Genome-Wide Identification, Expression, and Response to Fusarium Infection of the SWEET Gene Family in Garlic (Allium sativum L.)
Abstract
:1. Introduction
2. Results
2.1. Identification of SWEET Genes in the A. sativum Genome
2.2. Characterization of Putative SWEET Proteins in Garlic
2.3. Cis-Acting Elements in the AsSWEET Promoters
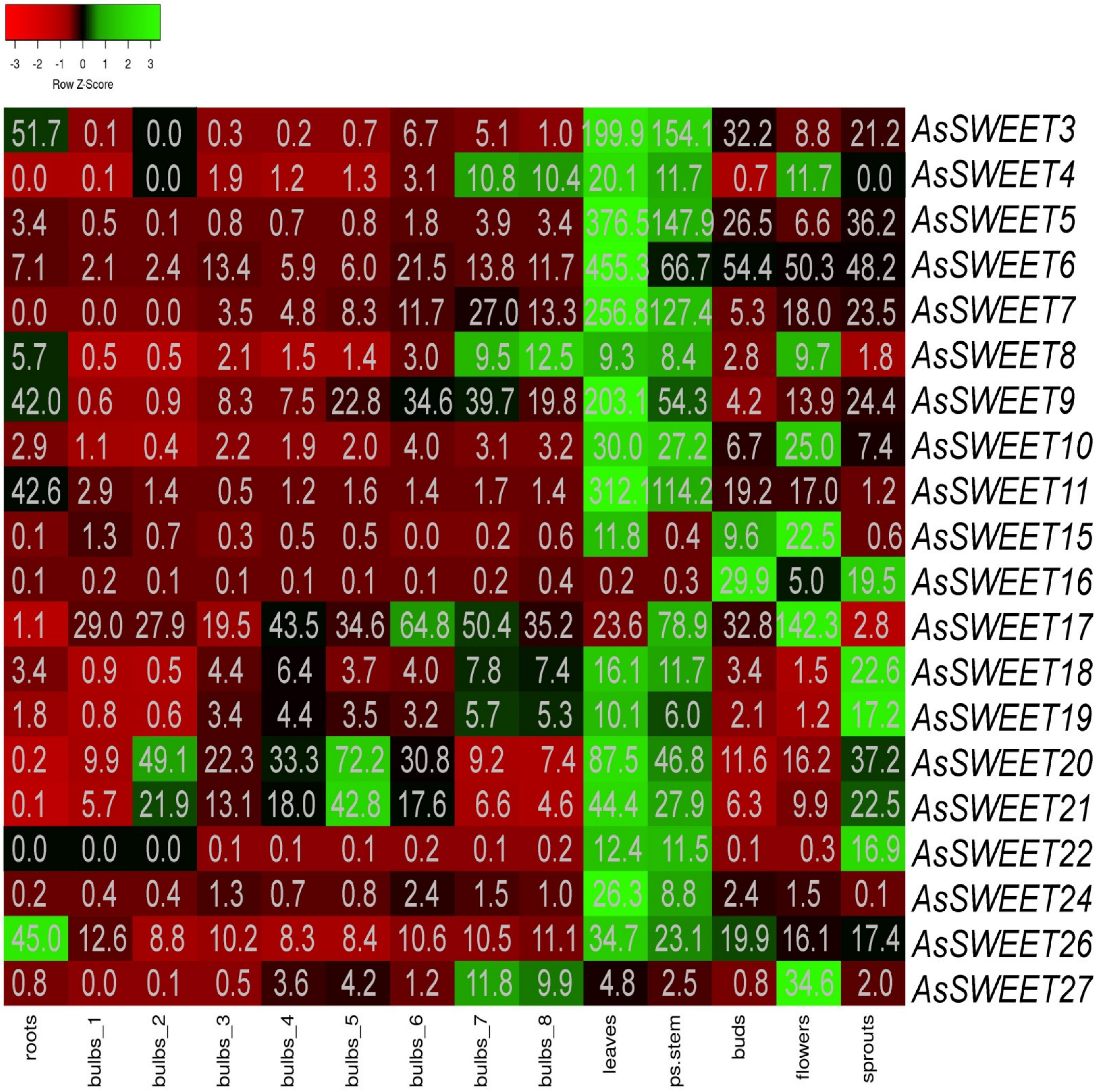
2.4. Tissue Expression Patterns of AsSWEET Genes
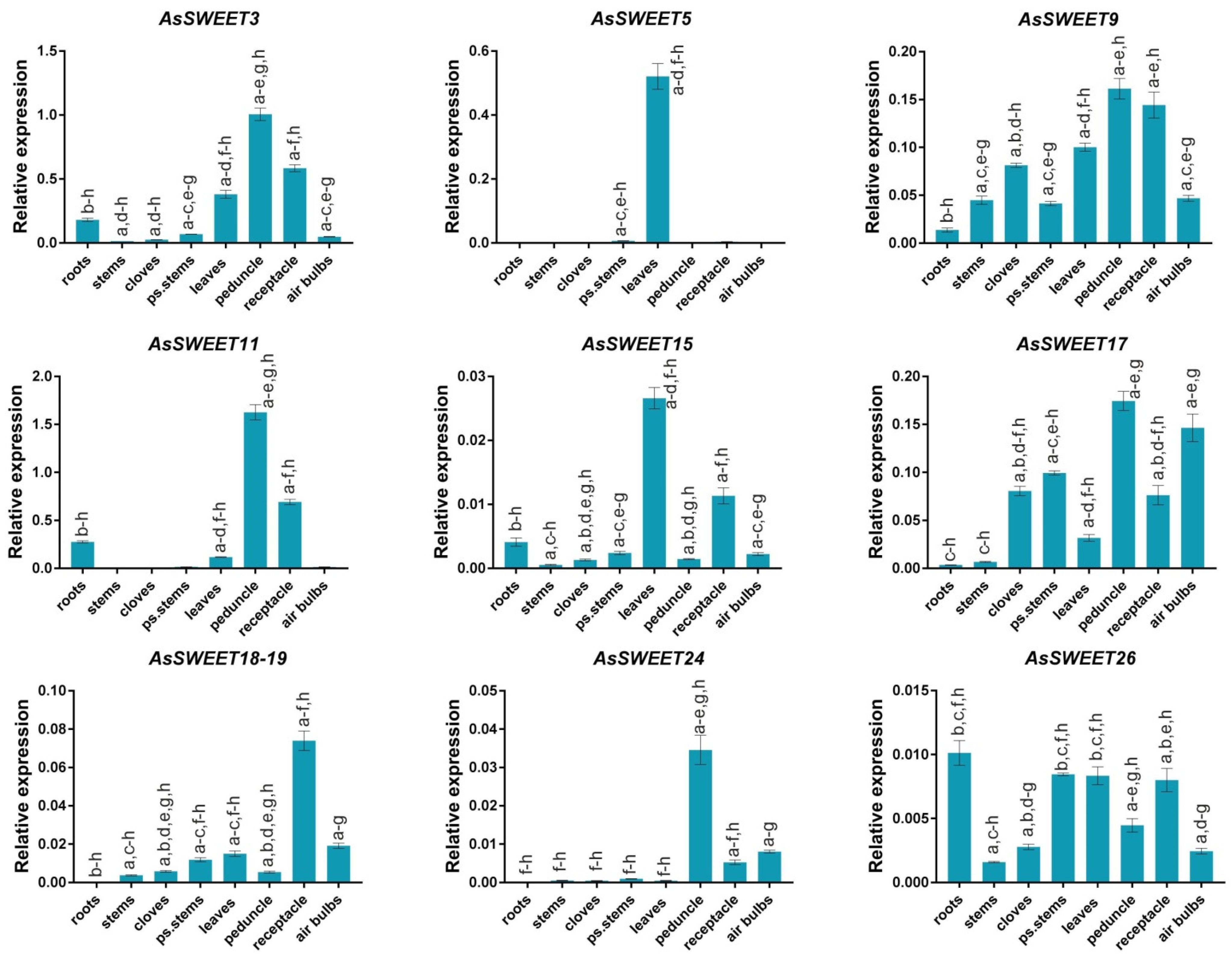
2.5. AsSWEET Gene Expression in Response to F. proliferatum Infection
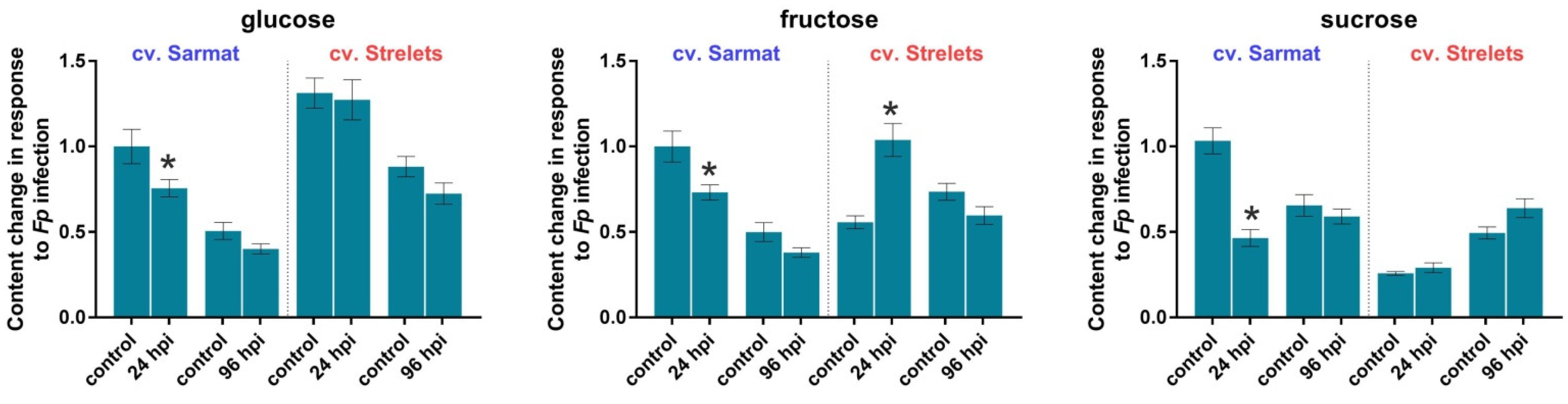
2.6. Effect of F. proliferatum Infection on Sucrose, Glucose, and Fructose Contents in Garlic
2.7. Cloning and Characterization of CDSs and Regulatory Regions of AsSWEET Genes Differentially Expressed in FBR-Sensitive and -Resistant Cultivars after F. proliferatum Infection
3. Discussion
4. Materials and Methods
4.1. Identification and Structural Analysis of the A. sativum SWEET Genes
4.2. In Silico mRNA Expression Analysis
4.3. Plant and Fungi Material and F. proliferatum Infection
4.4. RNA Extraction and qRT-PCR Analysis
4.5. Gene Amplification and Sequencing
4.6. Promoter Analysis
4.7. Sucrose, Glucose, and Fructose Contents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.-J.; Wang, G.-L.; Ma, J.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Transcript profiling of sucrose synthase genes involved in sucrose metabolism among four carrot (Daucus carota L.) cultivars reveals distinct patterns. BMC Plant Biol. 2018, 18, 8. [Google Scholar] [CrossRef]
- Duarte-Delgado, D.; Ñústez-López, C.E.; Narváez-Cuenca, C.E.; Restrepo-Sánchez, L.P.; Melo, S.E.; Sarmiento, F.; Kushalappa, A.C.; Mosquera-Vásquez, T. Natural variation of sucrose, glucose and fructose contents in Colombian genotypes of Solanum tuberosum Group Phureja at harvest. J. Sci. Food Agric. 2016, 96, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zou, L.; Ren, C.; Ren, F.; Wang, Y.; Fan, P.; Li, S.; Liang, Z. VvSWEET10 mediates sugar accumulation in grapes. Genes 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Nuñez Ocaña, D.; Choe, D.; Larsen, D.H.; Marcelis, L.F.M.; Heuvelink, E. Far-red radiation stimulates dry mass partitioning to fruits by increasing fruit sink strength in tomato. New Phytol. 2020, 228, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef]
- Sami, F.; Siddiqui, H.; Hayat, S. Interaction of glucose and phytohormone signaling in plants. Plant Physiol. Biochem. 2019, 135, 119–126. [Google Scholar] [CrossRef]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef]
- Li, X.; Si, W.; Qin, Q.; Wu, H.; Jiang, H. Deciphering evolutionary dynamics of SWEET genes in diverse plant lineages. Sci. Rep. 2018, 8, 13440. [Google Scholar] [CrossRef]
- Jeena, G.S.; Kumar, S.; Shukla, R.K. Structure, evolution and diverse physiological roles of SWEET sugar transporters in plants. Plant Mol. Biol. 2019, 100, 351–365. [Google Scholar] [CrossRef]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar transporters in plants: New insights and discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014, 201, 1150–1155. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Qu, X.-Q.; Hou, B.-H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Patil, G.; Valliyodan, B.; Deshmukh, R.; Prince, S.; Nicander, B.; Zhao, M.; Sonah, H.; Song, L.; Lin, L.; Chaudhary, J.; et al. Soybean (Glycine max) SWEET gene family: Insights through comparative genomics, transcriptome profiling and whole genome re-sequence analysis. BMC Genom. 2015, 16, 520. [Google Scholar] [CrossRef] [PubMed]
