A Decellularized Uterine Endometrial Scaffold Enhances Regeneration of the Endometrium in Rats
Abstract
:1. Introduction
2. Results
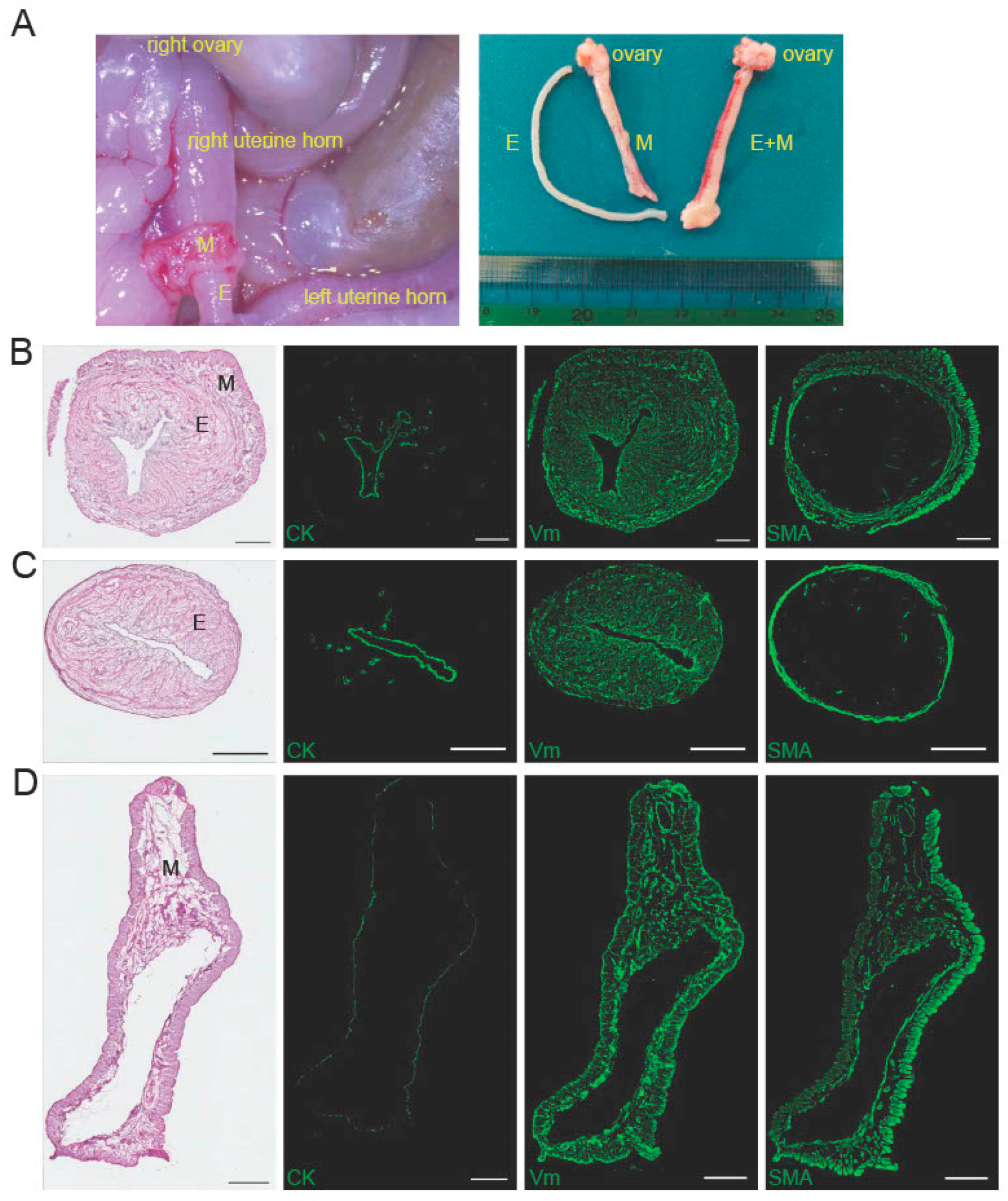
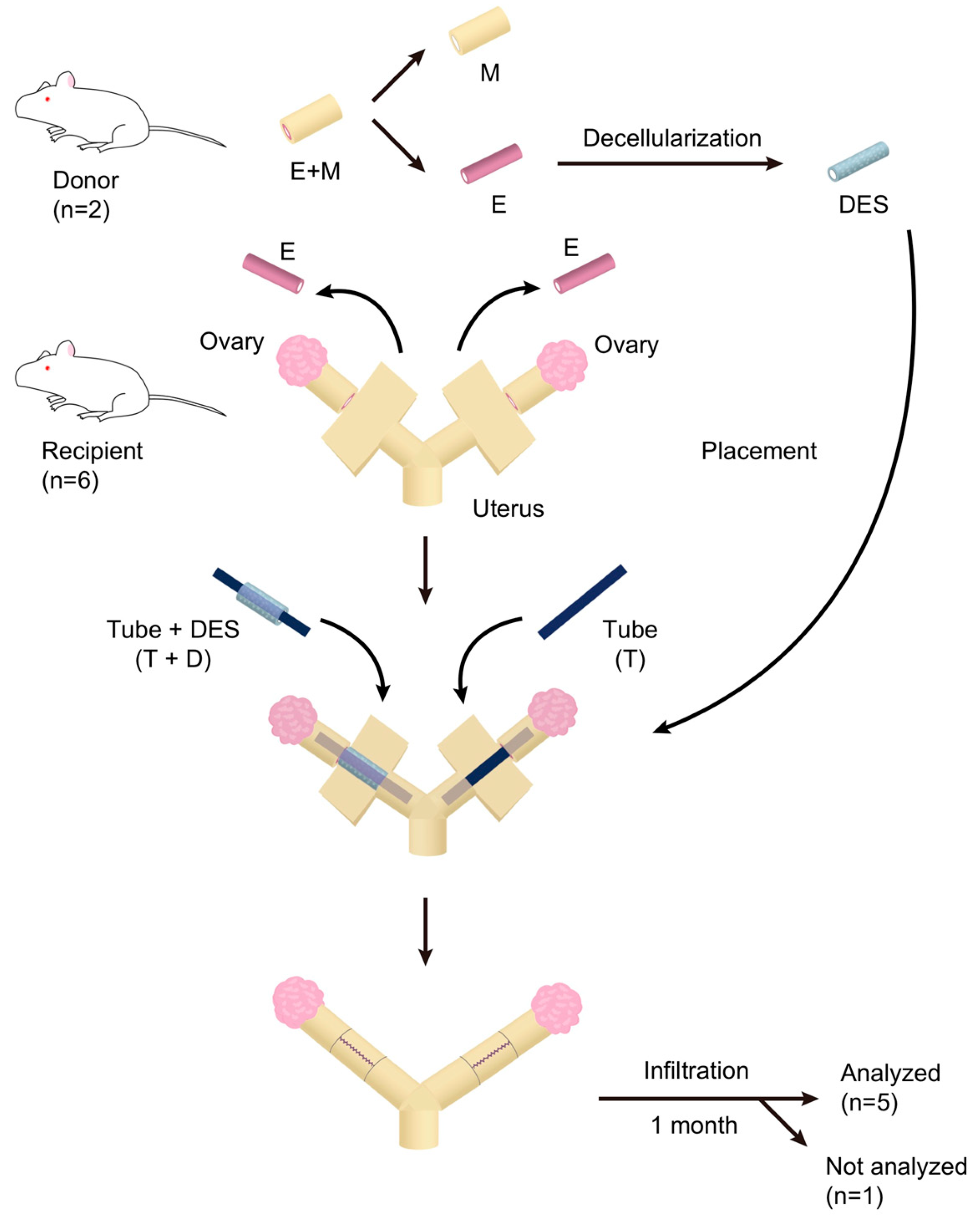
2.1. Characteristics of the Peeled-Off Endometrium
2.2. Preparation and Characterization of a Decellularized Endometrial Scaffold (DES)
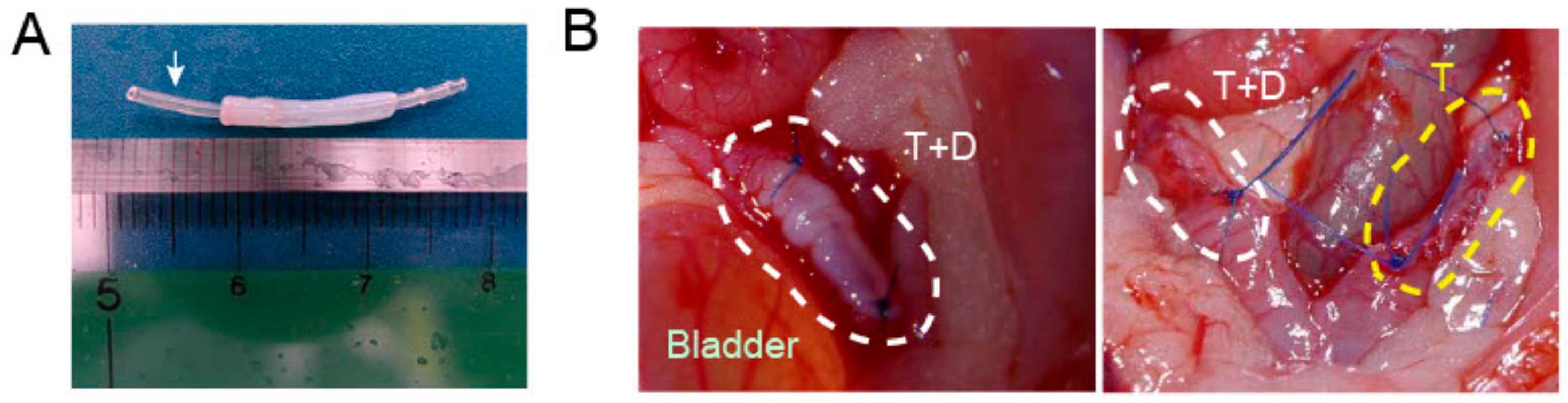
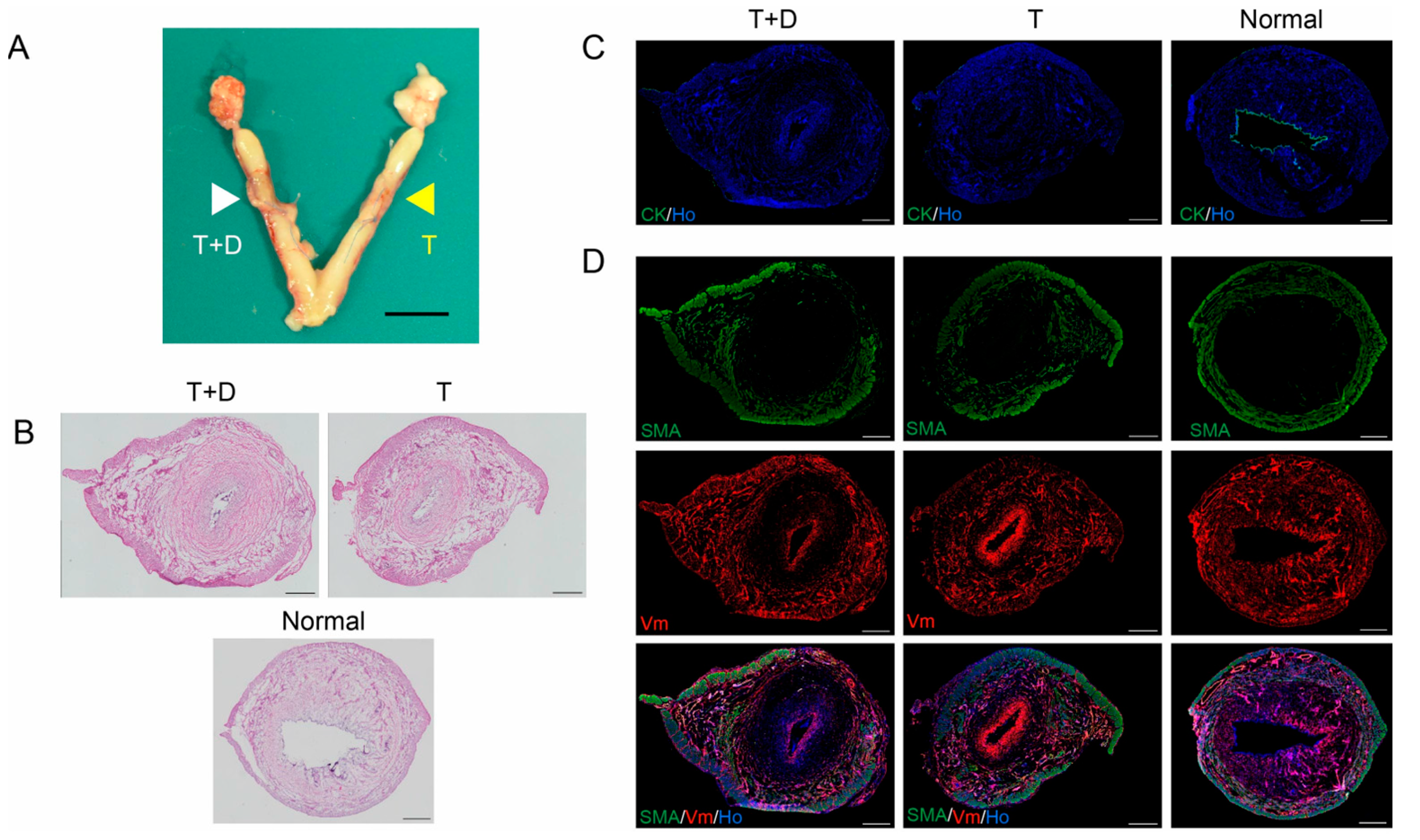
2.3. Transplantation of DES with and without a Silicone Tube
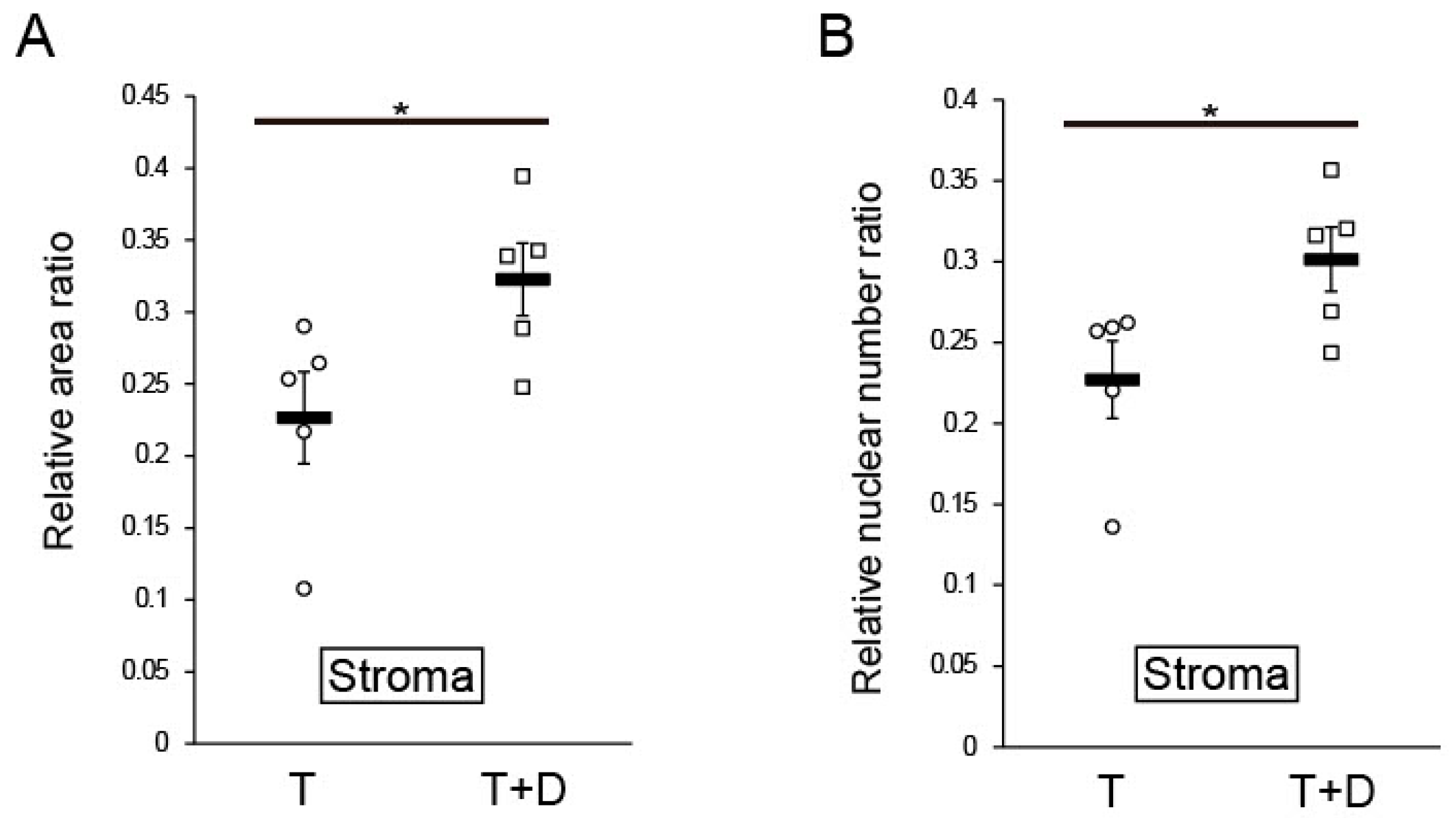
2.4. Histological and Immunofluorescent Analyses
3. Discussion
4. Materials and Methods
4.1. Collection of Rat Uterine Endometrium
4.2. Decellularization of the Uterine Endometrium
4.3. DES Transplantation
4.4. Histological and Immunofluorescent Analyses
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milliez, J. Uterine transplantation FIGO Committee for the Ethical Aspects of Human Reproduction and Women’s Health. Int. J. Gynaecol. Obstet. 2009, 106, 270. [Google Scholar] [CrossRef] [PubMed]
- Yoshimasa, Y.; Maruyama, T. Bioengineering of the Uterus. Reprod. Sci. 2021, 28, 1596–1611. [Google Scholar] [CrossRef]
- Hellstrom, M.; Moreno-Moya, J.M.; Bandstein, S.; Bom, E.; Akouri, R.R.; Miyazaki, K.; Maruyama, T.; Brannstrom, M. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil. Steril. 2016, 106, 487–496.e481. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, L.; Richards, E.; Reddy, V.; Walter, J.; Olthoff, K.; Quintini, C.; Tzakis, A.; Latif, N.; Porrett, P.; O’Neill, K.; et al. The First 5 Years of Uterus Transplant in the US: A Report From the United States Uterus Transplant Consortium. JAMA Surg. 2022, 157, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wu, B.; Li, Y.; Liu, Y.; Shi, L.; Gong, L.; Xia, Y.; Heng, B.C.; Wu, H.; Ouyang, H.; et al. Local Delivery of Silk-Cellulose Incorporated with Stromal Cell-Derived Factor-1α Functionally Improves the Uterus Repair. Tissue Eng. Part A 2019, 25, 1514–1526. