Composite Hydrogels Based on Poly(Ethylene Glycol) and Cellulose Macromonomers as Fortified Materials for Environmental Cleanup and Clean Water Safeguarding
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Chain-End-Modified PEGs
2.2. Synthesis and Characterization of Hydrogels Based on PEG-m, Cellobiose, and Cellulose Crosslinks
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Modified PEG (PEG-m)
3.2.2. Preparation of Reactive “Cellu-Mers” Based on Cellobiose and Cellulose Microfibers
3.2.3. Synthesis of Hydrogels Using PEG-m with Different Chain Lengths and “Cellu-Mers”
3.2.4. Characterization of Modified PEGs
- Size-Exclusion Chromatography (SEC)
- Nuclear Magnetic Resonance Spectroscopy (NMR)
- Ultraviolet–Visible (UV–Vis) Spectroscopy
3.2.5. Characterization of PEG-m Hydrogels
- Swelling Studies
- Scanning Electron Microscopy (SEM)
- Differential Scanning Calorimetry (DSC)
- Rheology Measurements
- Adsorption Capability, or Dye Binding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baggio, G.; Qadir, M.; Smakhtin, V. Freshwater availability status across countries for human and ecosystem needs. Sci. Total Environ. 2021, 792, 148230. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S. Freshwater and Human Population: A Global Perspective; Yale F & ES Bulletin Series; F & ES: Concord, CA, USA, 2002; pp. 149–157. [Google Scholar]
- Wu, Y.; Pang, H.; Liu, Y.; Wang, X.; Yu, S.; Fu, D.; Chen, J.; Wang, X. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Tratnyek, P.G.; Johnson, R.L. Nanotechnologies for environmental cleanup. Nano Today 2006, 1, 44–48. [Google Scholar] [CrossRef]
- Yadav, K.K.; Singh, J.K.; Gupta, N.; Kumar, V. A review of nanobioremediation technologies for environmental cleanup: A novel biological approach. J. Mater. Environ. Sci. 2017, 8, 740–757, ISSN 2028-2508. [Google Scholar]
- Pilon-Smits, E.A.H.; Freeman, J.L. Environmental cleanup using plants: Biotechnological advances and ecological considerations. Front. Ecol. Environ. 2006, 4, 203–210. [Google Scholar] [CrossRef]
- Weerasundara, L.; Gabriele, B.; Figoli, A.; Ok, Y.; Bundschuh, J. Hydrogels: Novel materials for contaminant removal in water—A review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1970–2014. [Google Scholar] [CrossRef]
- Hernández, N.; Williams, R.C.; Cochran, E.W. The battle for the “green” polymer. Different approaches for biopolymer synthesis: Bioadvantaged vs. bioreplacement. Org. Biomol. Chem. 2014, 12, 2834–2849. [Google Scholar] [CrossRef]
- Kukhar, V.P. Biomass—Renewable feedstock for organic chemicals (“white chemistry”). J. Chem. Chem. Eng. 2009, 58, 57–71. [Google Scholar] [CrossRef]
- Thakur, V.; Thakur, M. Handbook of Composites from Renewable Materials; V1; John Wiley & Sons: New York, NY, USA, 2017; pp. 190–191, 246–247. [Google Scholar]
- Maleki, L.; Edlund, U.; Albertsson, A.C. Green Semi-IPN Hydrogels by Direct Utilization of Crude Wood Hydrolysates. ACS Sustain. Chem. Eng. 2016, 4, 4370–4377. [Google Scholar] [CrossRef]
- Du, H.; Shi, S.; Liu, W.; Teng, H.; Piao, M. Processing and modification of hydrogel and its application in emerging contaminant adsorption and in catalyst immobilization: A review. J. Clean. Prod. 2020, 284, 12967–12994. [Google Scholar] [CrossRef]
- Jain, K.; Desai, C.; Tiwari, O.; Madamwar, D. Dyes: Effect on the Environment and Biosphere and Their Remediation Constraints. In Microbial Bioremediation & Biodegradation; Shah, M., Ed.; Springer: Singapore, 2020; pp. 73–94. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, F.; Li, L.; Cao, M.; Zuo, D.; Liu, H. Removal of anionic dyes from aqueous solutions by adsorption of chitosan-based semi-IPN hydrogel composites. Compos. B Eng. 2012, 43, 1570–1578. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, S.; Liu, H.; Yang, S.; Zhan, H. Removal of methylene blue dyes from wastewater using cellulose-based superadsorbent hydrogels. Polym. Eng. Sci. 2011, 51, 2417–2424. [Google Scholar] [CrossRef]