- Klemens, P.A.; Patzke, K.; Deitmer, J.; Spinner, L.; Le Hir, R.; Bellini, C.; Bedu, M.; Chardon, F.; Krapp, A.; Neuhaus, H.E. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol. 2013, 163, 1338–1352. [Google Scholar] [CrossRef]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef]
- Eom, J.S.; Chen, L.Q.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef]
- Isoda, R.; Palmai, Z.; Yoshinari, A.; Chen, L.Q.; Tama, F.; Frommer, W.B.; Nakamura, M. SWEET13 transport of sucrose, but not gibberellin, restores male fertility in Arabidopsis sweet13;14. Proc. Natl. Acad. Sci. USA 2022, 119, e2207558119. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Huh, J.H.; Yu, Y.C.; Ho, L.H.; Chen, L.Q.; Tholl, D.; Frommer, W.B.; Guo, W.J. The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant J. 2015, 83, 1046–1058. [Google Scholar] [CrossRef]
- Valifard, M.; Le Hir, R.; Müller, J.; Scheuring, D.; Neuhaus, H.E.; Pommerrenig, B. Vacuolar fructose transporter SWEET17 is critical for root development and drought tolerance. Plant Physiol. 2021, 187, 2716–2730. [Google Scholar] [CrossRef]
- Feng, C.Y.; Han, J.X.; Han, X.X.; Jiang, J. Genome-wide identification, phylogeny, and expression analysis of the SWEET gene family in tomato. Gene 2015, 573, 261–272. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.C.; Li, Y.; Chen, L.Q. Hexose transporter SWEET5 confers galactose sensitivity to Arabidopsis pollen germination via a galactokinase. Plant Physiol. 2022, 189, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.; Sosso, D.; Chen, L.Q.; Gase, K.; Kim, S.G.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.H.; Qu, X.Q.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549. [Google Scholar] [CrossRef]
- Chen, L.Q.; Lin, I.W.; Qu, X.Q.; Sosso, D.; McFarlane, H.E.; Londoño, A.; Samuels, A.L.; Frommer, W.B. A cascade of sequentially expressed sucrose transporters in the seed coat and endosperm provides nutrition for the Arabidopsis embryo. Plant Cell 2015, 27, 607–619. [Google Scholar] [CrossRef]
- Shammai, A.; Petreikov, M.; Yeselson, Y.; Faigenboim, A.; Moy-Komemi, M.; Cohen, S.; Cohen, D.; Besaulov, E.; Efrati, A.; Houminer, N.; et al. Natural genetic variation for expression of a SWEET transporter among wild species of Solanum lycopersicum (tomato) determines the hexose composition of ripening tomato fruit. Plant J. 2018, 96, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Levin, I.; Gilboa, N.; Yeselson, E.; Shen, S.; Schaffer, A.A. Fgr, a major locus that modulates fructose to glucose ratio in mature tomato fruit. Theor. Appl. Genet. 2000, 100, 256–262. [Google Scholar] [CrossRef]
- Ho, L.H.; Klemens, P.A.W.; Neuhaus, H.E.; Ko, H.Y.; Hsieh, S.Y.; Guo, W.J. SlSWEET1a is involved in glucose import to young leaves in tomato plants. J. Exp. Bot. 2019, 70, 3241–3254. [Google Scholar] [CrossRef]
- Antony, G.; Zhou, J.; Huang, S.; Li, T.; Liu, B.; White, F.; Yang, B. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell 2010, 22, 3864–3876. [Google Scholar] [CrossRef]
