Influence of Simulated Microgravity on Mammary Epithelial Cells Grown as 2D and 3D Cultures
Abstract
:1. Introduction
2. Results
2.1. Growth under Simulated Microgravity Causes a Reduction in Cell Diameter
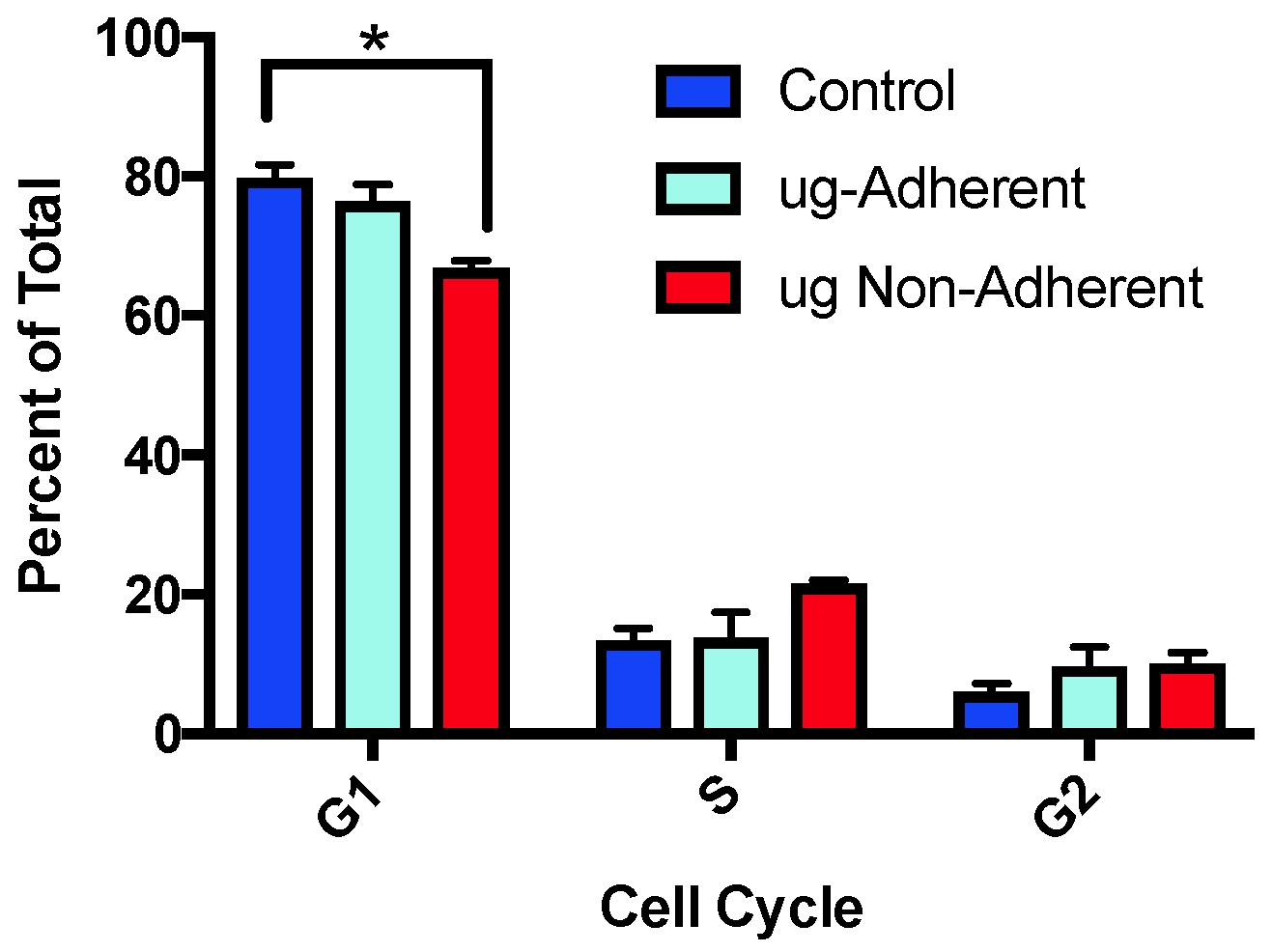
2.2. Cell Cycle Profiles Are Altered after Simulated Microgravity Treatment
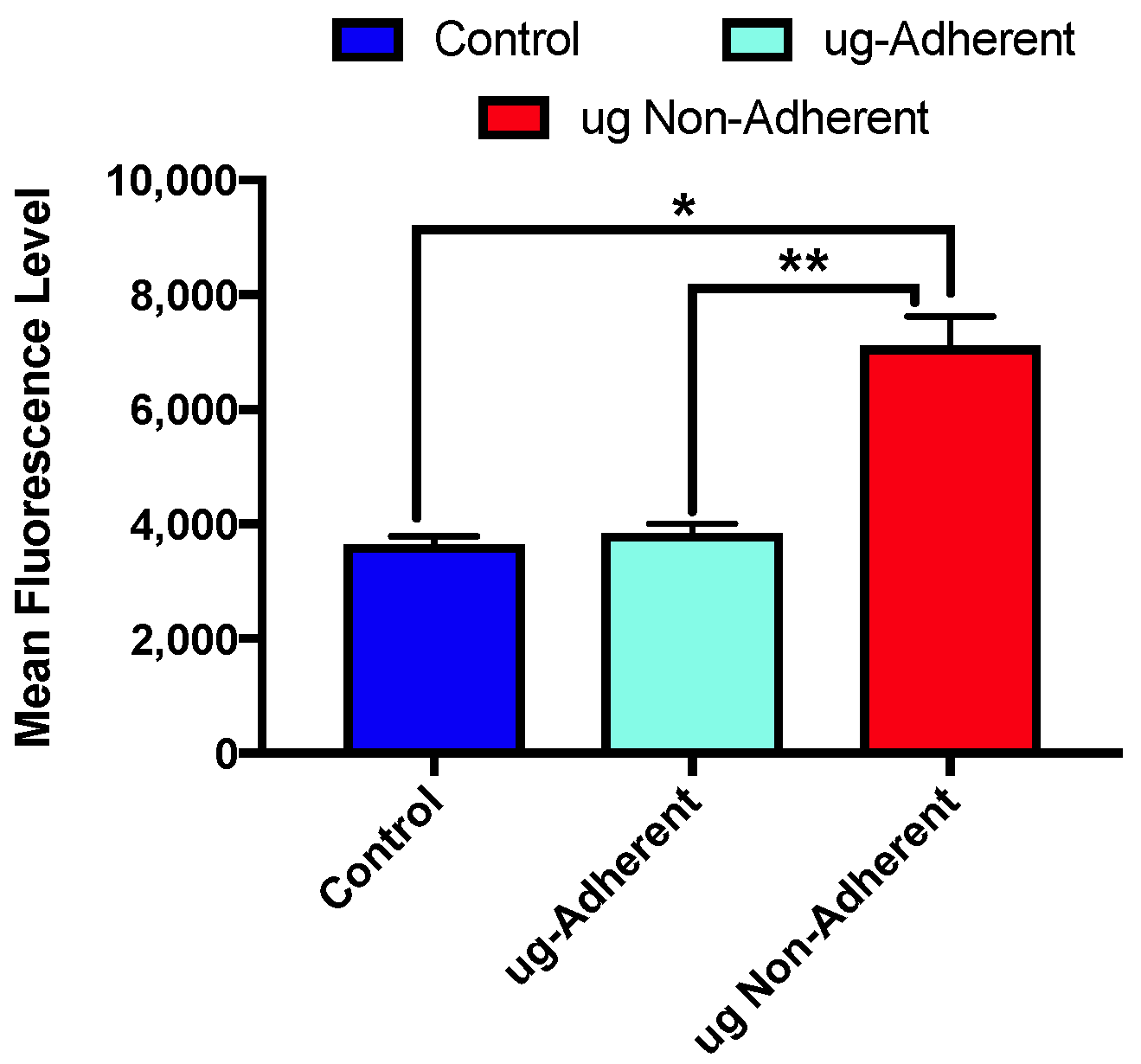