- Kabiri, K. Superabsorbent hydrogel composites. Polym. Adv. Technol. 2003, 14, 438–444. [Google Scholar] [CrossRef]
- Lee, W.; Huang, Y. Swelling and antibacterial properties for the superabsorbent hydrogels containing silver nanoparticles. J. Appl. Polym. Sci. 2007, 106, 1992–1999. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef] [PubMed]
- Sohni, S.; Hassan, T.; Khan, S.B.; Akhtar, K.; Bakhsh, E.M.; Hashim, R.; Nidaullah, H.; Khan, M.; Khan, S.A. Lignin nanoparticles-reduced graphene oxide based hydrogel: A novel strategy for environmental applications. Int. J. Biol. Macromol. 2022, 225, 1426–1436. [Google Scholar] [CrossRef]
- Hu, S.; Zhi, Y.; Shan, S.; Ni, Y. Research progress of smart response composite hydrogels based on nanocellulose. Carbohydr. Polym. 2022, 275, 118741. [Google Scholar] [CrossRef]
- Bonetti, L.; Demitri, C.; Riva, L. Editorial on the Special Issue “Advances in Cellulose-Based Hydrogels”. Gels 2022, 8, 790. [Google Scholar] [CrossRef]
- Li, K.; Yan, J.; Zhou, Y.; Li, B.; Xin, L. β-cyclodextrin and magnetic graphene oxide modified porous composite hydrogel as a superabsorbent for adsorption cationic dyes: Adsorption performance, adsorption mechanism and hydrogel column process investigates. J. Mol. Liq. 2021, 335, 116291. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.M.F. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels 2021, 7, 30. [Google Scholar] [CrossRef]
- Cheng, G.; Zhou, M.; Wei, Y.J.; Cheng, F.; Zhu, P.X. Comparison of mechanical reinforcement effects of cellulose nanocrystal, cellulose nanofiber, and microfibrillated cellulose in starch composites. Polym. Compos. 2019, 40, E365–E372. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.; Ibrahim, I.; Arun, D.; Holtzl, T.; Dumée, L.F.; Ngee, H.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Javadi, A. Fabrication and characterization of CMC-based magnetic superabsorbent hydrogel nanocomposites for crystal violet removal. Polym. Adv. Technol. 2016, 27, 1609–1616. [Google Scholar] [CrossRef]
- Getya, D.; Gitsov, I. Stronger together. Poly(styrene) gels reinforced by soft gellan gum. Gels 2022, 8, 607. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Shao, L.; Lau, C.H. High flux polyethylene glycol based nanofiltration membranes for water environmental remediation. J. Membr. Sci. 2015, 476, 95–104. [Google Scholar] [CrossRef]
- Margerrison, E. Polyethylene Glycol Safety Profile. 2021. Available online: https://www.fda.gov/media/155398/download (accessed on 20 February 2023).
- Balakrishnan, B.; Kumar, D.S.; Yoshida, Y.; Jayakrishnan, A. Chemical modification of poly(vinyl chloride) resin using poly(ethylene glycol) to improve blood compatibility. Biomaterials 2005, 26, 3495–3502. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 1, 64–82. [Google Scholar] [CrossRef]
- Johnson, W. Final report on the safety assessment of PEG-25 propylene glycol stearate, PEG-75 propylene glycol stearate, PEG-120 propylene glycol stearate, PEG-10 propylene glycol, PEG-8 propylene glycol cocoate, and PEG-55 propylene glycol oleate. Int. J. Toxicol. 2001, 20 (Suppl. 4), 13–26. [Google Scholar] [CrossRef]
- Sagar, A.; Rauf, F.; Mia, A.; Shabi, T.H.; Rahman, T.; Zakir, A.K.M. Polyethylene Glycol (PEG) induced drought stress on five rice genotypes at early seedling stage. J. Bangladesh Agril. Univ. 2020, 18, 606–614. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, S.; Duan, H.; He, J.; Luo, Y. Removal of anionic and cationic dyes using porous chitosan/carboxymethyl cellulose-PEG hydrogels: Optimization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2023, 231, 123213. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/epichlorohydrin.pdf (accessed on 27 March 2023).