- Chu, Z.; Yuan, M.; Yao, J.; Ge, X.; Yuan, B.; Xu, C.; Li, X.; Fu, B.; Li, Z.; Bennetzen, J.L.; et al. Promoter mutations of an essential gene for pollen development result in disease resistance in rice. Genes Dev. 2006, 20, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wu, H.; Huang, W.; Song, J.; Zhou, Y.; Lin, Y. SWEET gene family in Medicago truncatula: Genome-wide identification, expression and substrate specificity analysis. Plants 2019, 8, 338. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, J.; Lin, Y.; Chen, H. Gene silencing using the recessive rice bacterial blight resistance gene xa13 as a new paradigm in plant breeding. Plant Cell Rep. 2012, 31, 851–862. [Google Scholar] [CrossRef]
- Hennion, N.; Durand, M.; Vriet, C.; Doidy, J.; Maurousset, L.; Lemoine, R.; Pourtau, N. Sugars en route to the roots. Transport, metabolism and storage within plant roots and towards microorganisms of the rhizosphere. Physiol. Plant. 2019, 165, 44–57. [Google Scholar] [CrossRef]
- Gai, X.T.; Jiang, N.; Ma, J.; Wang, A.; Lu, C.; Xuan, Y.H.; Xia, Z.Y. NtSWEET1 promotes tobacco resistance to Fusarium oxysporum-induced root rot disease. Plant Signal. Behav. 2021, 16, 1970940. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Zhang, H.; Zhang, Q.; Zhai, H.; Liu, Q.; He, S. The Plasma Membrane-Localized Sucrose Transporter IbSWEET10 Contributes to the Resistance of Sweet Potato to Fusarium oxysporum. Front. Plant Sci. 2017, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Lan, G.; Si, F.; Zeng, Z.; Wang, C.; Yadav, V.; Wei, C.; Zhang, X. Systematic genome-wide study and expression analysis of SWEET gene family: Sugar transporter family contributes to biotic and abiotic stimuli in Watermelon. Int. J. Mol. Sci. 2021, 22, 8407. [Google Scholar] [CrossRef]
- Tamayo, E.; Figueira-Galán, D.; Manck-Götzenberger, J.; Requena, N. Overexpression of the potato monosaccharide transporter StSWEET7a promotes root colonization by symbiotic and pathogenic fungi by increasing root sink strength. Front. Plant Sci. 2022, 13, 837231. [Google Scholar] [CrossRef]
- Cohn, M.; Bart, R.S.; Shybut, M.; Dahlbeck, D.; Gomez, M.; Morbitzer, R.; Hou, B.H.; Frommer, W.B.; Lahaye, T.; Staskawicz, B.J. Xanthomonas axonopodis virulence is promoted by a transcription activator-like effector-mediated induction of a SWEET sugar transporter in Cassava. Mol. Plant Microbe Interact. 2014, 27, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Chandran, D. Co-option of developmentally regulated plant SWEET transporters for pathogen nutrition and abiotic stress tolerance. IUBMB Life 2015, 67, 461–471. [Google Scholar] [CrossRef]
- Chong, J.; Piron, M.C.; Meyer, S.; Merdinoglu, D.; Bertsch, C.; Mestre, P. The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J. Exp. Bot. 2014, 65, 6589–6601. [Google Scholar] [CrossRef]
- Ferrari, S.; Galletti, R.; Denoux, C.; De Lorenzo, G.; Ausubel, F.M.; Dewdney, J. Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol. 2007, 144, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Netzel, M.E. Garlic: Much more than a common spice. Foods 2020, 9, 1544. [Google Scholar] [CrossRef] [PubMed]
