50 Hz Magnetic Field Exposure Inhibited Spontaneous Movement of Zebrafish Larvae through ROS-Mediated syn2a Expression
Abstract
1. Introduction
2. Results
2.1. Exposure to a 50-Hz MF Did Not Affect the Basic Development on Zebrafish
2.2. Effects of 50-Hz MF Exposure on Locomotor Behavior
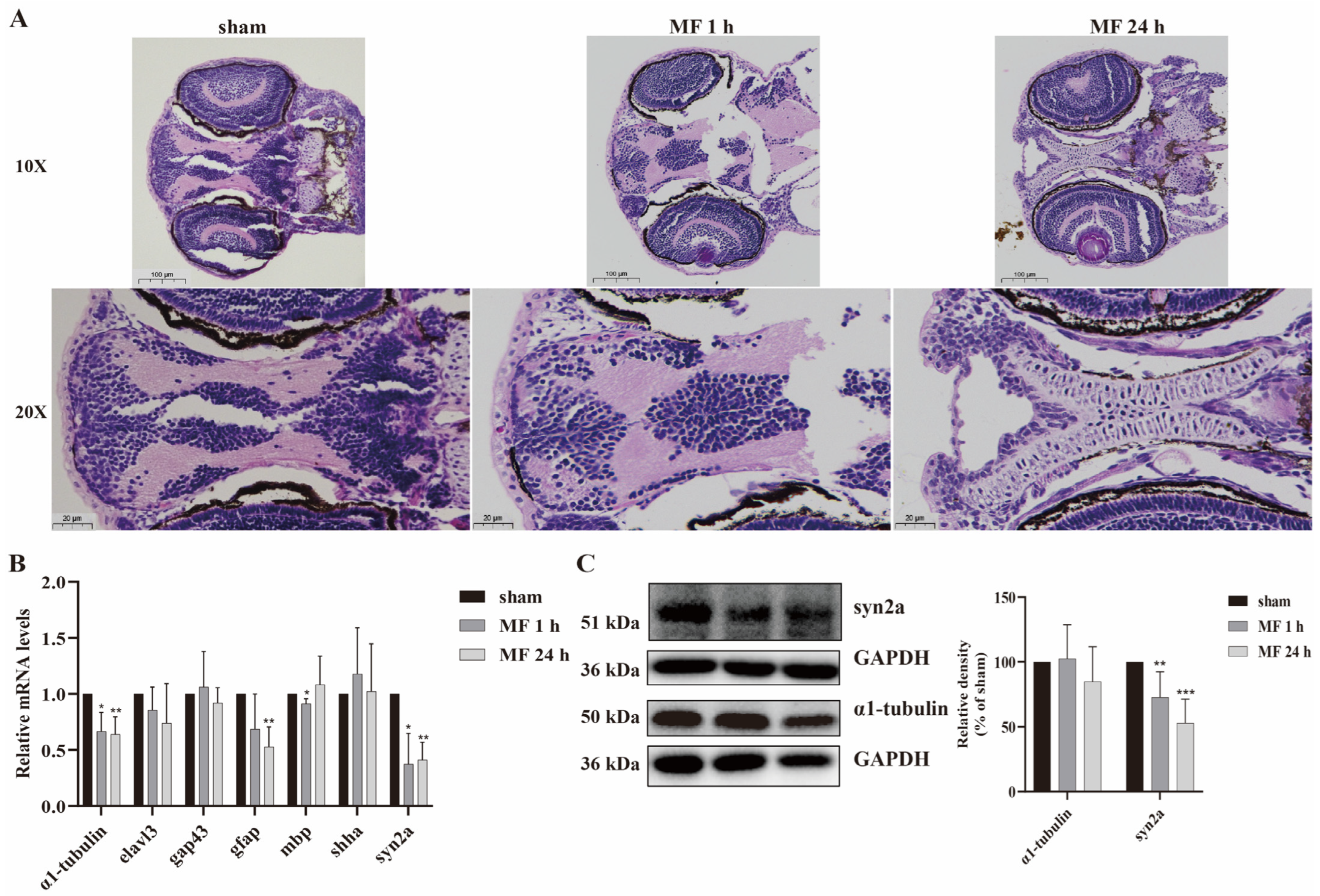
2.3. The Effects of 50-Hz MF Exposure on Brain Tissue Morphology and Neurodevelopment-Related Gene Expression in Zebrafish
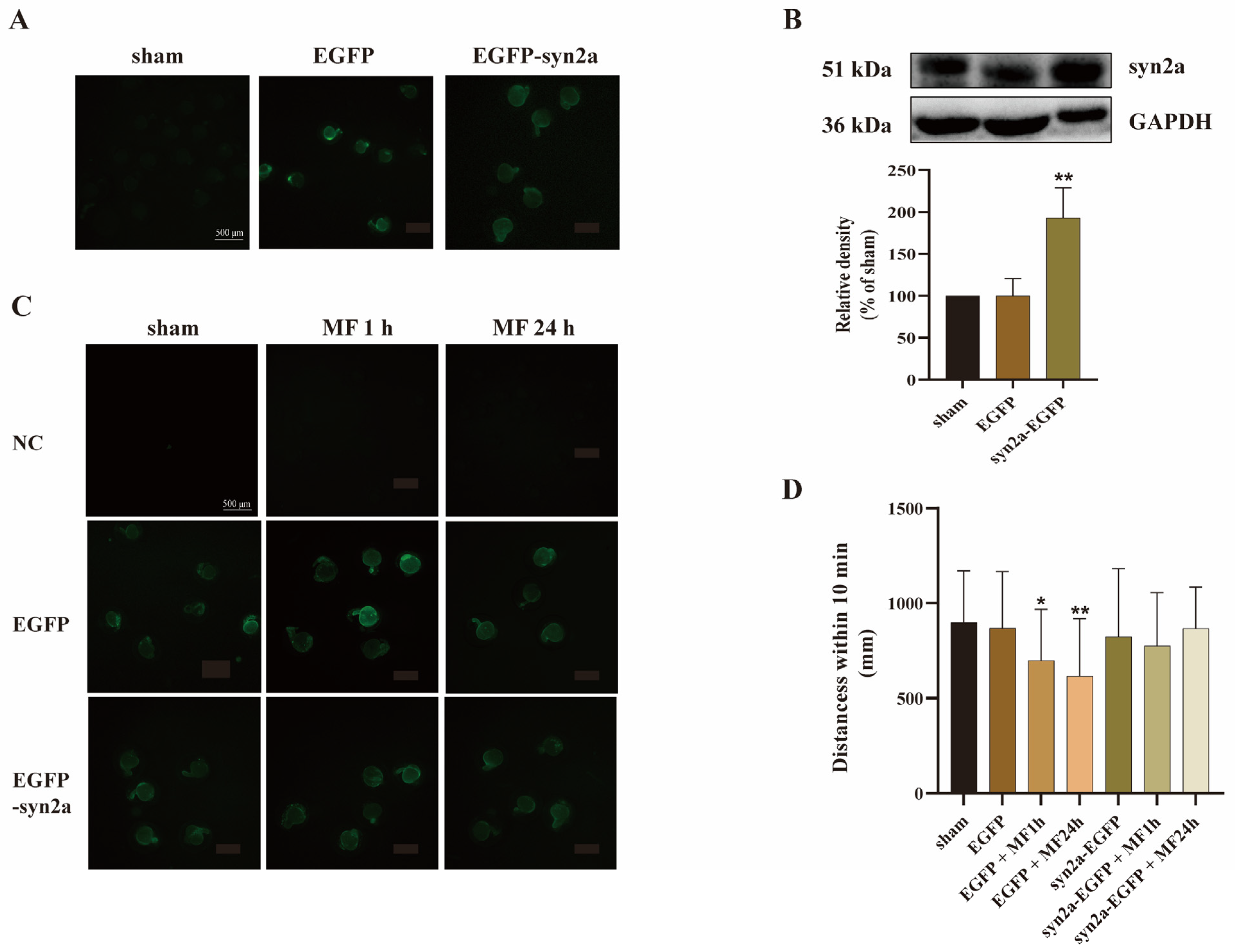
2.4. Overexpression of syn2a Restored MF-Induced SM Hypoactivity in Zebrafish
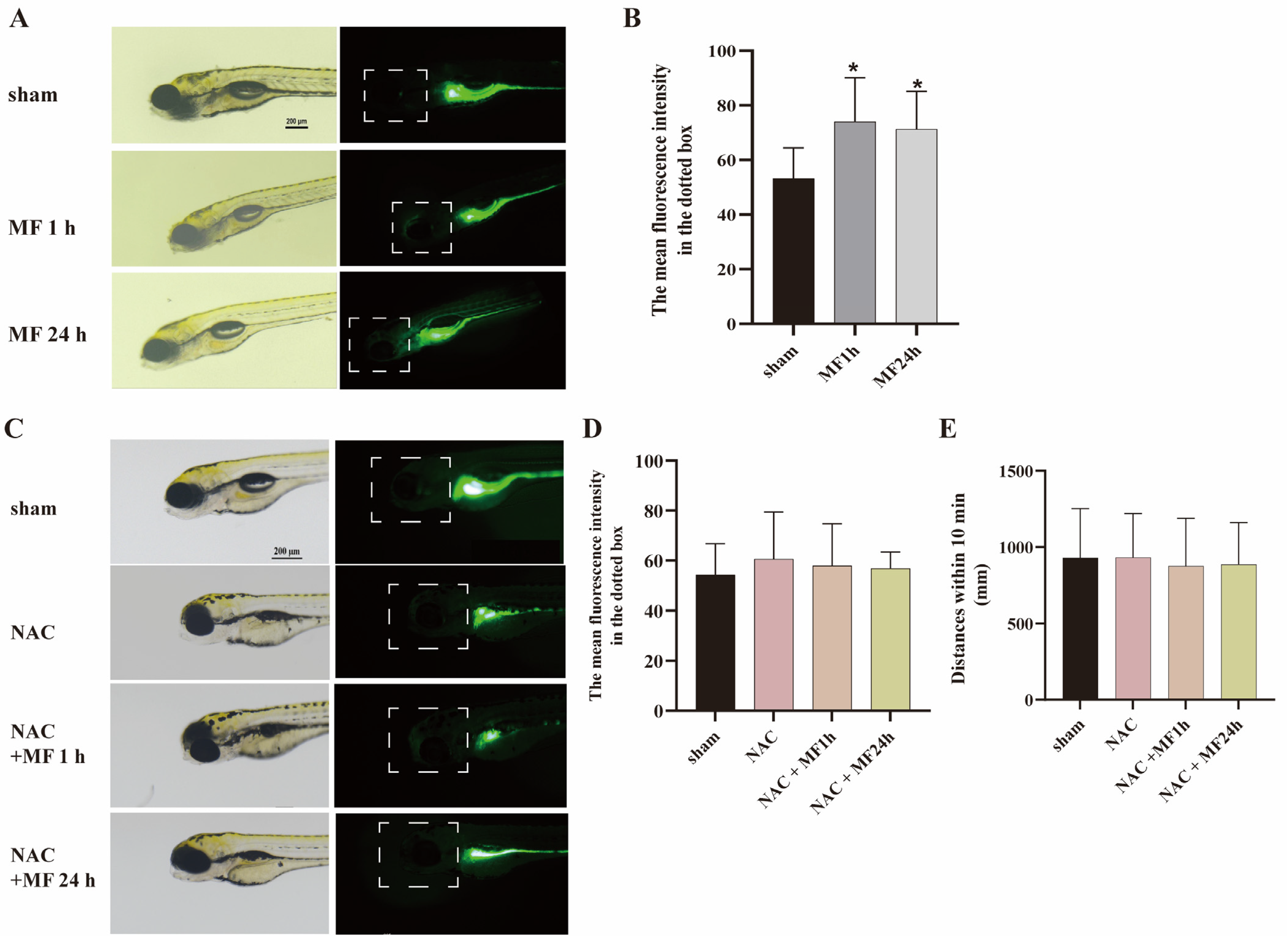
2.5. MF-Induced SM Hypoactivity Depended on ROS
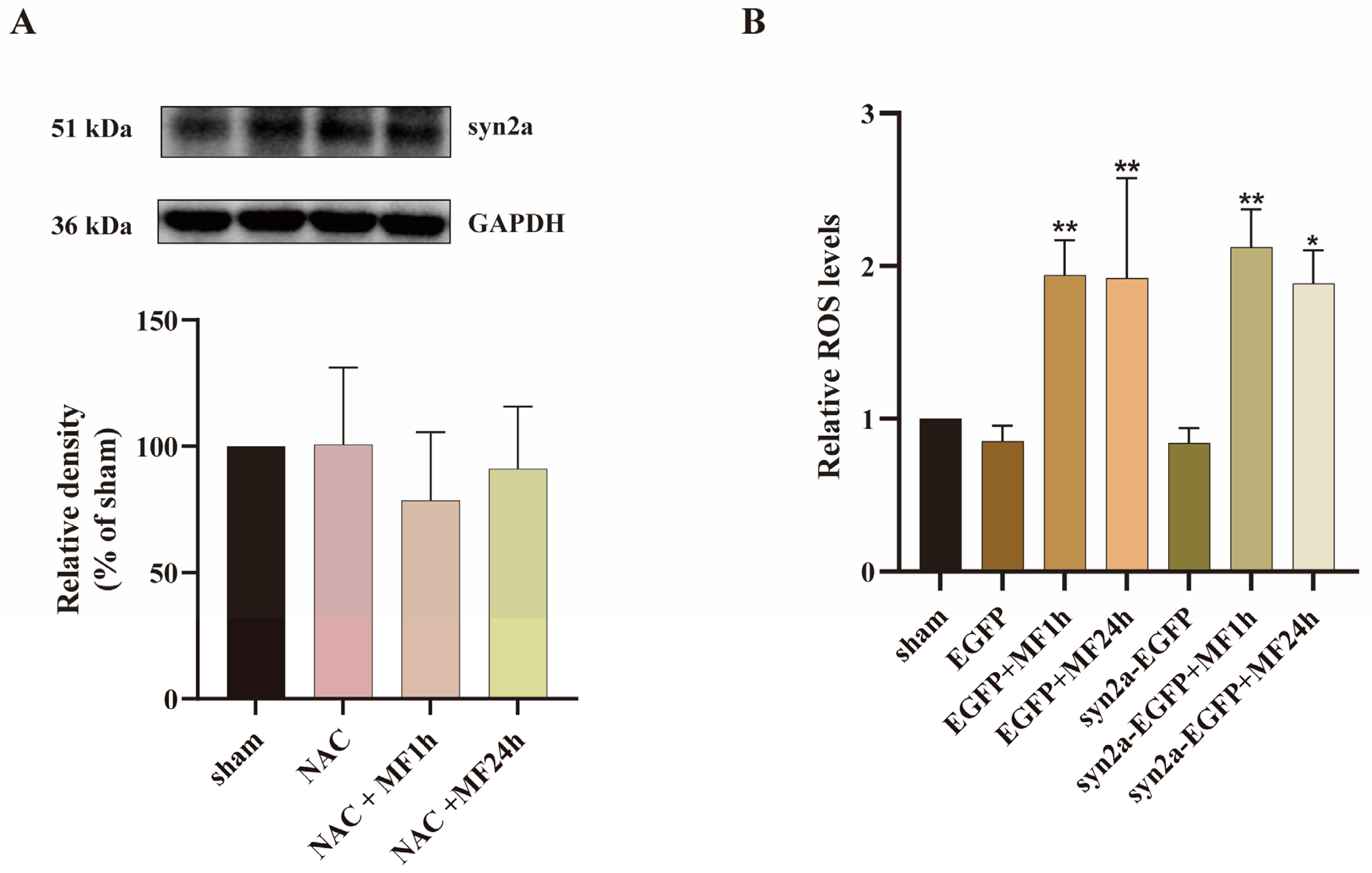
2.6. MF Regulated syn2a Expression via ROS
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Zebrafish Husbandry, Spawning and Egg Collection
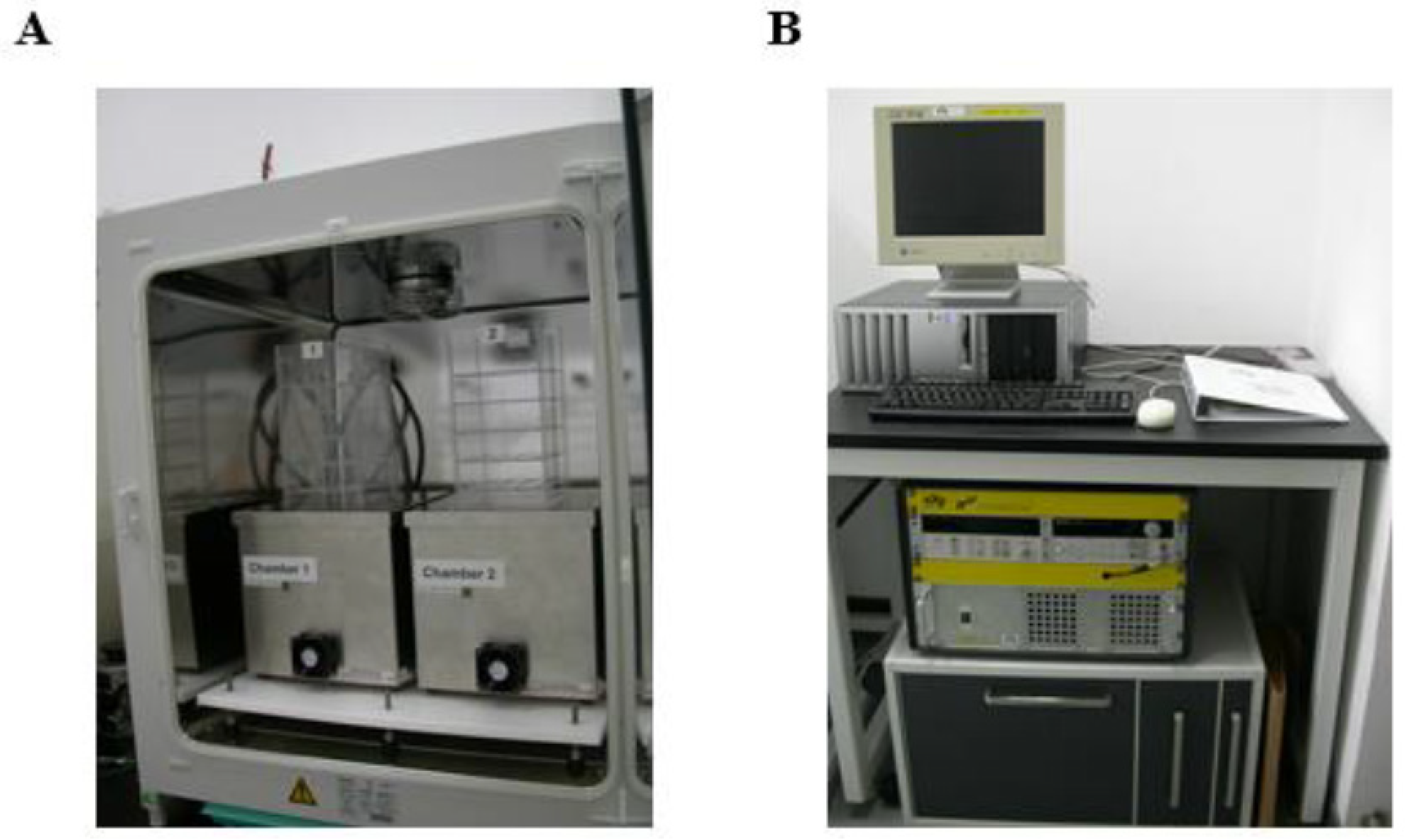
4.3. Magnetic Field Exposure System, Treatment of Zebrafish Embryos, and Observation of the Basic Developmental Parameters
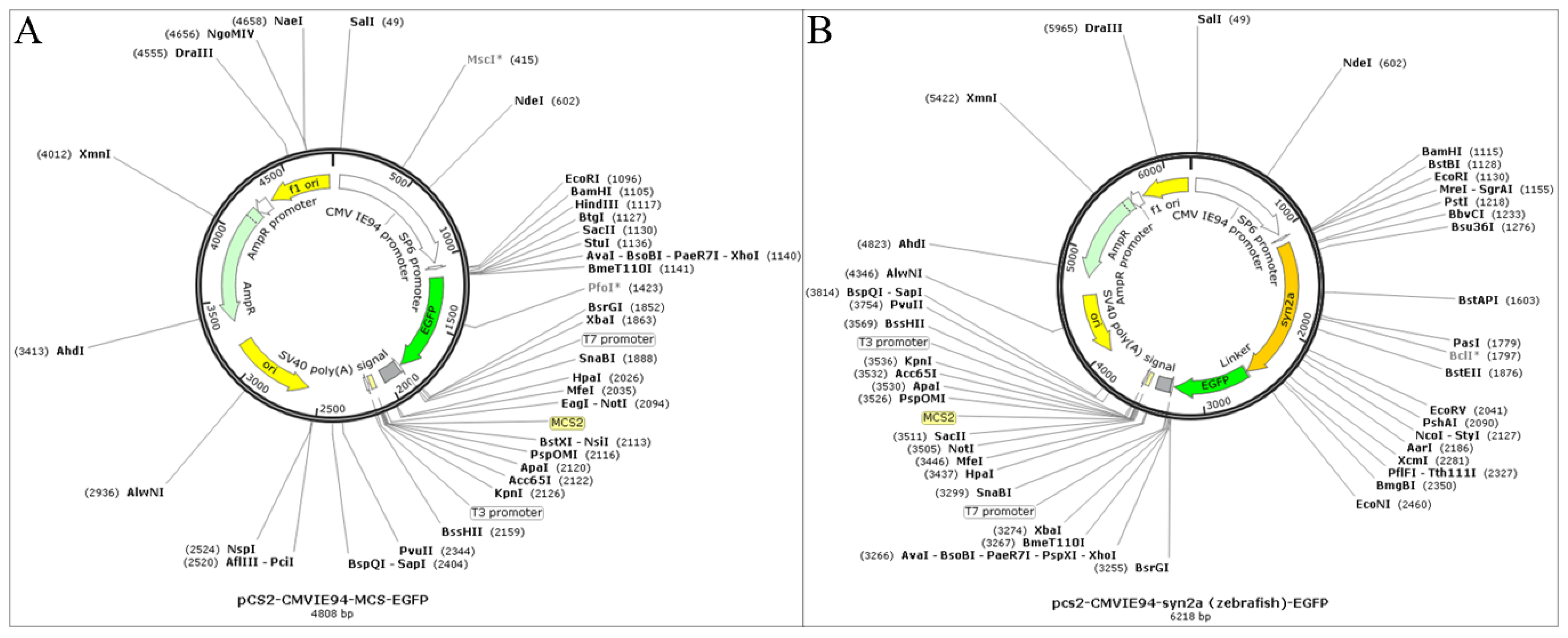
4.4. Overexpressing syn2a in Zebrafish
4.5. Locomotion Analysis
4.6. Histological Examination
4.7. ROS Assay
4.8. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wertheimer, N.; Leeper, E. Electrical Wiring Configurations and Childhood Cancer. Am. J. Epidemiol. 1979, 109, 273–284. [Google Scholar] [CrossRef]
- Ha, M.; Im, H.; Lee, M.; Kim, H.J.; Kim, B.C.; Gimm, Y.M.; Pack, J.K. Radiation Exposure from AM Radio Transmitters and Childhood Leukemia and Brain Cancer. Am. J. Epidemiol. 2007, 166, 270–927. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Sisternas, A.; Hoyos, S.P. Occupational Exposure to Extremely Low Frequency Electric and Magnetic Fields and Alzheimer Disease: A Meta-Analysis. Int. J. Epidemiol. 2008, 37, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Fratiglioni, L.; Karp, A.; Winblad, B.; Bellander, T. Occupational Exposure to Electromagnetic Fields and Risk of Alzheimer’s Disease. Epidemiology 2004, 15, 687–694. [Google Scholar] [CrossRef]
- Sobel, E.; Davanipour, Z.; Sulkava, R.; Erkinjuntti, T.; Wikstrom, J.; Henderson, V.W.; Buckwalter, G.; Bowman, J.D.; Lee, P.J. Occupations with Exposure to Electromagnetic Fields: A Possible Risk Factor for Alzheimer’s Disease. Am. J. Epidemiol. 1995, 142, 515–524. [Google Scholar] [CrossRef]
- Huss, A.; Peters, S.; Vermeulen, R. Occupational Exposure to Extremely Low-Frequency Magnetic Fields and the Risk of ALS: A Systematic Review And Meta-Analysis. Bioelectromagnetics 2018, 39, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Koeman, T.; Slottje, P.; Schouten, L.J.; Peters, S.; Huss, A.; Veldink, J.H.; Kromhout, H.; van den Brandt, P.A.; Vermeulen, R. Occupational Exposure and Amyotrophic Lateral Sclerosis in A Prospective Cohort. Occup. Environ. Med. 2017, 74, 578–585. [Google Scholar] [CrossRef]
