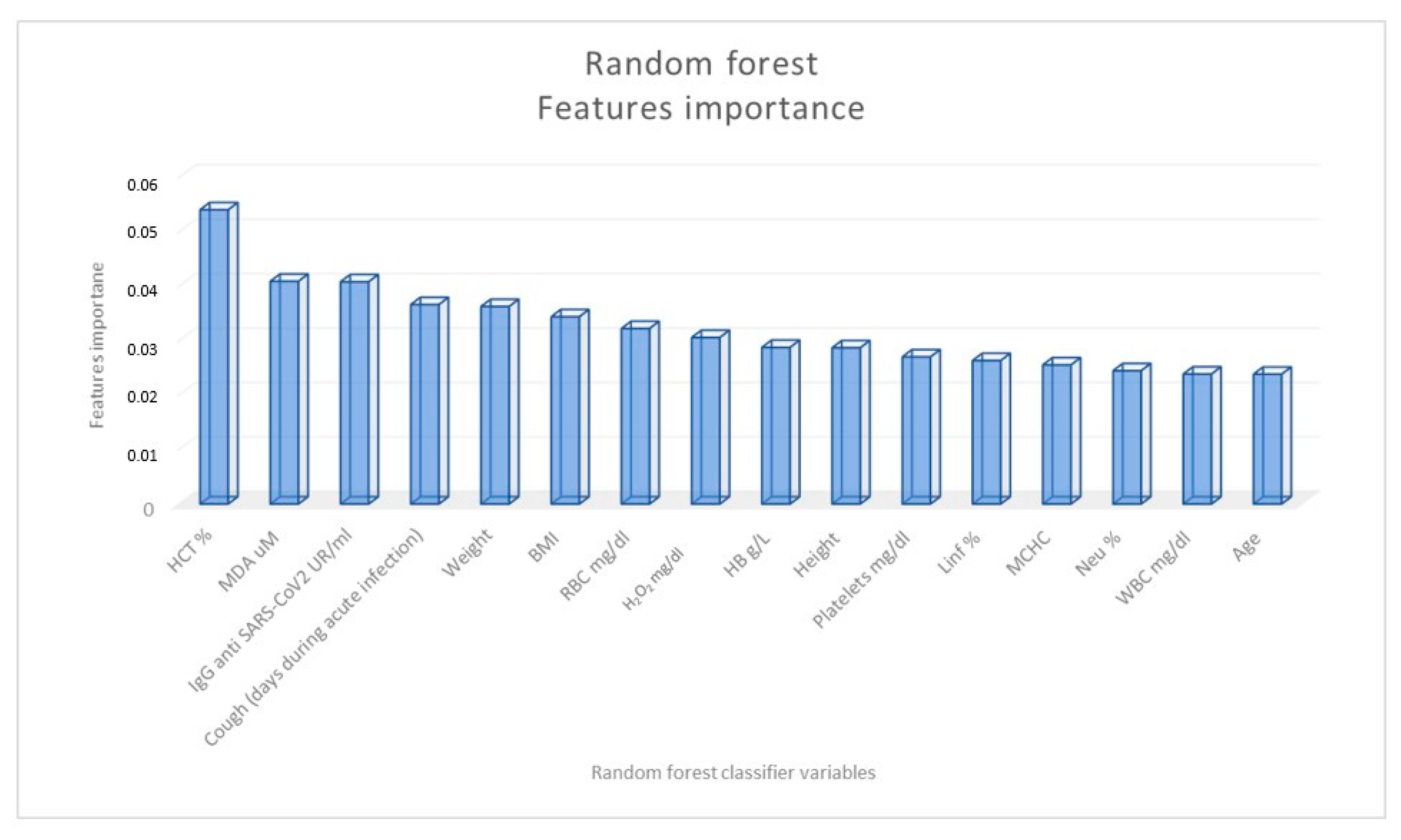
| General Characteristics | COVID-19 Patients (N 80) | Controls (N 47) | ||||
|---|---|---|---|---|---|---|
| N (%) | Mean ± SD | Range | N (%) | Mean ± SD | Range | |
| Age (years) | 50.6 ± 9.3 | 28–68 | 53.8 ± 12.6 | 31–73 | ||
| BMI (Kg/m2) | 25.8 ± 4.5 | 17.6–52.2 | 24.0 ± 3.3 | 18.1–32.4 | ||
| Gender | ||||||
| Men | 56 (70) | 33 (70) | ||||
| Women | 24 (30) | 14 (30) | ||||
| Smoking habits | ||||||
| Smokers | 25 (31) | 16 (34) | ||||
| Non-smokers | 55 (69) | 31 (66) | ||||
| Alcohol consumption (U/week) b | 2.1 ± 5.5 | 1–7 | 3.6 ± 4.6 | 0–14 | ||
| RBC (mg/dL) a | 4.9 ± 0.5 | 3.5–7.1 | 5.2 ± 0.5 | 4.4–7.6 | ||
| WBC (mg/dL) aa | 6.5 ± 1.3 | 4.1–11.1 | 7.3 ± 1.4 | 5.4–13.9 | ||
| Hb (g/dL) | 13.9 ± 1.2 | 10.8–16.4 | 13.9 ± 0.7 | 12.1–15.8 | ||
| HCT (%) a | 41.5 ± 4.5 | 15.8–48.0 | 42.8 ± 0.2 | 38.7–48.7 | ||
| MCV (fl/cell) a | 86.4 ± 4.8 | 61.1–97.0 | 83.8 ± 5.0 | 63.0–91.0 | ||
| MCHC (pg/dL) a | 29.2 ± 2.3 | 19.3–34.7 | 27.0 ± 1.9 | 18.9–29.1 | ||
| Neutrophils (%) | 55.0 ± 8.4 | 17–72 | 56.6 ± 6.8 | 44–78 | ||
| Lymphocytes (%) | 34.3 ± 8.1 | 16–76 | 32.5 ± 5.4 | 17–44 | ||
| Monocytes (%) | 8.0 ± 1.8 | 2–12 | 7.7 ± 2.1 | 2–14 | ||
| Eosinophils (%) | 2.4 ± 1.6 | 0–8 | 2.4 ± 1.4 | 1–8 | ||
| Basophils (%) | 0.3 ± 0.5 | 0–2 | 0.6 ± 0.6 | 0–2 | ||
| Platelets (mg/dL) a | 239.0 ± 52.2 | 97–398 | 251.3 ± 36.3 | 116–347 | ||
| H2O2 (mg/dL) | 65.4 ± 80.0 | 1.8–465.5 | 59.2 ± 78.7 | 1.5–313.0 | ||
| MDA (µm) a | 4.9 ± 3.5 | 1.2–24.9 | 2.8 ± 0.9 | 1.2–5.1 | ||
| General Characteristics | Long-COVID Patients (N. 34) | Non-Long-COVID Patients (N. 46) | ||||
|---|---|---|---|---|---|---|
| N (%) | Mean ± SD | Range | N (%) | Mean ± SD | Range | |
| Age (years) | 50.9 ± 9.4 | 28–66 | 50.3 ± 0.7 | 30–68 | ||
| Body Mass Index (Kg/m2) | 25.3 ± 4.1 | 17.6–34.6 | 26.1 ± 1.8 | 21.6–52.2 | ||