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Park, K.H.; Kim, Y.J.; Kim, M.S.; Liu, H.C.; Rosenwaks, Z.; Ku, S.Y. Synergistic regenerative effects of functionalized endometrial stromal cells with hyaluronic acid hydrogel in a murine model of uterine damage. Acta Biomater. 2019, 89, 139–151. [Google Scholar] [CrossRef]
- Magalhaes, R.S.; Williams, J.K.; Yoo, K.W.; Yoo, J.J.; Atala, A. A tissue-engineered uterus supports live births in rabbits. Nat. Biotechnol. 2020, 38, 1280–1287. [Google Scholar] [CrossRef]
- Kuramoto, G.; Shimizu, T.; Takagi, S.; Ishitani, K.; Matsui, H.; Okano, T. Endometrial regeneration using cell sheet transplantation techniques in rats facilitates successful fertilization and pregnancy. Fertil. Steril. 2018, 110, 172–181.e174. [Google Scholar] [CrossRef]
- Miyazaki, K.; Maruyama, T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 2014, 35, 8791–8800. [Google Scholar] [CrossRef]
- Young, R.C.; Goloman, G. Allo- and xeno-reassembly of human and rat myometrium from cells and scaffolds. Tissue Eng. Part A 2013, 19, 2112–2119. [Google Scholar] [CrossRef]
- Miki, F.; Maruyama, T.; Miyazaki, K.; Takao, T.; Yoshimasa, Y.; Katakura, S.; Hihara, H.; Uchida, S.; Masuda, H.; Uchida, H.; et al. The orientation of a decellularized uterine scaffold determines the tissue topology and architecture of the regenerated uterus in ratsdagger. Biol. Reprod. 2019, 100, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, M.; El-Akouri, R.R.; Sihlbom, C.; Olsson, B.M.; Lengqvist, J.; Backdahl, H.; Johansson, B.R.; Olausson, M.; Sumitran-Holgersson, S.; Brannstrom, M. Towards the development of a bioengineered uterus: Comparison of different protocols for rat uterus decellularization. Acta Biomater. 