- Stavělíková, H. Morphological characteristics of garlic (Allium sativum L.) genetic resources collection–Information. Hort. Sci. (Prague) 2008, 35, 130–135. [Google Scholar] [CrossRef]
- Gomez, C.D.; Aguilera, P.; Ortiz-Plata, A.; López, F.N.; Chánez-Cárdenas, M.E.; Flores-Alfaro, E.; Ruiz-Tachiquín, M.E.; Espinoza-Rojo, M. Aged garlic extract and S-allylcysteine increase the GLUT3 and GCLC expression levels in cerebral ischemia. Adv. Clin. Exp. Med. 2019, 28, 1609–1614. [Google Scholar] [CrossRef]
- Ban, J.O.; Lee, D.H.; Kim, E.J.; Kang, J.W.; Kim, M.S.; Cho, M.C.; Jeong, H.S.; Kim, J.W.; Yang, Y.; Hong, J.T.; et al. Antiobesity effects of a sulfur compound thiacremonone mediated via down-regulation of serum triglyceride and glucose levels and lipid accumulation in the liver of db/db mice. Phytother. Res. 2012, 26, 1265–1271. [Google Scholar] [CrossRef]
- Mylona, K.; Garcia-Cela, E.; Sulyok, M.; Medina, A.; Magan, N. Influence of two garlic-derived compounds, propyl propane thiosulfonate (PTS) and propyl propane thiosulfinate (PTSO), on growth and mycotoxin production by Fusarium species in vitro and in stored cereals. Toxins 2019, 11, 495. [Google Scholar] [CrossRef]
- Rizzalli, R.H.; Villalobos, F.J.; Orgaz, F. Radiation interception, radiation-use efficiency and dry matter partitioning in garlic (Allium sativum L.). Eur. J. Agron. 2002, 18, 33–43. [Google Scholar] [CrossRef]
- Baumgartner, S.; Dax, T.G.; Praznik, W.; Falk, H. Characterisation of the high-molecular weight fructan isolated from garlic (Allium sativum L.). Carbohydr. Res. 2000, 328, 177–183. [Google Scholar] [CrossRef]
- Guevara-Mashayekhi, K.; Mohammadi Chiane, S.; Mianabadi, M.; Ghaderifar, F.; Mousavizadeh, S.J. Change in carbohydrate and enzymes from harvest to sprouting in garlic. Food Sci. Nutr. 2015, 4, 370–376. [Google Scholar] [CrossRef]
- Figueroa, T.; López-Hernández, L.; Lopez, M.G.; Hurtado, M.D.; Vázquez-Barrios, M.E.; Guevara-Olvera, L.; Guevara González, R.G.; Rivera-Pastrana, D.M.; Torres-Robles, H.; Mercado-Silva, E.M. Conditioning garlic “seed” cloves at low temperature modifies plant growth, sugar, fructan content, and sucrose sucrose fructosyl transferase (1-SST) expression. Sci. Hortic. 2015, 189, 150–158. [Google Scholar] [CrossRef]
- Vinodh Kumar, P.N.; Mallikarjuna, M.G.; Jha, S.K.; Mahato, A.; Lal, S.K.; Yathish, K.R.; Lohithaswa, H.C.; Chinnusamy, V. Unravelling structural, functional, evolutionary and genetic basis of SWEET transporters regulating abiotic stress tolerance in maize. Int. J. Biol. Macromol. 2023, 229, 539–560. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, S.; Li, N.; Cheng, Y.; Zhao, J.; Qiao, X.; Lu, L.; Liu, S.; Wang, Y.; Liu, C.; et al. A Chromosome-level genome assembly of garlic (Allium sativum) provides insights into genome evolution and allicin biosynthesis. Mol. Plant 2020, 13, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0. molecular biology and evolution. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, L.; Li, T.; Ruan, Y.; Zhang, A.; Dong, X.; Zhu, Y.; Li, C.; Fan, J. Genome-wide investigation and characterization of sweet gene family with focus on their evolution and expression during hormone and abiotic stress response in maize. Genes 2022, 13, 1682. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z.; Filyushin, M.A. Pathogenesis-related genes of PR1, PR2, PR4, and PR5 families are involved in the response to Fusarium infection in garlic (Allium sativum L.). Int. J. Mol. Sci. 2021, 22, 6688. [Google Scholar] [CrossRef]