- Bagheri Hosseinabadi, M.; Khanjani, N.; Ebrahimi, M.H.; Haji, B.; Abdolahfard, M. The Effect of Chronic Exposure to Extremely Low-Frequency Electromagnetic Fields on Sleep Quality, Stress, Depression and Anxiety. Electromagn. Biol. Med. 2019, 38, 96–101. [Google Scholar] [CrossRef]
- Li, M.; Wu, Q.; Wang, Q.; Xiang, D.; Zhu, G. Effect of Titanium Dioxide Nanoparticles on the Bioavailability and Neurotoxicity of Cypermethrin in Zebrafish Larvae. Aquat. Toxicol. 2018, 199, 212–219. [Google Scholar] [CrossRef]
- Lai, H. Neurological Effects of Static and Extremely-Low Frequency Electromagnetic Fields. Electromagn. Biol. Med. 2022, 41, 201–221. [Google Scholar] [CrossRef]
- Lundberg, L.; Sienkiewicz, Z.; Anthony, D.C.; Broom, K.A. Effects of 50 Hz magnetic fields on circadian rhythm control in mice. Bioelectromagnetics 2019, 40, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Shahani, M.; Zarrindast, M.R.; Semnanian, S.; Rahmati Roudsari, M.; Rezaei Tavirani, M.; Hasanzadeh, H. Effects of 3 Hz and 60 Hz Extremely Low Frequency Electromagnetic Fields on Anxiety-Like Behaviors, Memory Retention of Passive Avoidance and Electrophysiological Properties of Male Rats. J. Lasers Med. Sci. 2016, 7, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, D.; Savić, T.; Anđelković, M.; Prolić, Z.; Janać, B. Extremely Low Frequency Magnetic Field (50 Hz, 0.5 Mt) Modifies Fitness Components and Locomotor Activity of Drosophila subobscura. Int. J. Radiat. Biol. 2014, 90, 337–343. [Google Scholar] [PubMed]
- Mahdavi, S.M.; Sahraei, H.; Yaghmaei, P.; Tavakoli, H. Effects of Electromagnetic Radiation Exposure on Stress-Related Behaviors and Stress Hormones in Male Wistar Rats. Biomol. Ther. 2014, 22, 570–576. [Google Scholar] [CrossRef]
- Das, S.; Kumar, S.; Jain, S.; Avelev, V.D.; Mathur, R. Exposure to ELF-Magnetic Field Promotes Restoration of Sensori-Motor Functions in Adult Rats with Hemisection of Thoracic Spinal Cord. Electromagn. Biol. Med. 2012, 31, 180–194. [Google Scholar] [CrossRef]
- Shin, E.J.; Nguyen, X.K.; Nguyen, T.T.; Pham, D.T.; Kim, H.C. Exposure to Extremely Low Frequency Magnetic Fields Induces Fos-Related Antigen-Immunoreactivity via Activation of Dopaminergic D1 Receptor. Exp. Neurobiol. 2011, 20, 130–136. [Google Scholar] [CrossRef]
- Madjid Ansari, A.; Farzampour, S.; Sadr, A.; Shekarchi, B.; Majidzadeh-A, K. Effects of Short Term and Long Term Extremely Low Frequency Magnetic Field on Depressive Disorder in Mice: Involvement of Nitric Oxide Pathway. Life Sci. 2016, 146, 52–57. [Google Scholar] [CrossRef]
- Zhao, Q.R.; Lu, J.M.; Yao, J.J.; Zhang, Z.Y.; Ling, C.; Mei, Y.A. Neuritin Reverses Deficits in Murine Novel Object Associative Recognition Memory Caused by Exposure to Extremely Low-Frequency (50 Hz) Electromagnetic Fields. Sci. Rep. 2015, 5, 11768. [Google Scholar] [CrossRef]