| Gender a | ||||||
| Men | 15 (44) | 41 (89) | ||||
| Women | 19 (56) | 6 (11) | ||||
| Smoking habits | ||||||
| Smokers | 11 (32) | 14 (30) | ||||
| Nonsmokers | 23 (68) | 32 (70) | ||||
| Alcohol consumption(U/week) | 2.1 ± 2.7 | 1–7 | 2.2 ± 3.5 | 0–6 | ||
| Comorbidities | ||||||
| Cardiovascular diseases | 6 (17.6) | 10 (21.7) | ||||
| Respiratory diseases | 6 (17.6) | 4 (8.6) | ||||
| Autoimmune diseases | 4 (11.7) | 1 (2.1) | ||||
| Neoplasms | 2 (5.8) | 3 (6.5) | ||||
| RBC (mg/dL) | 4.7 ± 0.4 | 3.7–5.4 | 4.9 ± 0.1 | 3.5–7.1 | ||
| WBC (mg/dL) | 6.5 ± 1.2 | 4.1–9.7 | 6.5 ± 1.4 | 4.6–11.1 | ||
| Hb (g/dL) | 13.5 ± 1.3 | 10.8–16.1 | 14.1 ± 1.6 | 11.2–16.4 | ||
| HCT (%) a | 39.7 ± 5.6 | 15.9–46.8 | 42.7 ± 1.4 | 33.0–48.0 | ||
| MCV (fl/cell) | 86.3 ± 4.2 | 75.0–97.0 | 86.4 ± 2.5 | 61.0–96.8 | ||
| MCHC (pg/dL) | 28.9 ± 1.4 | 25.5–32.3 | 29.3 ± 3.5 | 19.3–34.7 | ||
| Platelets (mg/dL) | 241.0 ± 60.9 | 97–398 | 238.2 ± 62.1 | 152–347 | ||
| IgG Anti-SARS-CoV-2 (UR/mL) a | 68.1 ± 72.7 | 0.0–223.0 | 26.6 ± 9.0 | 0.0–246.0 | ||
| H2O2 (mg/dL) | 61.9 ± 66.1 | 1.7–456.4 | 67.9 ± 70.6 | 2.6–294.0 | ||
| MDA (µm) a | 5.7 ± 5.9 | 1.2–24.8 | 3.8 ± 2.4 | 1.1–12.6 | ||
| Therapy | ||||||
| Paracetamol | 11 (32.3) | 12 (26.1) | ||||
| Corticosteroids | 13 (38.2) | 12 (26.1) | ||||
| Oxygen therapy | 4 (11.7) | 0 (0.0) | ||||
| Heparin | 6 (17.6) | 2 (4.3) | ||||
| Antiviral drugs | 0 (0.0) | 0 (0.0) | ||||
| Prevalence and Duration of Symptoms during COVID-19 Infection (Days) | Long-COVID Patients (N. 34) | Non-Long-COVID Patients (N. 46) | ||||
|---|---|---|---|---|---|---|
| N(%) | Mean | Range | N (%) | Mean | Range | |
| Duration of COVID-19 infection (days) | 23.3 ± 1.4 | 10–64 | 22.6 ± 1.3 | 2–52 | ||
| Presence of symptoms during infection | 32 (94.1) | 41 (89.1) | ||||
| Fever | 24 (70) | 0.7 ± 5.6 | 0–24 | 27 (58) | 2.4 ± 7 | 0–20 |