- Filyushin, M.A.; Anisimova, O.K.; Kochieva, E.Z.; Shchennikova, A.V. Genome-wide identification and expression of chitinase class I genes in garlic (Allium sativum L.) cultivars resistant and susceptible to Fusarium proliferatum. Plants 2021, 10, 720. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Kochieva, E.Z.; Shchennikova, A.V.; Filyushin, M.A. Thaumatin-like protein (TLP) genes in garlic (Allium sativum L.): Genome-wide identification, characterization, and expression in response to Fusarium proliferatum infection. Plants 2022, 11, 748. [Google Scholar] [CrossRef]
- Bartholomew, E.S.; Black, K.; Feng, Z.; Liu, W.; Shan, N.; Zhang, X.; Wu, L.; Bailey, L.; Zhu, N.; Qi, C.; et al. Comprehensive analysis of the chitinase gene family in cucumber (Cucumis sativus L.): From gene identification and evolution to expression in response to Fusarium oxysporum. Int. J. Mol. Sci. 2019, 20, 5309. [Google Scholar] [CrossRef]
- Manck-Götzenberger, J.; Requena, N. Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Front. Plant Sci. 2016, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Gautam, T.; Saripalli, G.; Gahlaut, V.; Kumar, A.; Sharma, P.K.; Balyan, H.S.; Gupta, P.K. Further studies on sugar transporter (SWEET) genes in wheat (Triticum aestivum L.). Mol. Biol. Rep. 2019, 46, 2327–2353. [Google Scholar] [CrossRef] [PubMed]
- Labate, J.A.; Robertson, L.D. Evidence of cryptic introgression in tomato (Solanum lycopersicum L.) based on wild tomato species alleles. BMC Plant Biol. 2012, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, X.; Li, Y.; Xu, J.; Bi, A.; Kang, L.; Xu, D.; Chen, H.; Wang, Y.; Wang, Y.G.; et al. Triticum population sequencing provides insights into wheat adaptation. Nat. Genet. 2020, 52, 1412–1422. [Google Scholar] [CrossRef]
- Kıraç, H.; Dalda Şekerci, A.; Coşkun, Ö.F.; Gülşen, O. Morphological and molecular characterization of garlic (Allium sativum L.) genotypes sampled from Turkey. Genet. Resour. Crop Evol. 2022, 69, 1833–1841. [Google Scholar] [CrossRef]
- Kalman, B.; Abraham, D.; Graph, S.; Perl-Treves, R.; Meller Harel, Y.; Degani, O. Isolation and identification of Fusarium spp., the causal agents of onion (Allium cepa) basal rot in northeastern Israel. Biology 2020, 9, 69. [Google Scholar] [CrossRef]
- Cramer, C.S. Breeding and genetics of Fusarium basal rot resistance in onion. Euphytica 2000, 115, 159–166. [Google Scholar] [CrossRef]
- Gálvez, L.; Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł.; Palmero, D. Fusarium proliferatum—Causal agent of garlic bulb rot in Spain: Genetic variability and mycotoxin production. Food Microbiol. 2017, 67, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.K.; Nanda, S.; Mishra, R.; Joshi, R.K. Multiple garlic (Allium sativum L.) microRNAs regulate the immunity against the basal rot fungus Fusarium oxysporum f. sp. cepae. Plant Sci. 2017, 257, 9–21. [Google Scholar] [CrossRef]