- Alsaeed, I.; Al-Somali, F.; Sakhnini, L.; Aljarallah, O.S.; Hamdan, R.M.; Bubishate, S.A.; Sarfaraz, Z.K.; Kamal, A. Autism-Relevant Social Abnormalities in Mice Exposed Perinatally to Extremely Low Frequency Electromagnetic Fields. Int. J. Dev. Neurosci. 2014, 37, 58–64. [Google Scholar] [CrossRef]
- Korpinar, M.A.; Kalkan, M.T.; Tuncel, H. The 50 Hz (10 Mt) Sinusoidal Magnetic Field: Effects on Stress-Related Behavior of Rats. Bratisl. Lek. Listy 2012, 113, 521–524. [Google Scholar] [CrossRef]
- He, L.H.; Shi, H.M.; Liu, T.T.; Xu, Y.C.; Ye, K.P.; Wang, S. Effects of Extremely Low Frequency Magnetic Field on Anxiety Level and Spatial Memory of Adult Rats. Chin. Med. J. 2011, 124, 3362–3366. [Google Scholar] [PubMed]
- Fu, Y.; Wang, C.; Wang, J.; Lei, Y.; Ma, Y. Long-Term Exposure to Extremely Low-Frequency Magnetic Fields Impairs Spatial Recognition Memory in Mice. Clin. Exp. Pharm. Physiol. 2008, 35, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, S.; He, L.; Ye, K. Anxiogenic Effect of Chronic Exposure to Extremely Low Frequency Magnetic Field in Adult Rats. Neurosci. Lett. 2008, 434, 12–17. [Google Scholar] [CrossRef]
- Ozgun, A.; Marote, A.; Behie, L.A.; Salgado, A.; Garipcan, B. Extremely Low Frequency Magnetic Field Induces Human Neuronal Differentiation Through NMDA Receptor Activation. J. Neural. Transm. 2019, 126, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Bianco, B.; Cugnoli, C.; Panfoli, I.; Calzia, D.; Morelli, A.; Pepe, I.M. Sinusoidal ELF Magnetic Fields Affect Acetylcholinesterase Activity in Cerebellum Synaptosomal Membranes. Bioelectromagnetics 2010, 31, 270–276. [Google Scholar] [CrossRef]
- Lai, H. Spatial Learning Deficit in the Rat After Exposure to A 60 Hz Magnetic Field. Bioelectromagnetics 1996, 17, 494–496. [Google Scholar] [CrossRef]
- Sieron, A.; Brus, R.; Szkilnik, R.; Plech, A.; Kubański, N.; Cieślar, G. Influence of Alternating Low Frequency Magnetic Fields on Reactivity of Central Dopamine Receptors in Neonatal 6-Hydroxydopamine Treated Rats. Bioelectromagnetics 2001, 22, 479–486. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Lei, Y.; Zhou, D.; Fu, Y.; Che, Y.; Xu, R.; Yu, H.; Hu, X.; Ma, Y. Extremely Low-Frequency Electromagnetic Field Exposure During Chronic Morphine Treatment Strengthens Downregulation of Dopamine D2 Receptors in Rat Dorsal Hippocampus after Morphine Withdrawal. Neurosci. Lett. 2008, 433, 178–182. [Google Scholar] [CrossRef]
- Touitou, Y.; Selmaoui, B.; Lambrozo, J. Assessment of Cortisol Secretory Pattern in Workers Chronically Exposed to ELF-EMF Generated by High Voltage Transmission Lines and Substations. Environ. Int. 2022, 161, 107103. [Google Scholar] [CrossRef]
- Djordjevic, N.Z.; Paunovic, M.G.; Peulic, A.S. Anxiety-Like Behavioural Effects of Extremely Low-Frequency Electromagnetic Field in Rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 21693–21699. [Google Scholar] [CrossRef]