- Getya, D.; Gitsov, I. Reactive cellu-mers—A novel approach to improved cellulose/polymer composites. Gels 2022, 14, 1670. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.; Gatenholm, P.; Oksman, K. The effect of crosslinking on the properties of polyethylene/wood flour composites. J. Compos. Sci. 2005, 65, 1468–1479. [Google Scholar] [CrossRef]
- Chiou, B.S.; Schoen, P.E. Effects of crosslinking on thermal and mechanical properties of polyurethanes. J. Appl. Polym. Sci. 2002, 83, 212–223. [Google Scholar] [CrossRef]
- Safranski, D.L.; Gall, K. Effect of chemical structure and crosslinking density on the thermo-mechanical properties and toughness of (meth)acrylate shape memory polymer networks. Polymer 2008, 49, 4446–4455. [Google Scholar] [CrossRef]
- Schlögl, S.; Trutschel, M.; Chassé, W.; Riess, G.; Saalwächter, K. Entanglement Effects in Elastomers: Macroscopic vs Microscopic Properties. Macromolecules 2014, 47, 2759–2773. [Google Scholar] [CrossRef]
- Branca, C.; Magazù, S.; Maisano, G.; Migliardo, F.; Migliardo, P.; Romeo, G. Hydration study of PEG/water mixtures by quasi elastic light scattering, acoustic and rheological measurements. J. Phys. Chem. B 2002, 106, 10272–10276. [Google Scholar] [CrossRef]
- Craig, Q.M. A review of thermal methods used for the analysis of the crystal form, solution thermodynamics and glass transition behavior of polyethylene glycols. Thermochim. Acta 1995, 248, 189–203. [Google Scholar] [CrossRef]
- Buckley, C.P. Kinetic theory of polymer crystal growth applied to melt crystallization of low molecular weight poly(ethylene oxide). Polymer 1980, 21, 444–457. [Google Scholar] [CrossRef]
- Gitsov, I.; Zhu, C. Amphiphilic hydrogels constructed by poly(ethylene glycol) and shape-persistent dendritic fragments. Macromolecules 2002, 35, 8418–8427. [Google Scholar] [CrossRef]
- Kou, J.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Minea, A.A. State of the Art in PEG-Based Heat Transfer Fluids and Their Suspensions with Nanoparticles. Nanomaterials 2021, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, M.S.; Albertsson, A.C.; Ranucci, E.; Laus, M.; Giani, E. Biodegradable polymers from renewable sources: Rheological characterization of hemicellulose-based hydrogels. Biomacromolecules 2005, 6, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Hoti, G.; Caldera, F.; Cecone, C.; Pedrazzo, A.R.; Anceschi, A.; Appleton, S.L.; Monfared, Y.K.; Trotta, F. Effect of the cross-linking density on the swelling and rheological behavior of ester-bridged β-cyclodextrin nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Horkay, F.; Douglas, J.F. Chapter 1—Polymer Gels: Basics, Challenges, and Perspectives. In Gels and Other Amorphous Solids; Horkay, F., Douglas, J.F., Del Gado, E., Eds.; ACS Symp. Ser. Vol. 1296; American Chemical Society: Washington, DC, USA, 2018; pp. 1–13. [Google Scholar] [CrossRef]
- Hamdaoui, O. Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J. Hazard. Mater. 2006, 135, 264–273. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R.N. Exposure to Crystal Violet, Its Toxic, Genotoxic and Carcinogenic Effects on Environment and Its Degradation and Detoxification for Environmental Safety. Rev. Environ. Contam. Toxicol. 2016, 237, 71–104. [Google Scholar] [CrossRef]
- Pandiyarajan, C.K.; Genzer, J. Effect of Network Density in Surface-Anchored Poly (N-isopropylacrylamide) Hydrogels on Adsorption of Fibrinogen. Langmuir 2017, 33, 1974–1983. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Wattanakornsiri, A.; Wachirawongsakorn, P.; Kaewpirom, S. Effects of crosslinking degree of poly(vinyl alcohol) hydrogel in aqueous solution: Kinetics and mechanism. Polym. Bull. 2014, 71, 1081–1100. [Google Scholar] [CrossRef]
- Mahdavinia, R.G.; Hasanpour, J.; Rahmani, Z.; Karami, S.; Etemadi, H. Nanocomposite hydrogel from grafting of acrylamide onto HPMC using sodium montmorillonite nanoclay and removal of crystal violet dye. Cellulose 2013, 20, 2591–2604. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Z.Y.; Su, X.P.; Chen, X.; Jiang, L.; Yao, J.M. Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent. ACS Sustain. Chem. Eng. 2015, 3, 432–442. [Google Scholar] [CrossRef]
- Zhu, C.; Hard, C.; Lin, C.; Gitsov, I. Novel materials for bioanalytical and biomedical applications. Environmental response and binding/release capabilities of amphiphilic hydrogels with shape-persistent dendritic junctions. J. Polym. Sci., Part A Polym. Chem. 2005, 43, 4017–4029. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Massoumi, B.; Jalili, K.; Kiani, G. Effect of sodium montmorillonite nanoclay on the water absorbency and cationic dye removal of carrageenan-based nanocomposite superabsorbents. J. Polym. Res. 2012, 19, 9947. [Google Scholar] [CrossRef]
- Baughman, G.L.; Banerjee, S.; Perenich, T.A. Chapter 5—Dye solubility. In Physico-Chemical Principles of Color Chemistry, Advances in Color Chemistry Series; Peters, A.T., Freeman, H.S., Eds.; Springer: Dordrecht, Germany, 1996; Volume 4, pp. 145–195. [Google Scholar] [CrossRef]
- Ramos, G.R.T.M.; da Costa, D.M.; Shiguihara, A.L.; Amim, J., Jr. Partitioning behavior of methylene blue in aqueous two phase system. J. Chem. Pharm. Res. 2016, 8, 928–933. [Google Scholar]
- Pielichowski, K.; Flejtuch, K. Differential scanning calorimetry studies on poly(ethylene glycol) with different molecular weights for thermal energy storage materials. Polym. Adv. Technol. 2003, 13, 690–696. [Google Scholar] [CrossRef]
- Mayerhöfer, T.G.; Pahlow, S.; Popp, J. The Bouguer-Beer-Lambert Law: Shining Light on the Obscure. ChemPhysChem 2020, 21, 2029–2046. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mu, B.; Yang, Y. Feasibility of industrial scale treatment of due wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable Technologies for Water Purification from Heavy Metals: Review and Analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/water-and-wastewater-treatment-equipment-market (accessed on 27 March 2023).