2014, 10, 5034–5042. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, T.T.; Padma, A.M.; Sehic, E.; Bäckdahl, H.; Oltean, M.; Song, M.J.; Brännström, M.; Hellström, M. Towards uterus tissue engineering: A comparative study of sheep uterus decellularisation. Mol. Hum. Reprod. 2020, 26, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Campo, H.; Baptista, P.M.; Lopez-Perez, N.; Faus, A.; Cervello, I.; Simon, C. De- and recellularization of the pig uterus: A bioengineering pilot study. Biol. Reprod. 2017, 96, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Campo, H.; Garcia-Dominguez, X.; Lopez-Martinez, S.; Faus, A.; Vicente Anton, J.S.; Marco-Jimenez, F.; Cervello, I. Tissue-specific decellularized endometrial substratum mimicking different physiological conditions influences in vitro embryo development in a rabbit model. Acta Biomater. 2019, 89, 126–138. [Google Scholar] [CrossRef]
- Daryabari, S.S.; Kajbafzadeh, A.M.; Fendereski, K.; Ghorbani, F.; Dehnavi, M.; Rostami, M.; Garajegayeh, B.A.; Tavangar, S.M. Development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. J. Assist. Reprod. Genet. 2019, 36, 1211–1223. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, Y.W.; Lin, H.L.; Lan, Q.H.; Huang, Z.W.; Wang, L.F.; Chen, R.; Xiao, J.; Kou, L.; Xu, H.L.; et al. Exploiting crosslinked decellularized matrix to achieve uterus regeneration and construction. Artif. Cells Nanomed. Biotechnol. 2020, 48, 218–229. [Google Scholar] [CrossRef]
- Guo, E.J.; Chung, J.P.W.; Poon, L.C.Y.; Li, T.C. Reproductive outcomes after surgical treatment of asherman syndrome: A systematic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 59, 98–114. [Google Scholar] [CrossRef]