- Kitaoka, K.; Kawata, S.; Yoshida, T.; Kadoriku, F.; Kitamura, M. Exposure to an Extremely-Low-Frequency Magnetic Field Stimulates Adrenal Steroidogenesis via Inhibition of Phosphodiesterase Activity in a Mouse Adrenal Cell Line. PLoS ONE 2016, 11, e0154167. [Google Scholar] [CrossRef]
- Szemerszky, R.; Zelena, D.; Barna, I.; Bárdos, G. Stress-Related Endocrinological and Psychopathological Effects of Short- and Long-Term 50 Hz Electromagnetic Field Exposure in Rats. Brain Res. Bull. 2010, 81, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Eimon, P.M.; Ashkenazi, A. The Zebrafish as A Model Organism for the Study of Apoptosis. Apoptosis 2010, 15, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, C.; Wang, J.; Hu, W.; Cao, Z.; Sun, D.; Xia, H.; Ma, X. Developmental Mechanisms of Arsenite Toxicity in Zebrafish (Danio rerio) Embryos. Aquat. Toxicol. 2009, 91, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- Brustein, E.; Saint-Amant, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Drapeau, P. Steps During the Development of the Zebrafish Locomotor Network. J. Physiol.-Paris 2003, 97, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X. Magnetic Fields and Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 2175. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to time-varying electric, magnetic, and electromagnetic fields (up to 300 GHz). Health Phys. 1998, 74, 494–522. [Google Scholar]
- Blackman, C.F.; Benane, S.G.; Elder, J.A.; House, D.E.; Lampe, J.A.; Faulk, J.M. Induction of Calcium-Ion Efflux from Brain Tissue by Radiofrequency Radiation: Effect of Sample Number and Modulation Frequency on the Power-Density Window. Bioelectromagnetics 1980, 1, 35–43. [Google Scholar] [CrossRef]
- Teglas, T.; Dörnyei, G.; Bretz, K.; Nyakas, C. Whole-Bodye Pulsed EMF Stimulation Improves Cognitive and Psychomotor Activity in Senescent Rats. Behav. Brain Res. 2018, 349, 163–168. [Google Scholar] [CrossRef]
- Agrawal, N.; Verma, K.; Baghel, D.; Chauhan, A.; Prasad, D.N.; Sharma, S.K.; Kohli, E. Effects of Extremely Low-Frequency Electromagnetic Field on Different Developmental Stages of Drosophila melanogaster. Int. J. Radiat. Biol. 2021, 97, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Su, M.; Ba, H.; Chen, G.; Luo, J.; Liu, F.; Liao, X.; Cao, Z.; Zeng, J.; Lu, H.; et al. ZIF-8 Nanoparticles Induce Neurobehavioral Disorders Through the Regulation of ROS-Mediated Oxidative Stress in Zebrafish Embryos. Chemosphere 2022, 305, 135453. [Google Scholar] [CrossRef] [PubMed]