- Forbes. New Technology Trends In The Wastewater Management Industry. Available online: https://www.forbes.com/sites/theyec/2022/10/25/new-technology-trends-in-the-wastewater-management-industry/?sh=4db1f51b4199 (accessed on 27 March 2023).
- Das, P.; Ganguly, S.; Saravanan, A.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Naturally Derived Carbon Dots in Situ Confined Self-Healing and Breathable Hydrogel Monolith for Anomalous Diffusion-Driven Phytomedicine Release. ACS Appl. Bio Mater. 2022, 5, 5617–5633. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhao, J.; Wang, S. Multifunctional hydrogels based on chitosan, hyaluronic acid and other biological macromolecules for the treatment of inflammatory bowel disease: A review. Int. J. Biol. Macromol. 2023, 227, 505–523. [Google Scholar] [CrossRef]
- Yin, H.; Song, P.; Chen, X.; Huang, Q.; Huang, H. A self-healing hydrogel based on oxidized microcrystalline cellulose and carboxymethyl chitosan as wound dressing material. Int. J. Biol. Macromol. 2022, 221, 1606–1617. [Google Scholar] [CrossRef]



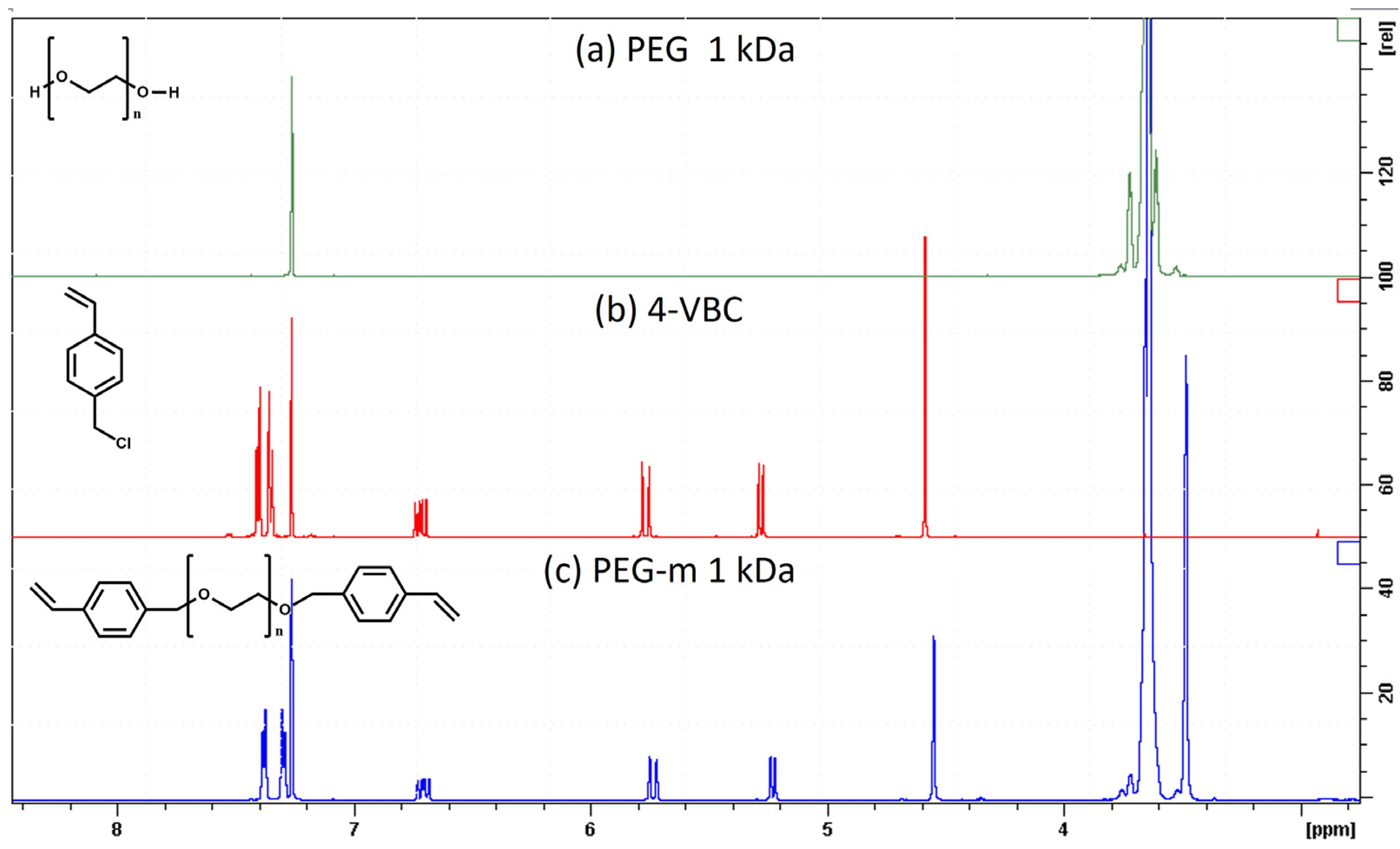
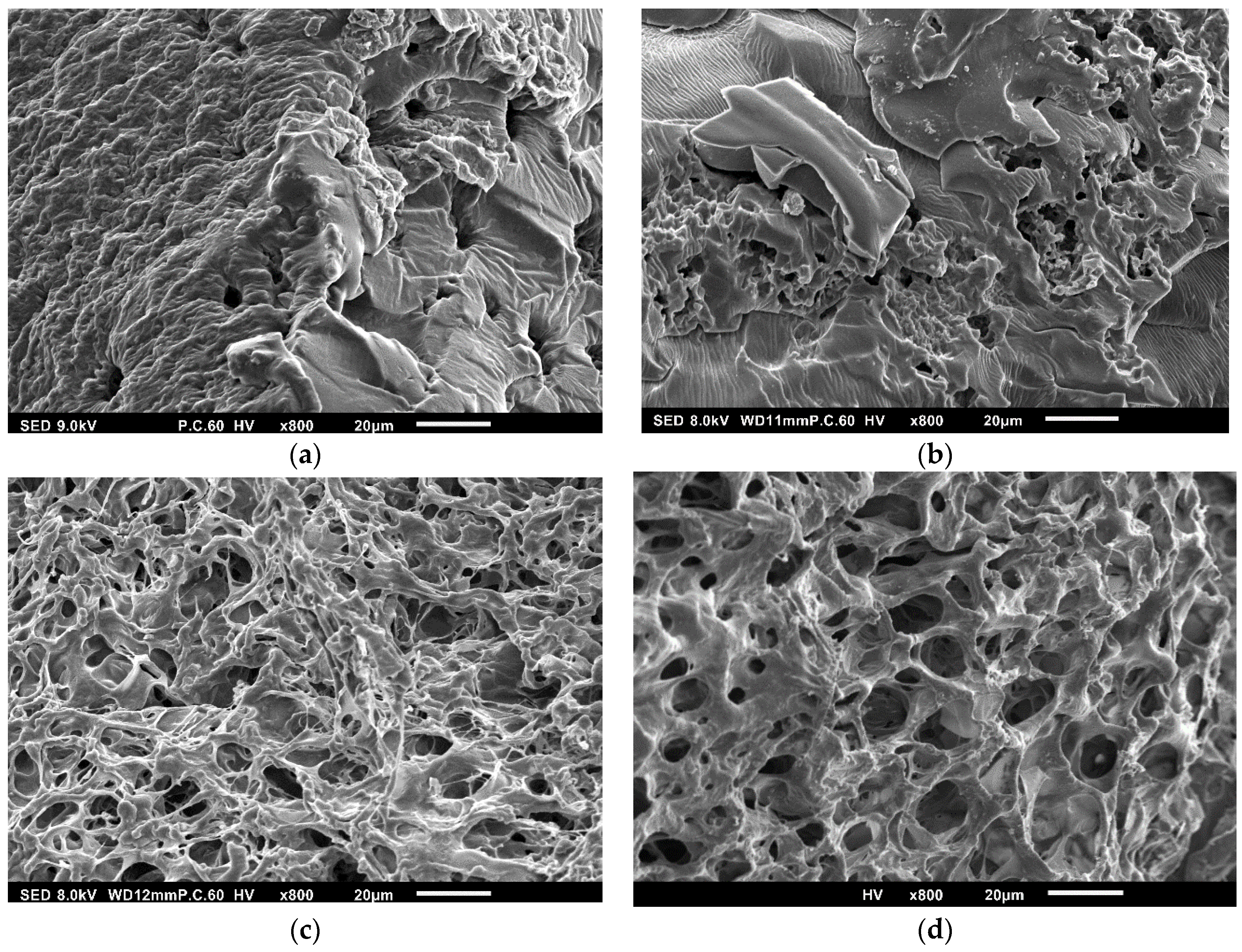




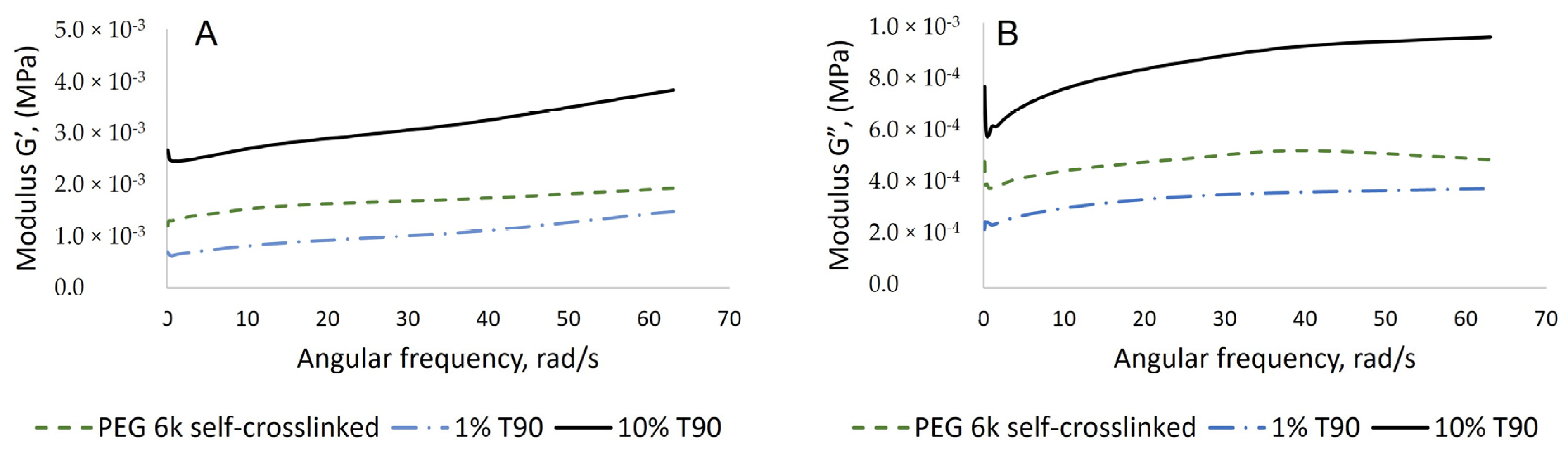
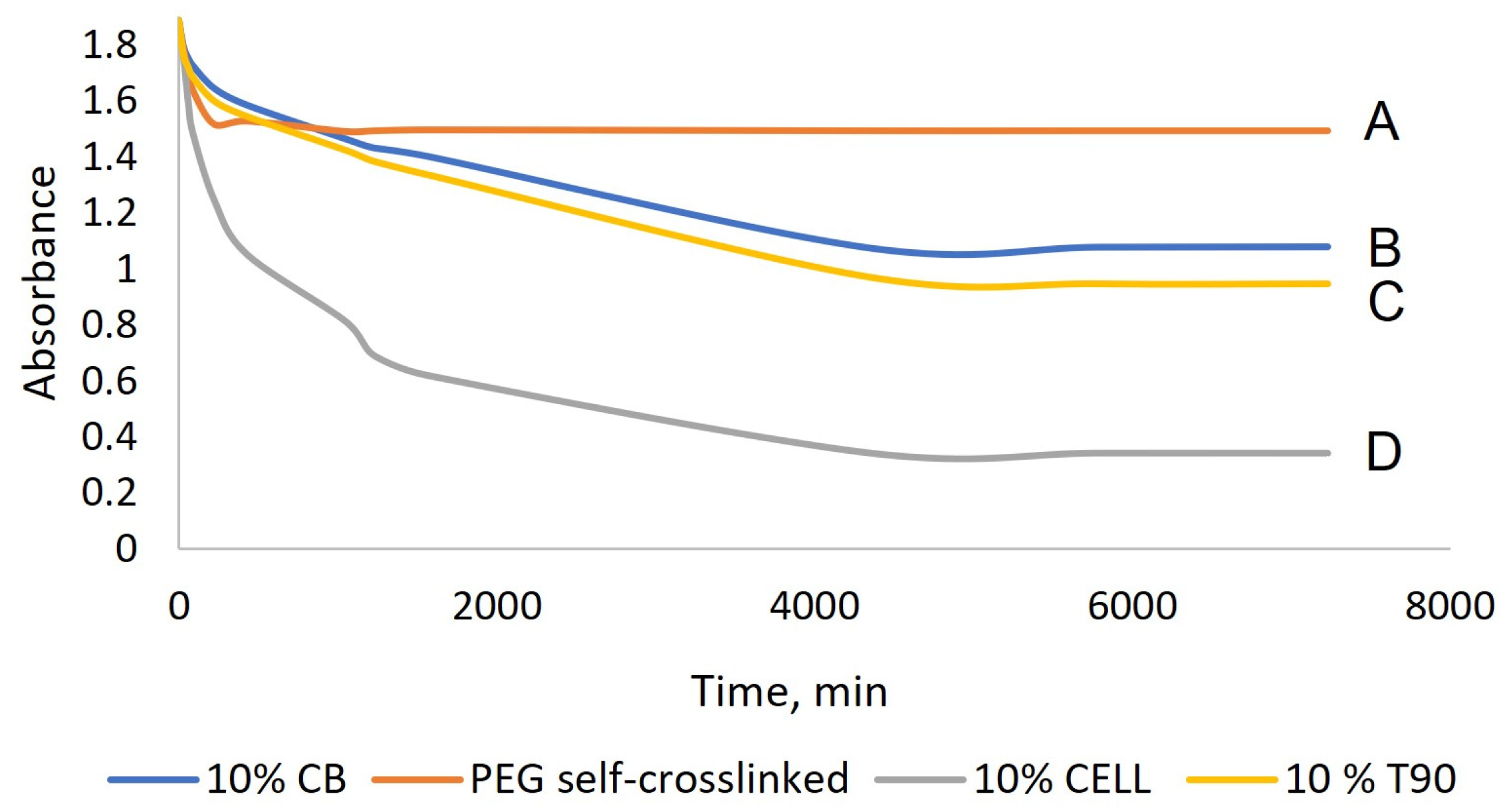
| Sample | Mn (a) | Mw (b) | Mp (c) | Ð (d) |
|---|---|---|---|---|
| PEG 1k | 1300 | 1400 | 1500 | 1.09 |
| PEG 1k-m | 1500 | 1600 | 1700 | 1.08 |
| PEG 5k | 6500 | 7000 | 7200 | 1.05 |
| PEG 5k-m | 6900 | 7100 | 7600 | 1.04 |
| PEG 6k | 8100 | 8400 | 8800 | 1.04 |
| PEG 6k-m | 8700 | 9100 | 9500 | 1.04 |
| PEG 10k | 11,600 | 12,200 | 13,400 | 1.05 |
| PEG 10k-m | 15,100 | 15,600 | 15,700 | 1.03 |
| Hydrogel | Crosslinker, wt% | PEG-m, wt% | AIBN, wt% | SD in THF, % | SD in H2O, % | Extractables, % | Gel Yield, % |
|---|---|---|---|---|---|---|---|
| No crosslinker | |||||||