- Li, X.; Lv, H.F.; Zhao, R.; Ying, M.F.; Samuriwo, A.T.; Zhao, Y.Z. Recent developments in bio-scaffold materials as delivery strategies for therapeutics for endometrium regeneration. Mater. Today Bio 2021, 11, 100101. [Google Scholar] [CrossRef]
- Keyhanvar, N.; Zarghami, N.; Bleisinger, N.; Hajipour, H.; Fattahi, A.; Nouri, M.; Dittrich, R. Cell-based endometrial regeneration: Current status and future perspectives. Cell Tissue Res. 2021, 384, 241–254. [Google Scholar] [CrossRef]
- Zhang, X.R.; Chien, P.N.; Trinh, X.T.; Nam, S.Y.; Heo, C.Y. Comparison of Formation of Capsule Among Different Breast Silicone Implants. Vivo 2022, 36, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Wick, G.; Backovic, A.; Rabensteiner, E.; Plank, N.; Schwentner, C.; Sgonc, R. The immunology of fibrosis: Innate and adaptive responses. Trends Immunol. 2010, 31, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Liebgott, B. Mesometrial smooth muscle in the mouse: Its control of uterine blood flows. Anat. Rec. 1984, 208, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Haase, E.B.; Buchman, J.; Tietz, A.E.; Schramm, L.P. Pregnancy-induced uterine neuronal degeneration in the rat. Cell Tissue Res. 1997, 288, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Santoso, E.G.; Yoshida, K.; Hirota, Y.; Aizawa, M.; Yoshino, O.; Kishida, A.; Osuga, Y.; Saito, S.; Ushida, T.; Furukawa, K.S. Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PLoS ONE 2014, 9, e103201. [Google Scholar] [CrossRef]
- Hiraoka, T.; Hirota, Y.; Saito-Fujita, T.; Matsuo, M.; Egashira, M.; Matsumoto, L.; Haraguchi, H.; Dey, S.K.; Furukawa, K.S.; Fujii, T.; et al. STAT3 accelerates uterine epithelial regeneration in a mouse model of decellularized uterine matrix transplantation. JCI Insight 2016, 1, e87591. [Google Scholar] [CrossRef]