| Dyspnea | 9 (26) | 4.7 ± 0.7 | 0–65 | 10 (21) | 9.2 ± 11.3 | 0–160 |
| Cough a | 20 (58) | 9.2 ± 7.7 | 0–39 | 16 (34) | 3.1 ± 6.3 | 0–24 |
| Myalgia | 21 (61) | 5.9 ± 7.0 | 0–45 | 21 (45) | 11.6 ± 22.6 | 0–151 |
| Pharyngodynia | 6 (17) | 0.9 ± 0.0 | 0–10 | 7 (15) | 1.3 ± 11.3 | 0–38 |
| Ageusia | 17 (50) | 7.6 ± 1.4 | 0–41 | 21 (46) | 8.7 ± 5.6 | 0–115 |
| Anosmia | 19 (55) | 13.5 ± 0.0 | 0–141 | 24 (52) | 8.1 ± 5.6 | 0–115 |
| Diarrhea | 8 (23) | 0.7 ± 1.4 | 0–10 | 8 (17) | 1.2 ± 0.0 | 0–22 |
| Headache b | 11 (32) | 2.5 ± 0.0 | 0–19 | 10 (22) | 0.7 ± 0.0 | 0–7 |
| Dermatitis | 1 (3) | 0.2 ± 0.0 | 0–7 | 2 (5) | 2.3 ± 0.0 | 0–103 |
| Asthenia | 8 (23) | 4.0 ± 7.7 | 0–31 | 4 (9) | 2.1 ± 0.7 | 0–48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stufano, A.; Isgrò, C.; Palese, L.L.; Caretta, P.; De Maria, L.; Lovreglio, P.; Sardanelli, A.M. Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers. Int. J. Mol. Sci. 2023, 24, 7445. https://doi.org/10.3390/ijms24087445
Stufano A, Isgrò C, Palese LL, Caretta P, De Maria L, Lovreglio P, Sardanelli AM. Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers. International Journal of Molecular Sciences. 2023; 24(8):7445. https://doi.org/10.3390/ijms24087445
Chicago/Turabian StyleStufano, Angela, Camilla Isgrò, Luigi Leonardo Palese, Paolo Caretta, Luigi De Maria, Piero Lovreglio, and Anna Maria Sardanelli. 2023. "Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers" International Journal of Molecular Sciences 24, no. 8: 7445. https://doi.org/10.3390/ijms24087445
APA StyleStufano, A., Isgrò, C., Palese, L. L., Caretta, P., De Maria, L., Lovreglio, P., & Sardanelli, A. M. (2023). Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers. International Journal of Molecular Sciences, 24(8), 7445. https://doi.org/10.3390/ijms24087445