| 1 kDa | 0 | 99.5 | 0.5 | 837 ± 16 | 1236 ± 75 | 5.30 | 94.70 |
| 6 kDa | 0 | 99.5 | 0.5 | 1048 ± 371 | 1682 ± 132 | 7.6 | 92.4 |
| CB-m | |||||||
| 1 kDa | 1 | 98.5 | 0.5 | 427 ± 31 | 628 ± 89 | 17.65 | 82.35 |
| 10 | 89.5 | 0.5 | 356 ± 17 | 871 ± 95 | 20.20 | 79.80 | |
| 6 kDa | 1 | 98.5 | 0.5 | 561 ± 41 | 655 ± 181 | 13.7 | 86.3 |
| 10 | 100 | 0.5 | 237 ± 46 | 779 ± 30 | 11.1 | 88.9 | |
| CELL-m | |||||||
| 1 kDa | 1 | 98.5 | 0.5 | 338 ± 24 | 568 ± 74 | 24.40 | 75.60 |
| 10 | 89.5 | 0.5 | 125 ± 9 | 296 ± 9 | 13.25 | 86.75 | |
| 6 kDa | 1 | 98.5 | 0.5 | 652 ± 185 | 738 ± 12 | 10.1 | 89.9 |
| 10 | 89.5 | 0.5 | 387 ± 35 | 1102 ± 170 | 8.4 | 91.6 | |
| T-90-m | |||||||
| 1 kDa | 1 | 98.5 | 0.5 | 411 ± 50 | 683 ± 136 | 3.50 | 96.50 |
| 10 | 89.5 | 0.5 | 144 ± 4 | 323 ± 48 | 4.70 | 95.30 | |
| 6 kDa | 1 | 98.5 | 0.5 | 467 ± 45 | 376 ± 27 | 7.9 | 92.1 |
| 10 | 89.5 | 0.5 | 305 ± 97 | 948 ± 122 | 21.4 | 78.6 |
| Sample | Tm, °C (a) | ∆Hm, J/g (b) | DC, % (c) |