- Pullaguri, N.; Grover, P.; Abhishek, S.; Rajakumara, E.; Bhargava, Y.; Bhargava, A. Triclosan Affects Motor Function in Zebrafish Larva by Inhibiting Ache and Syn2a Genes. Chemosphere 2021, 266, 128930. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, Z.; Liu, Q.; Chen, L. Adverse Effects of Bisphenol B Exposure on the Thyroid and Nervous System in Early Life Stages of Zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 250, 109167. [Google Scholar] [CrossRef]
- Xia, M.; Wang, X.; Xu, J.; Qian, Q.; Gao, M.; Wang, H. Tris (1-Chloro-2-Propyl) Phosphate Exposure to Zebrafish Causes Neurodevelopmental Toxicity and Abnormal Locomotor Behavior. Sci. Total Environ. 2021, 758, 143694. [Google Scholar] [CrossRef]
- Cheng, B.; Jiang, F.; Su, M.; Zhou, L.; Zhang, H.; Cao, Z.; Liao, X.; Xiong, G.; Xiao, J.; Liu, F.; et al. Effects of Lincomycin Hydrochloride on the Neurotoxicity of Zebrafish. Ecotoxicol. Environ. Saf. 2020, 201, 110725. [Google Scholar] [CrossRef]
- Fu, J.; Guo, Y.; Yang, L.; Han, J.; Zhou, B. Nano-TiO2 Enhanced Bioaccumulation and Developmental Neurotoxicity of Bisphenol A in Zebrafish Larvae. Environ. Res. 2020, 187, 109682. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, L.; Meng, Z.; Su, M.; Zhang, S.; Huang, P.; Jiang, F.; Liao, X.; Cao, Z.; Lu, H. Clethodim Exposure Induced Development Toxicity and Behaviour Alteration in Early Stages of Zebrafish Life. Environ. Pollut. 2019, 255, 113218. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, M.; Shi, F.; Yang, L.; Guo, Y.; Feng, C.; Liu, J.; Zhou, B. Developmental Neurotoxicity of Triphenyl Phosphate in Zebrafish Larvae. Aquat. Toxicol. 2018, 203, 80–87. [Google Scholar] [CrossRef]
- Song, S.H.; Augustine, G.J. Synapsin Isoforms and Synaptic Vesicle Trafficking. Mol. Cells 2015, 38, 936–940. [Google Scholar]
- Cesca, F.; Baldelli, P.; Valtorta, F.; Benfenati, F. The Synapsins: Key Actors of Synapse Function and Plasticity. Prog. Neurobiol. 2010, 91, 313–348. [Google Scholar] [CrossRef]
- Gitler, D.; Cheng, Q.; Greengard, P.; Augustine, G.J. Synapsin IIa Controls the Reserve Pool of Glutamatergic Synaptic Vesicles. J. Neurosci. 2008, 28, 10835–10843. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Sahraei, H.; Aliyari, H.; Tekieh, E.; Saberi, M.; Tavacoli, H.; Meftahi, G.H.; Ghanaati, H.; Salehi, M.; Hajnasrollah, M. Effects of the Extremely Low Frequency Electromagnetic Fields on NMDA-Receptor Gene Expression and Visual Working Memory in Male Rhesus Macaques. Basic Clin. Neurosci. 2018, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Salunke, B.P.; Umathe, S.N.; Chavan, J.G. Involvement of NMDA Receptor in Low-Frequency Magnetic Field-Induced Anxiety in Mice. Electromagn. Biol. Med. 2014, 33, 312–326. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Z.; Zhang, H.; He, Y.; Fan, R.; Cheng, Y.; Sun, G.; Sun, X. Extremely Low Frequency Electromagnetic Field Exposure Causes Cognitive Impairment Associated with Alteration of the Glutamate Level, MAPK Pathway Activation and Decreased CREB Phosphorylation in Mice Hippocampus: Reversal by Procyanidins Extracted from the Lotus Seedpod. Food Funct. 2014, 5, 2289–2297. [Google Scholar] [PubMed]