- Leyronas, C.; Chrétien, P.L.; Troulet, C.; Duffaud, M.; Villeneuve, F.; Morris, C.E.; Hunyadi, H. First report of Fusarium proliferatum causing garlic clove rot in France. Plant Dis. 2018, 102, 2658. [Google Scholar] [CrossRef]
- Tonti, S.; Prà, M.D.; Nipoti, P.; Prodi, A.; Alberti, I. First report of Fusarium proliferatum causing rot of stored garlic bulbs (Allium sativum L.) in Italy. J. Phytopathol. 2012, 160, 761–763. [Google Scholar] [CrossRef]
- Lalak-Kańczugowska, J.; Witaszak, N.; Waśkiewicz, A.; Bocianowski, J.; Stępień, Ł. Plant metabolites affect Fusarium proliferatum metabolism and in vitro fumonisin biosynthesis. Int. J. Mol. Sci. 2023, 24, 3002. [Google Scholar] [CrossRef]
- Wei, Y.; Xiao, D.; Zhang, C.; Hou, X. The expanded SWEET gene family following whole genome triplication in Brassica rapa. Genes 2019, 10, 722. [Google Scholar] [CrossRef]
- Morkunas, I.; Ratajczak, L. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol. Plant. 2014, 36, 1607–1619. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An Upgraded Gene Feature Visualization Server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucl. Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Schwinn, K.E.; Ngo, H.; Kenel, F.; Brummell, D.A.; Albert, N.W.; McCallum, J.A.; Pither-Joyce, M.; Crowhurst, R.N.; Eady, C.; Davies, K.M. The onion (Allium cepa L.) R2R3-MYB gene MYB1 regulates anthocyanin biosynthesis. Front. Plant Sci. 2016, 7, 1865. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, Z.; Jiang, F. Selection and validation of garlic reference genes for quantitative real-time PCR normalization. Plant Cell Tissue Organ Cult. 2015, 122, 435–444. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Q.; Li, Y.; Gao, L.; Lv, F.; Yang, R.; Wang, P. Candidate reference genes for quantitative gene expression analysis in Lagerstroemia indica. Mol. Biol. Rep. 2021, 48, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
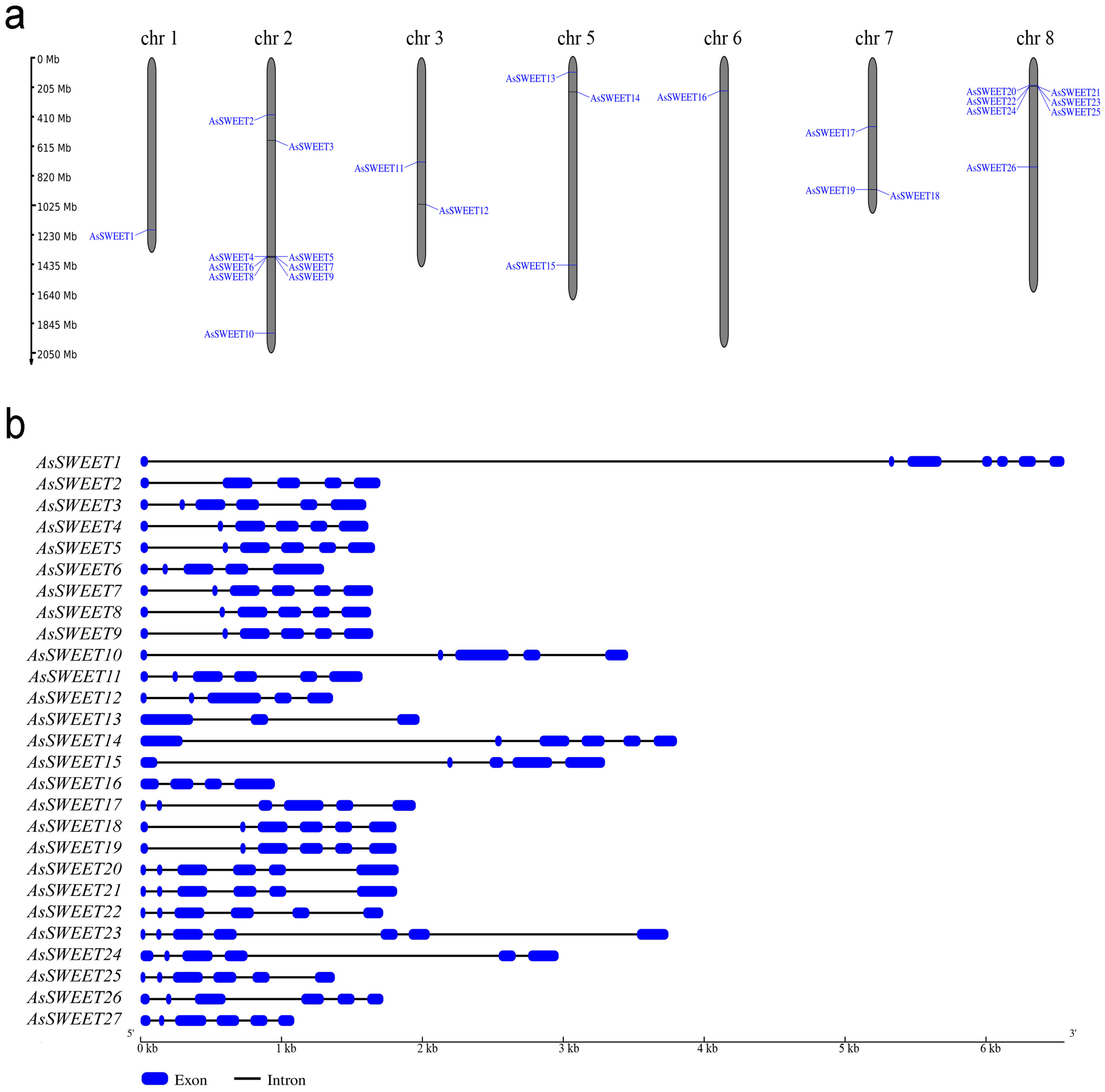
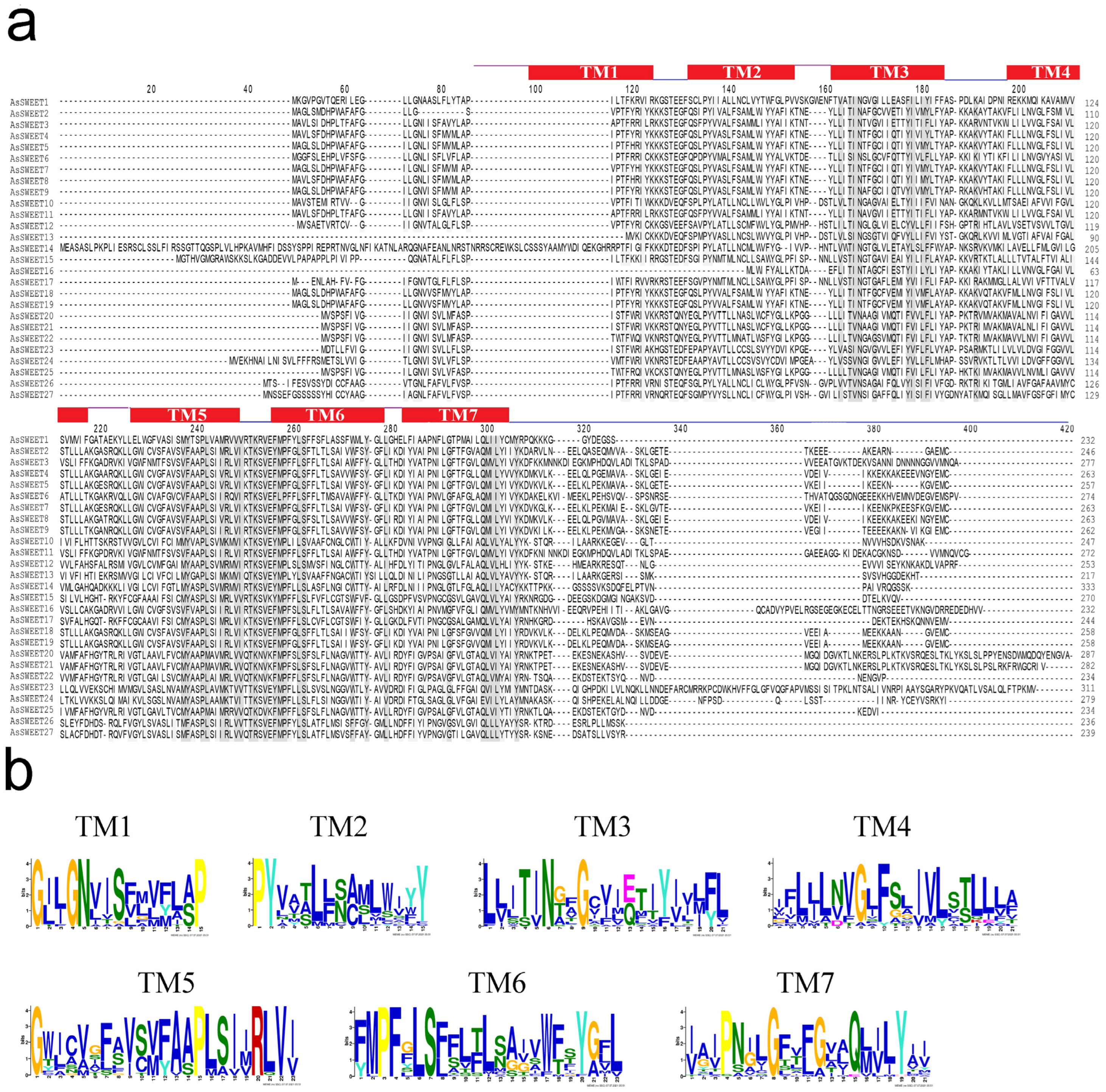

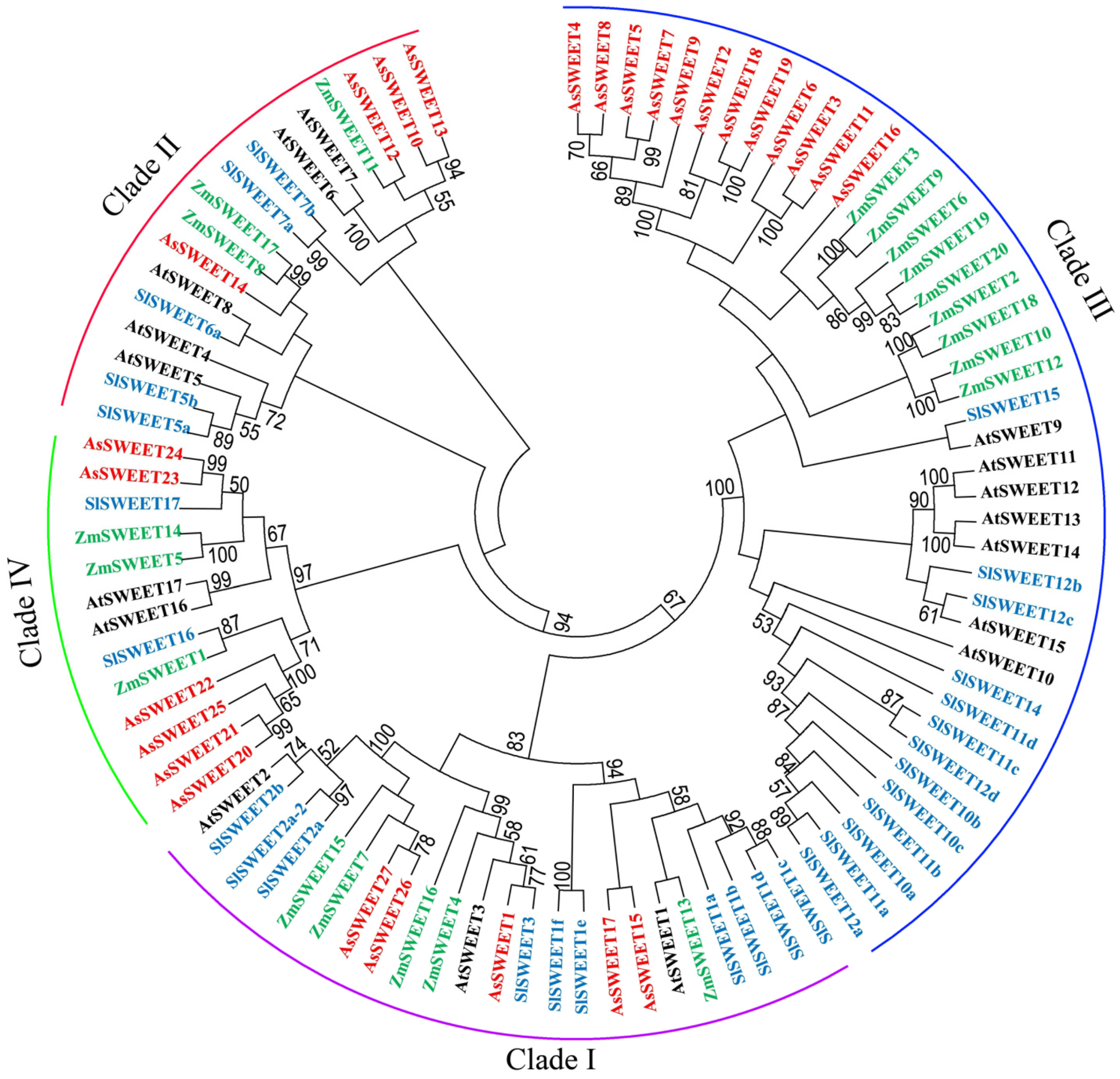
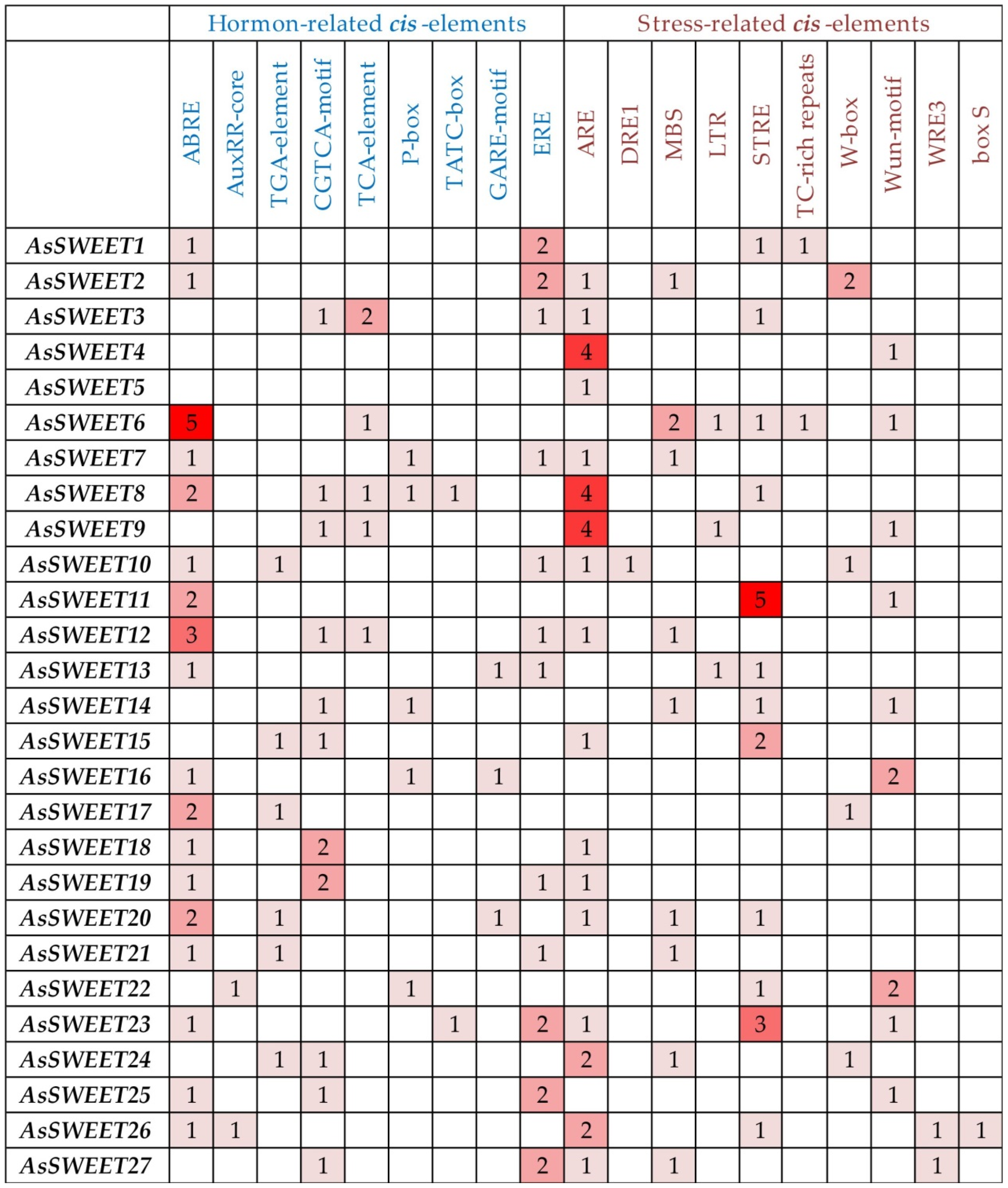

| Gene | Gene/Transcript ID [31] | Localization | Length, bp | Number of Exons | CDS, bp | Protein, aa | MW, kDa | pI | GRAVY | MtN3_slv Domain | TM Helix |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AsSWEET1 | Asa1G04426.1/Asa2G00182.1 | ch1: 1194647983–1194654538 (−) | 6556 | 7 | 699 | 232 | 25.998 | 9.18 | 0.607 | 15–99, 138–223 | 7 |
| AsSWEET2 | Asa2G01431.1/Asa3G01319.1 | ch2: 399216486–399218187 (+) | 1702 | 5 | 741 | 246 | 27.647 | 8.95 | 0.628 | 12–89, 123–205 | 7 |
| AsSWEET3 | Asa2G02061.1/Asa3G02137.1 | ch2: 572338389–572339990 (−) | 1602 | 6 | 834 | 277 | 31.033 | 9.43 | 0.666 | 12–99, 133–215 | 7 |
| AsSWEET4 | Asa2G05047.1/Asa3G05154.1 | ch2: 1381249160–1381250776 (−) | 1617 | 6 | 792 | 263 | 29.748 | 9.12 | 0.759 | 12–99, 133–215 | 7 |
| AsSWEET5 | Asa2G05048.1/Asa3G05155.1 | ch2: 1382002246–1382003909 (+) | 1664 | 6 | 774 | 257 | 29.044 | 9.37 | 0.748 | 12–99, 133–216 | 7 |
| AsSWEET6 | Asa2G05049.1/Asa3G05156.1 | ch2: 1382472847–1382474148 (+) | 1302 | 5 | 825 | 274 | 30.711 | 8.55 | 0.604 | 12–98, 133–215 | 7 |
| AsSWEET7 | Asa2G05056.1/Asa3G05153.1 | ch2: 1383831994–1383833643 (+) | 1650 | 6 | 792 | 263 | 29.754 | 9.20 | 0.698 | 12–99, 133–215 | 7 |