- Li, X.A.; Sun, H.; Lin, N.; Hou, X.; Wang, J.; Zhou, B.; Xu, P.; Xiao, Z.; Chen, B.; Dai, J.; et al. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials 2011, 32, 8172–8181. [Google Scholar] [CrossRef]
- Lin, N.; Li, X.; Song, T.; Wang, J.; Meng, K.; Yang, J.; Hou, X.; Dai, J.; Hu, Y. The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials 2012, 33, 1801–1807. [Google Scholar] [CrossRef]
- Ding, L.; Li, X.; Sun, H.; Su, J.; Lin, N.; Peault, B.; Song, T.; Yang, J.; Dai, J.; Hu, Y. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials 2014, 35, 4888–4900. [Google Scholar] [CrossRef]
- Song, T.; Zhao, X.; Sun, H.; Li, X.A.; Lin, N.; Ding, L.; Dai, J.; Hu, Y. Regeneration of Uterine Horns in Rats Using Collagen Scaffolds Loaded with Human Embryonic Stem Cell-Derived Endometrium-Like Cells. Tissue Eng. Part A 2015, 21, 353–361. [Google Scholar] [CrossRef]
- Xue, B.; Liu, D.; Song, M.; Zhao, G.; Cao, Y.; Yan, G.; Dai, J.; Hu, Y. Leukemia inhibitory factor promotes the regeneration of rat uterine horns with full-thickness injury. Wound Repair Regen. 2019, 27, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yan, G.; Diao, Q.; Yu, F.; Li, X.A.; Sheng, X.; Liu, Y.; Dai, Y.; Zhou, H.; Zhen, X.; et al. Transplantation of human endometrial perivascular cells with elevated CYR61 expression induces angiogenesis and promotes repair of a full-thickness uterine injury in rat. Stem Cell. Res. Ther. 2019, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Wang, H. Uterine disorders and pregnancy complications: Insights from mouse models. J. Clin. Investig. 