- Sako, W.; Izumi, Y.; Abe, T.; Haji, S.; Murakami, N.; Osaki, Y.; Matsumoto, Y.; Harada, M.; Kaji, R. MR Spectroscopy and Imaging-Derived Measurements in the Supplementary Motor Area for Biomarkers of Amyotrophic Lateral Sclerosis. Neurol. Sci. 2021, 42, 4257–4263. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.; Skatchkov, S.N.; Szabó, Z.; Qahtan, S.; Méndez-González, M.P.; Malpica-Nieves, C.J.; Eaton, M.J.; Kardos, J.; Héja, L. Putrescine Intensifies Glu/GABA Exchange Mechanism and Promotes Early Termination of Seizures. Int. J. Mol. Sci. 2022, 23, 8191. [Google Scholar] [CrossRef]
- Yang, K.C.; Chen, Y.Y.; Liu, M.N.; Yang, B.H.; Chou, Y.H. Interactions Between Dopamine Transporter and N-Methyl-D-Aspartate Receptor-Related Amino Acids on Cognitive Impairments in Schizophrenia. Schizophr. Res. 2022, 248, 263–270. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, G.; Chen, C.; Yu, Y.; Xu, Z. Association Between Extremely Low-Frequency Electromagnetic Fields Occupations and Amyotrophic Lateral Sclerosis: A Meta-Analysis. PLoS ONE 2012, 7, e48354. [Google Scholar] [CrossRef]
- Ye, A.F.; Liu, X.C.; Chen, L.J.; Xia, Y.P.; Yang, X.B.; Sun, W.J. Endogenous Ca2+ Release Was Involved in 50-Hz MF-Induced Proliferation via Akt-SK1 Signal Cascade in Human Amniotic Epithelial Cells. Electromagn. Biol. Med. 2022, 41, 142–151. [Google Scholar] [CrossRef]
- Yang, X.; Ye, A.; Chen, L.; Xia, Y.; Jiang, W.; Sun, W. Involvement of Calcium In 50-Hz Magnetic Field-Induced Activation of Sphingosine Kinase 1 Signaling Pathway. Bioelectromagnetics 2019, 40, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.L.; Bill, C.A.; Simsek-Duran, F.; Lonart, G.; Samigullin, D.; Bykhovskaia, M. Synapsin II and Calcium Regulate Vesicle Docking and the Cross-Talk Between Vesicle Pools at the Mouse Motor Terminals. J. Physiol. 2008, 586, 4649–4673. [Google Scholar] [CrossRef]
- Masoudian, N.; Riazi, G.H.; Afrasiabi, A.; Modaresi, S.M.; Dadras, A.; Rafiei, S.; Yazdankhah, M.; Lyaghi, A.; Jarah, M.; Ahmadian, S.; et al. Variations of Glutamate Concentration within Synaptic Cleft in the Presence of Electromagnetic Fields: An Artificial Neural Networks Study. Neurochem. Res. 2015, 40, 629–642. [Google Scholar] [CrossRef]
- Bouchez, C.; Devin, A. Mitochondrial Biogenesis and Mitochondrial Reactive Oxygen Species (ROS): A Complex Relationship Regulated by the cAMP/PKA Signaling Pathway. Cells 2019, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Giachello, C.N.; Fiumara, F.; Giacomini, C.; Corradi, A.; Milanese, C.; Ghirardi, M.; Benfenati, F.; Montarolo, P.G. MAPK/Erk-Dependent Phosphorylation of Synapsin Mediates Formation of Functional Synapses and Short-Term Homosynaptic Plasticity. J. Cell Sci. 2010, 123, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, M.; Hammer, R.E.; Sudhof, T.C. A Phospho-Switch Controls the Dynamic Association of Synapsins with Synaptic Vesicles. Neuron 1999, 24, 377–387. [Google Scholar] [CrossRef]