| AsSWEET8 | Asa2G05057.1/Asa3G05172.1 | ch2: 1383951410–1383953045 (+) | 1636 | 6 | 792 | 263 | 29.750 | 9.23 | 0.749 | 12–99, 133–215 | 7 |
| AsSWEET9 | Asa2G05060.1/Asa3G05167.1 | ch2: 1384535061–1384536711 (+) | 1651 | 6 | 789 | 262 | 29.544 | 9.26 | 0.714 | 12–99, 133–215 | 7 |
| AsSWEET10 | Asa2G07032.1/Asa3G07188.1 | ch2: 1914150050–1914153509 (+) | 3460 | 5 | 744 | 247 | 27.098 | 9.35 | 0.972 | 11–98, 134–218 | 7 |
| AsSWEET11 | Asa3G02607.1/Asa8G00525.1 | ch3: 721990358–721991933 (+) | 1576 | 6 | 819 | 272 | 30.456 | 9.30 | 0.696 | 12–99, 133–215 | 7 |
| AsSWEET12 | Asa3G03715.1/Asa8G02144.1 | ch3: 1020592700–1020594065 (−) | 1366 | 5 | 762 | 253 | 28.012 | 8.89 | 0.860 | 11–94, 132–218 | 7 |
| AsSWEET13 | Asa5G00452.1/Asa6G00626.1 | ch5: 108642296–108644275 (−) | 1980 | 3 | 654 | 217 | 23.991 | 9.36 | 0.835 | 1–65, 103–189 | 6 |
| AsSWEET14 | Asa5G01067.1/Asa6G01212.1 | ch5: 246733367–246737173 (−) | 3807 | 6 | 1002 | 333 | 37.185 | 9.75 | 0.203 | 116–183, 220–301 | 6 |
| AsSWEET15 | Asa5G05357.1/Asa5G00822.1 | ch5: 1446881843–1446885138 (−) | 3296 | 5 | 813 | 270 | 29.239 | 9.36 | 0.552 | 40–122, 159–241 | 6 |
| AsSWEET16 | Asa6G00924.1/Asa6G07296.1 | ch6: 237675722–237676674 (+) | 953 | 4 | 699 | 232 | 25.961 | 5.84 | 0.613 | 1–42, 76–162 | 5 |
| AsSWEET17 | Asa7G01759.1/Asa1G02255.1 | ch7: 480861997–480863949 (+) | 1953 | 6 | 735 | 244 | 27.281 | 9.28 | 0.644 | 7–95, 129–214 | 7 |
| AsSWEET18 | Asa7G03377.1/Asa1G00572.1 | ch7: 914393920–914395735 (−) | 1816 | 6 | 777 | 258 | 29.040 | 9.07 | 0.796 | 12–99, 133–214 | 7 |
| AsSWEET19 | Asa7G03379.1/Asa1G00574.1 | ch7: 914587432–914589248 (+) | 1817 | 6 | 777 | 258 | 29.040 | 9.07 | 0.796 | 12–99, 133–215 | 7 |
| AsSWEET20 | Asa8G00607.1/Asa6G05670.1 | ch8: 193855534–193857365 (+) | 1832 | 6 | 864 | 287 | 31.852 | 9.54 | 0.515 | 6–93, 129–212 | 7 |
| AsSWEET21 | Asa8G00611.1/Asa7G00384.1 | ch8: 194959619–194961439 (+) | 1821 | 6 | 849 | 282 | 31.369 | 10.01 | 0.612 | 6–93, 129–212 | 7 |
| AsSWEET22 | Asa8G00624.1/Asa7G00398.1 | ch8: 197356527–197358249 (−) | 1722 | 6 | 705 | 234 | 25.832 | 9.68 | 0.765 | 6–93, 129–210 | 7 |
| AsSWEET23 | Asa8G00626.1/Asa7G00406.1 | ch8: 197411881–197415626 (−) | 3746 | 7 | 936 | 311 | 34.077 | 8.87 | 0.797 | 6–91, 127–208 | 6 |
| AsSWEET24 | Asa8G00629.1/Asa8G00420.1 | ch8: 198119328–198122294 (−) | 2967 | 6 | 840 | 279 | 30.696 | 6.82 | 0.778 | 25–111, 157–233 | 6 |
| AsSWEET25 | Asa8G00631.1/Asa7G00403.1 | ch8: 198345649–198347027 (+) | 1379 | 6 | 705 | 234 | 25.907 | 9.58 | 0.795 | 6–93, 129–213 | 7 |
| AsSWEET26 | Asa8G02736.1/Asa7G02662.1 | ch8: 756634184–756635906 (−) | 1723 | 6 | 711 | 236 | 26.380 | 8.79 | 0.852 | 19–105, 139–224 | 7 |
| AsSWEET27 | Asa0G02772.1/Asa8G01762.1 | scaffold24049: 67334–68424 (−) | 1091 | 5 | 720 | 239 | 26.761 | 8.19 | 0.741 | 21–102, 141–225 | 7 |
| Gene | NCBI ID (Sarmat/Strelets) | SNP (aa Substitution) in CDS | SNPs and Indels in Regulatory Region | ||
|---|---|---|---|---|---|
| cv. Sarmat | cv. Strelets | cv. Sarmat | cv. Strelets | ||
| AsSWEET3 | OQ607029/OQ607030 | c. 282T>C, c. 293A>G (p. K98R), c. 351G>A, c. 387T>C, c. 711T>C | -768A>T, -769A>G, -778C>T, del. ˗239.. ˗579 (340 bp), insertion 3 bp (˗704) | ˗188 T>A, ˗768A>T, ˗769A>G, ˗778C>T, ˗832T>C, del. ˗239.. ˗579 (340 bp) | |
| c. 758T>C (p. V253A) | |||||