|---|---|---|---|
| PEG 6k | 61.6 | 179.0 | 90.9 |
| PEG-m 6k self-crosslinked | 50.6 | 106.7 | 54.2 |
| 1% CB | 49.5 | 41.6 | 21.1 |
| 10% CB | 51.5 | 14.6 | 7.4 |
| 1% CELL | 52.4 | 85.6 | 43.5 |
| 10% CELL | 52.4 | 73.4 | 37.3 |
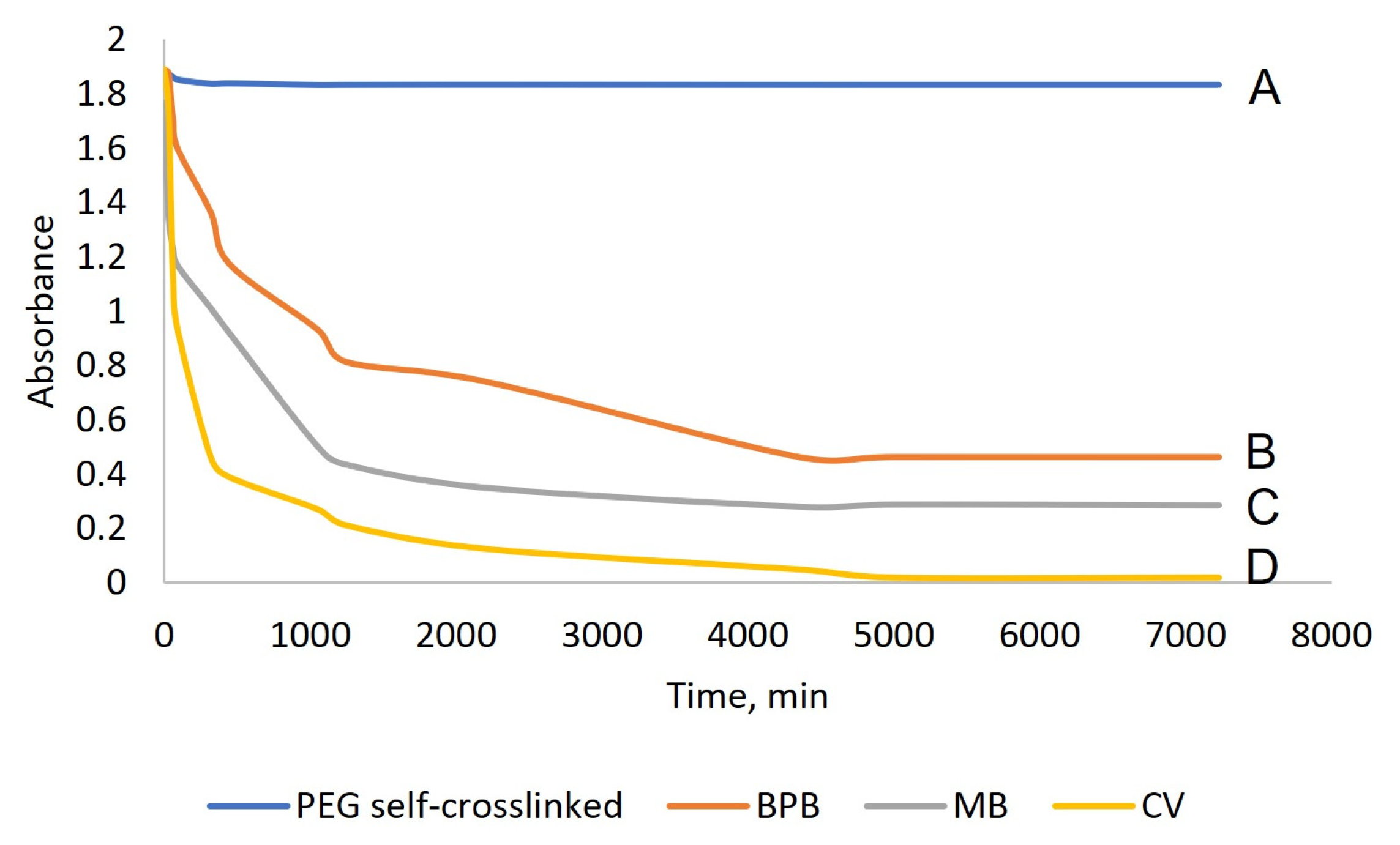
| Dye | Adsorption Capability, mg/g |
|---|---|
| BPB | 5.3 |
| MB | 8.5 |
| CV | ~67.0 |
| Bromophenol Blue (BPB) | Crystal Violet (CV) | Methylene Blue (MB) |
|---|---|---|
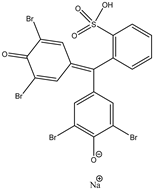 | 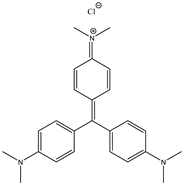 | 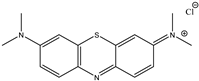 |
 |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getya, D.; Lucas, A.; Gitsov, I. Composite Hydrogels Based on Poly(Ethylene Glycol) and Cellulose Macromonomers as Fortified Materials for Environmental Cleanup and Clean Water Safeguarding. Int. J. Mol. Sci. 2023, 24, 7558. https://doi.org/10.3390/ijms24087558
Getya D, Lucas A, Gitsov I. Composite Hydrogels Based on Poly(Ethylene Glycol) and Cellulose Macromonomers as Fortified Materials for Environmental Cleanup and Clean Water Safeguarding. International Journal of Molecular Sciences. 2023; 24(8):7558. https://doi.org/10.3390/ijms24087558
Chicago/Turabian StyleGetya, Dariya, Alec Lucas, and Ivan Gitsov. 2023. "Composite Hydrogels Based on Poly(Ethylene Glycol) and Cellulose Macromonomers as Fortified Materials for Environmental Cleanup and Clean Water Safeguarding" International Journal of Molecular Sciences 24, no. 8: 7558. https://doi.org/10.3390/ijms24087558
APA StyleGetya, D., Lucas, A., & Gitsov, I. (2023). Composite Hydrogels Based on Poly(Ethylene Glycol) and Cellulose Macromonomers as Fortified Materials for Environmental Cleanup and Clean Water Safeguarding. International Journal of Molecular Sciences, 24(8), 7558. https://doi.org/10.3390/ijms24087558