- He, Y.L.; Liu, D.D.; Fang, Y.J.; Zhan, X.Q.; Yao, J.J.; Mei, Y.A. Exposure to Extremely Low-Frequency Electromagnetic Fields Modulates Na+ Currents in Rat Cerebellar Granule Cells through Increase Of AA/PGE2 and EP Receptor-Mediated Camp/PKA Pathway. PLoS ONE 2013, 8, e54376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, Y.; Cheng, Y.; He, Y.; Manyakara, Z.; Duan, Y.; Sun, G.; Sun, X. Influence of Extremely Low Frequency Magnetic Fields on Ca2+ Signaling and Double Messenger System in Mice Hippocampus and Reversal Function of Procyanidins Extracted from Lotus Seedpod. Bioelectromagnetics 2017, 38, 436–446. [Google Scholar] [CrossRef]
- Li, J.; Wu, G.; Song, W.; Liu, Y.; Han, Z.; Shen, Z.; Li, Y. Prophylactic Melatonin Treatment Ameliorated Propofol-Induced Cognitive Dysfunction in Aged Rats. Neurotox. Res. 2021, 39, 227–239. [Google Scholar] [CrossRef]
- Acevedo, J.; Santana-Almansa, A.; Matos-Vergara, N.; Marrero-Cordero, L.R.; Cabezas-Bou, E.; Díaz-Ríos, M. Caffeine Stimulates Locomotor Activity in the Mammalian Spinal Cord via Adenosine A1 Receptor-Dopamine D1 Receptor Interaction and PKA-Dependent Mechanisms. Neuropharmacology 2016, 101, 490–505. [Google Scholar] [CrossRef]
- Foster, S.L.; Lustberg, D.J.; Harbin, N.H.; Bramlett, S.N.; Hepler, J.R.; Weinshenker, D. RGS14 Modulates Locomotor Behavior and ERK Signaling Induced by Environmental Novelty and Cocaine within Discrete Limbic Structures. Psychopharmacology 2021, 238, 2755–2773. [Google Scholar] [CrossRef]
- Wang, J.Q.; Mao, L. The ERK Pathway: Molecular Mechanisms and Treatment of Depression. Mol. Neurobiol. 2019, 56, 6197–6205. [Google Scholar] [CrossRef]
- Hutton, S.R.; Otis, J.M.; Kim, E.M.; Lamsal, Y.; Stuber, G.D.; Snider, W.D. ERK/MAPK Signaling Is Required for Pathway-Specific Striatal Motor Functions. J. Neurosci. 2017, 37, 8102–8115. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ma, J.; Yang, Y.; Shi, W.; Luo, L. Epcam is an Endoderm-Specific Wnt Derepressor that Licenses Hepatic Development. Dev. Cell 2013, 24, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Schuderer, J.; Oesch, W.; Felber, N.; Spät, D.; Kuster, N. In vitro Exposure Apparatus for ELF Magnetic Fields. Bioelectromagnetics 2004, 25, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Ma, Y.; Luo, Y.; Cao, Z.; Lu, H. Protective Effects of Isorhamnetin on N2a Cell against Endoplasmic Reticulum Stress-Induced Injury is Mediated by Pkcepsilon. Biomed. Pharmacother. 2017, 93, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

| Developmental Index | 100 μT | 200 μT | 400 μT | 800 μT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | MF1h | MF24h | Sham | MF1h | MF24h | Sham | MF1h | MF24h | Sham | MF1h | MF24h | |