| AsSWEET9 | OQ607031/OQ607032 | c. 153C>T, c. 336A>T, c. 582G>A | ˗307G>A, ˗411C>T, ˗412T>C, ˗419A>G, ˗429C>T, ˗450T>G, ˗454A>G, ˗456C>G, ˗459A>T, ˗475A>G, ˗910C>T, del. ˗595.. ˗596 (2 bp) | ˗307G>A, ˗411C>T, ˗412T>C, ˗419A>G, ˗429C>T, ˗450T>G, ˗454A>G, ˗456C>G, ˗459A>T, ˗475A>G, ˗910C>T, del. ˗595.. ˗596 (2 bp) | |
| AsSWEET11 | OQ607033/OQ607034 | c. 72C>T, c. 75T>C, c. 324G>A, c. 813C>A, c. 734A>T | ˗501C>T, ˗551T>C | ˗501C>T, ˗551T>C | |
| c. 734A>T (p. E245V) | c. 69C>T, c. 761T>C (p. I254T) | ||||
| AsSWEET26 | OQ607035/OQ607036 | c. 204C>A, c. 534T>C | ˗225G>A, ˗291T>A, ˗515T>G, ˗528C>T, ˗564G>A, ˗578A>G, ˗596T>G, ˗671G>T, ˗673T>C, ˗791T>A, ˗849T>C, ˗856T>C, del. ˗624.. ˗633 (10 bp) | ˗222T>C, ˗259G>A, ˗355A>G, ˗515T>G, ˗516T>C, ˗533T>A, ˗539T>G, ˗578A>G, ˗591G>A, ˗596T>G, ˗604A>C, ˗653A>G, ˗667T>C, ˗671G>T, ˗673T>C, ˗791T>A, ˗849T>C, ˗856T>C | |
| Elements | AsSWEET3 | AsSWEET9 | AsSWEET11 | AsSWEET26 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ershuizao | Sarmat | Strelets | Ershuizao | Sarmat | Strelets | Ershuizao | Sarmat | Strelets | Ershuizao | Sarmat | Strelets | ||
| Hormone-related | ABRE | 1 | 1 | 1 | |||||||||
| AuxRR-core | 1 | 1 | |||||||||||
| TGA-element | 1 | 1 | |||||||||||
| CGTCA-motif | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| TCA-element | 2 | 2 | 2 | 1 | 1 | 1 | |||||||
| ERE | 1 | 1 | 1 | ||||||||||
| P-box | 1 | 1 | |||||||||||
| Stress-related | ARE | 1 | 1 | 1 | 4 | 4 | 4 | 2 | 1 | 1 | |||
| STRE | 1 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||
| LTR | 1 | 1 | 1 | ||||||||||
| WRE3 | 1 | 1 | 1 | 1 | |||||||||
| WUN-motif | 1 | 1 | 1 | ||||||||||
| GC-motif | 2 | 2 | 2 | ||||||||||
| Other | AAGAA-motif | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Box 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| F-box | 1 | ||||||||||||
| AE-box | 3 | 2 | 2 | 1 | 1 | 1 | |||||||
| CAT-box | 1 | 1 | 1 | ||||||||||
| G-box | 1 | 1 | 1 | 2 | 2 | 2 | |||||||
| ATC-motif | 1 | 1 | 1 | ||||||||||
| TCT-motif | |||||||||||||
| Circadian | 1 | 1 | 1 | ||||||||||
| AT-rich element | 1 | 1 | 1 | ||||||||||
| CTAG-motif | 1 | 1 | 1 | ||||||||||
| O2-site | 1 | 1 | 1 | ||||||||||
| Box S | 1 | 1 | 1 | ||||||||||
| MYC | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 2 | 2 | ||||
| MYB | 2 | 2 | 2 | 3 | 1 | 2 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filyushin, M.A.; Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z. Genome-Wide Identification, Expression, and Response to Fusarium Infection of the SWEET Gene Family in Garlic (Allium sativum L.). Int. J. Mol. Sci. 2023, 24, 7533. https://doi.org/10.3390/ijms24087533
Filyushin MA, Anisimova OK, Shchennikova AV, Kochieva EZ. Genome-Wide Identification, Expression, and Response to Fusarium Infection of the SWEET Gene Family in Garlic (Allium sativum L.). International Journal of Molecular Sciences. 2023; 24(8):7533. https://doi.org/10.3390/ijms24087533
Chicago/Turabian StyleFilyushin, Mikhail A., Olga K. Anisimova, Anna V. Shchennikova, and Elena Z. Kochieva. 2023. "Genome-Wide Identification, Expression, and Response to Fusarium Infection of the SWEET Gene Family in Garlic (Allium sativum L.)" International Journal of Molecular Sciences 24, no. 8: 7533. https://doi.org/10.3390/ijms24087533