2010, 120, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Lin, X.; Pan, Y.; Zheng, X.; Shi, L.; Zhang, Y.; Ma, L.; Gao, C.; Zhang, S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019, 92, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Xia, W.T.; Xu, J.; Xu, H.L.; Lu, C.T.; Zhao, Y.Z.; Wu, X.Q. Three-dimensional structure micelles of heparin-poloxamer improve the therapeutic effect of 17beta-estradiol on endometrial regeneration for intrauterine adhesions in a rat model. Int. J. Nanomed. 2017, 12, 5643–5657. [Google Scholar] [CrossRef]
- Xu, H.L.; Xu, J.; Zhang, S.S.; Zhu, Q.Y.; Jin, B.H.; ZhuGe, D.L.; Shen, B.X.; Wu, X.Q.; Xiao, J.; Zhao, Y.Z. Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Deliv. 2017, 24, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L.; Xu, J.; Shen, B.-X.; Zhang, S.-S.; Jin, B.-H.; Zhu, Q.-Y.; Zhuge, D.-L.; Wu, X.-Q.; Xiao, J.; Zhao, Y.-Z. Dual Regulations of Thermosensitive Heparin–Poloxamer Hydrogel Using ε-Polylysine: Bioadhesivity and Controlled KGF Release for Enhancing Wound Healing of Endometrial Injury. ACS Appl. Mater. Interfaces 2017, 9, 29580–29594. [Google Scholar] [CrossRef]
- Yang, H.; Wu, S.; Feng, R.; Huang, J.; Liu, L.; Liu, F.; Chen, Y. Vitamin C plus hydrogel facilitates bone marrow stromal cell-mediated endometrium regeneration in rats. Stem Cell. Res. Ther. 2017, 8, 267. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, F.; Yu, Y.; Liu, Y.; Shao, C.; Gu, H.; Li, M.; Zhao, Y. Porous scaffolds from droplet microfluidics for prevention of intrauterine adhesion. Acta Biomater. 2019, 84, 222–230. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, W.; Lei, D.; Huang, J.; Yin, Y.; Zhu, Y.; You, Z.; Wang, F.; Sun, S. PGS Scaffolds Promote the In Vivo Survival and Directional Differentiation of Bone Marrow Mesenchymal Stem Cells Restoring the Morphology and Function of Wounded Rat Uterus. Adv. Health Mater. 2019, 8, 1801455. [Google Scholar] [CrossRef]