| Hatching rate (72 hpf) | 95.40 ±2.17 | 96.17 ±1.36 | 92.90 ±1.57 | 84.64 ±4.12 | 89.52 ±4.28 | 94.11 ±2.91 | 95.99 ±2.07 | 94.71 ±2.24 | 94.38 ±2.41 | 94.50 ±2.01 | 93.30 ±1.59 | 92.80 ±1.47 |
| Mortality (120 hpf) | 9.72 ±0.83 | 8.82 ±2.36 | 7.10 ±1.57 | 4.84 ±3.30 | 5.99 ±2.30 | 8.72 ±2.30 | 7.63 ±1.76 | 9.63 ±0.74 | 11.01 ±1.19 | 7.60 ±3.10 | 6.50 ±0.95 | 9.44 ±1.25 |
| Malformation rate (120 hpf) | 5.57 ±0.74 | 5.39 ±0.33 | 6.67 ±0.53 | 6.43 ±1.50 | 5.89 ±2.10 | 7.41 ±1.70 | 6.51 ±0.73 | 5.88 ±1.53 | 5.04 ±1.02 | 5.78 ±0.94 | 6.33 ±0.23 | 6.82 ±0.91 |
| Index | 100 μT | 200 μT | 400 μT | 800 μT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | MF1h | MF24h | Sham | MF1h | MF24h | Sham | MF1h | MF24h | Sham | MF1h | MF24h | |
| Distances (mm) | 1410.68 ±202.78 | 1417.06 ±274.08 | 1330.37 ±252.76 | 1471.30 ±163.95 | 1152.28 ±182.56 * | 1132.64 ±195.43 * | 1566.67 ±160.14 | 1512.73 ±33.98 | 1661.28 ±213.11 | 1237.34 ±275.94 | 1339.10 ±371.74 | 1437.65 ±169.00 |
| Average speed (mm/s) | 2.35 ±0.34 | 2.36 ±0.46 | 2.22 ±0.42 | 2.45 ±0.23 | 1.96 ±0.37 * | 2.01 ±0.22 * | 2.61 ±0.26 | 2.52 ±0.06 | 2.77 ±0.36 | 2.06 ±0.46 | 2.23 ±0.62 | 2.40 ±0.28 |
| Gene Name | Primer Sequence (5′-3′) | |
|---|---|---|
| Forward | Reverse | |
| elavl3 | GTCAGAAAGACATGGAGCAGTTG | GAACCGAATGAAACCTACCCC |
| gap43 | TGCTGCATCAGAAGAACTAA | CCTCCGGTTTGATTCCATC |
| gfap | GGATGCAGCCAATCGTAAT | TTCCAGGTCACAGGTCAG |
| mbp | AATCAGCAGGTTCTTCGGAGGAGA | AAGAAATGCACGACAGGGTTGACG |
| shha | AGACCGAGACTCCACGACGC | TGCAGTCACTGGTGCGAACG |
| syn2a | GTACCATGCCAGCATTTC | TGGTTCTCCACTTTCACCTT |
| α1-tubulin | AATCACCAATGCTTGCTTCGAGCC | TTCACGTCTTTGGGTACCACGTCA |
| β-actin | ATGGATGAGGAAATCGCTGCC | CTCCCTGATGTCTGGGTCGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Fu, Y.; Sun, W. 50 Hz Magnetic Field Exposure Inhibited Spontaneous Movement of Zebrafish Larvae through ROS-Mediated syn2a Expression. Int. J. Mol. Sci. 2023, 24, 7576. https://doi.org/10.3390/ijms24087576
Guo Y, Fu Y, Sun W. 50 Hz Magnetic Field Exposure Inhibited Spontaneous Movement of Zebrafish Larvae through ROS-Mediated syn2a Expression. International Journal of Molecular Sciences. 2023; 24(8):7576. https://doi.org/10.3390/ijms24087576
Chicago/Turabian StyleGuo, Yixin, Yiti Fu, and Wenjun Sun. 2023. "50 Hz Magnetic Field Exposure Inhibited Spontaneous Movement of Zebrafish Larvae through ROS-Mediated syn2a Expression" International Journal of Molecular Sciences 24, no. 8: 7576. https://doi.org/10.3390/ijms24087576
APA StyleGuo, Y., Fu, Y., & Sun, W. (2023). 50 Hz Magnetic Field Exposure Inhibited Spontaneous Movement of Zebrafish Larvae through ROS-Mediated syn2a Expression. International Journal of Molecular Sciences, 24(8), 7576. https://doi.org/10.3390/ijms24087576