- Kuramoto, G.; Takagi, S.; Ishitani, K.; Shimizu, T.; Okano, T.; Matsui, H. Preventive effect of oral mucosal epithelial cell sheets on intrauterine adhesions. Hum. Reprod. 2015, 30, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lu, J.; Li, B.; Chen, S.; Xiao, X.; Wang, J.; Wang, J.; Wang, X. Partial regeneration of uterine horns in rats through adipose-derived stem cell sheets. Biol. Reprod. 2018, 99, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Y. Preventive effects of transplantation of oral mucosal epithelial cells seeded on a decellularized amniotic membrane in a model of intrauterine adhesion. Int. J. Clin. Exp. Pathol. 2018, 11, 1510–1519. [Google Scholar] [PubMed]
- Chen, X.; Sun, J.; Li, X.; Mao, L.; Zhou, Y.; Cui, L.; Bai, W. Antifibrotic Effects of Decellularized and Lyophilized Human Amniotic Membrane Transplant on the Formation of Intrauterine Adhesion. Exp. Clin. Transplant. 2019, 17, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, J.; Li, X.; Mao, L.; Cui, L.; Bai, W. Transplantation of oral mucosal epithelial cells seeded on decellularized and lyophilized amniotic membrane for the regeneration of injured endometrium. Stem Cell. Res. Ther. 2019, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zheng, Y.W.; Lan, Q.H.; Wang, L.F.; Huang, Z.W.; Chen, R.; Yang, Y.; Xu, H.L.; Kou, L.; Zhao, Y.Z. Aloe/poloxamer hydrogel as an injectable beta-estradiol delivery scaffold with multi-therapeutic effects to promote endometrial regeneration for intrauterine adhesion treatment. Eur. J. Pharm. Sci. 2020, 148, 105316. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, S.; Yang, H.; Li, Z.; Huang, K.; Su, T.; Wang, S.; Cheng, K. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv. Health Mater. 2019, 8, e1900411. [Google Scholar] [CrossRef]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef]
- Frances-Herrero, E.; Lopez, R.; Hellstrom, M.; De Miguel-Gomez, L.; Herraiz, S.; Brannstrom, M.; Pellicer, A.; Cervello, I. Bioengineering trends in female reproduction: A systematic review. Hum. Reprod. Update 2022, 28, 798–837. [Google Scholar] [CrossRef]
- Azumaguchi, A.; Henmi, H.; Saito, T. Efficacy of silicone sheet as a personalized barrier for preventing adhesion reformation after hysteroscopic adhesiolysis of intrauterine adhesions. Reprod. Med. Biol. 2019, 18, 378–383. [Google Scholar] [CrossRef]
- Goto, Y.; Noda, Y.; Narimoto, K.; Umaoka, Y.; Tokura, T.; Mori, T. Pregnancy achieved by transferring blastocysts into endometrial stroma in mice. Hum. Reprod. 1992, 7, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, H.C.; Schust, D.J.; Spencer, T.E. In vitro models of the human endometrium: Evolution and application for women’s health. Biol. Reprod. 2021, 104, 282–293. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimasa, Y.; Takao, T.; Katakura, S.; Tomisato, S.; Masuda, H.; Tanaka, M.; Maruyama, T. A Decellularized Uterine Endometrial Scaffold Enhances Regeneration of the Endometrium in Rats. Int. J. Mol. Sci. 2023, 24, 7605. https://doi.org/10.3390/ijms24087605
Yoshimasa Y, Takao T, Katakura S, Tomisato S, Masuda H, Tanaka M, Maruyama T. A Decellularized Uterine Endometrial Scaffold Enhances Regeneration of the Endometrium in Rats. International Journal of Molecular Sciences. 2023; 24(8):7605. https://doi.org/10.3390/ijms24087605
Chicago/Turabian StyleYoshimasa, Yushi, Tomoka Takao, Satomi Katakura, Shoko Tomisato, Hirotaka Masuda, Mamoru Tanaka, and Tetsuo Maruyama. 2023. "A Decellularized Uterine Endometrial Scaffold Enhances Regeneration of the Endometrium in Rats" International Journal of Molecular Sciences 24, no. 8: 7605. https://doi.org/10.3390/ijms24087605