2.3. Levels of γH2AX Differ with Sim µg Exposure
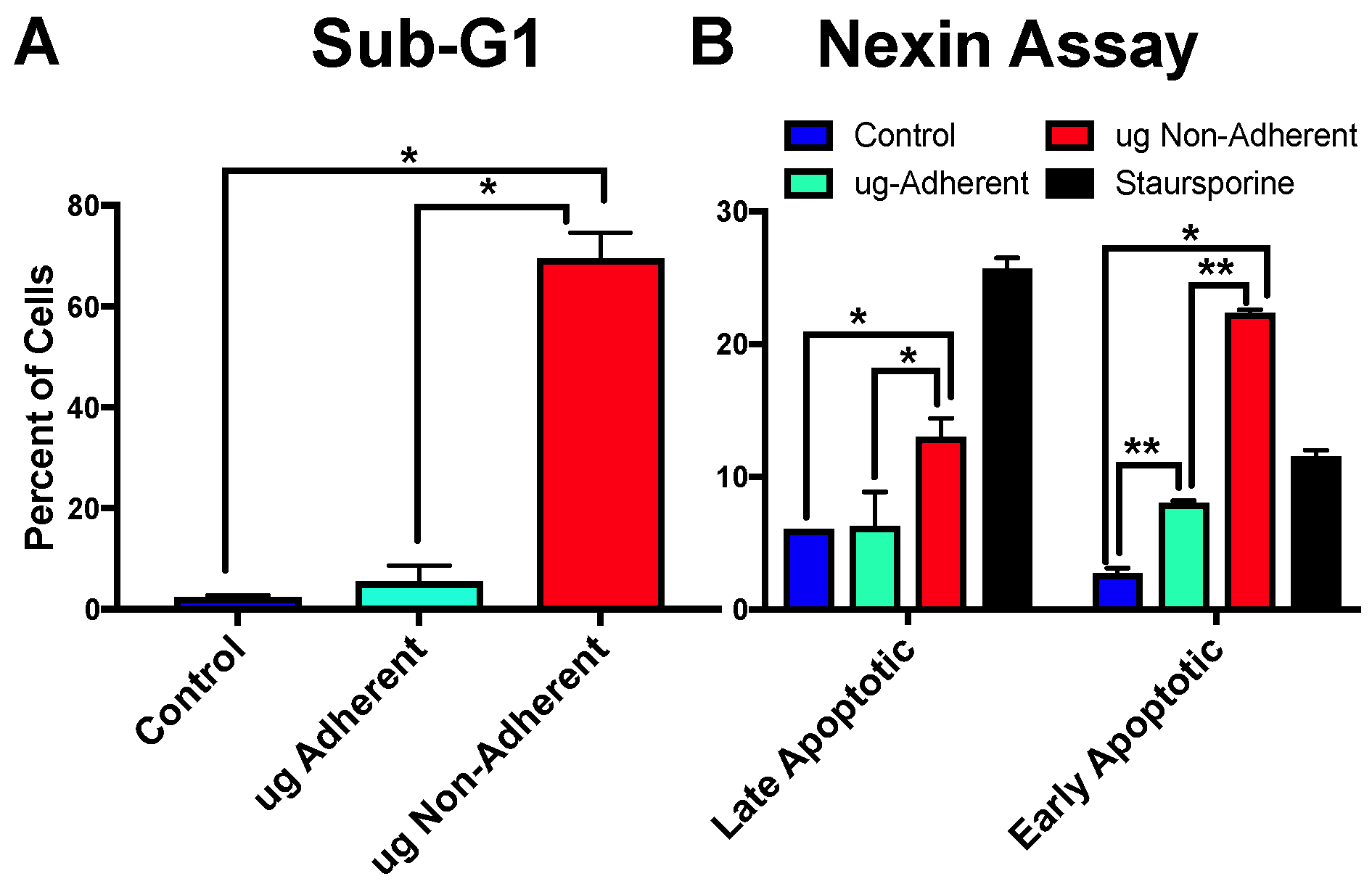
2.4. Simulated Microgravity Causes an Increase in Apoptosis Specifically in Non-Adherent Cells
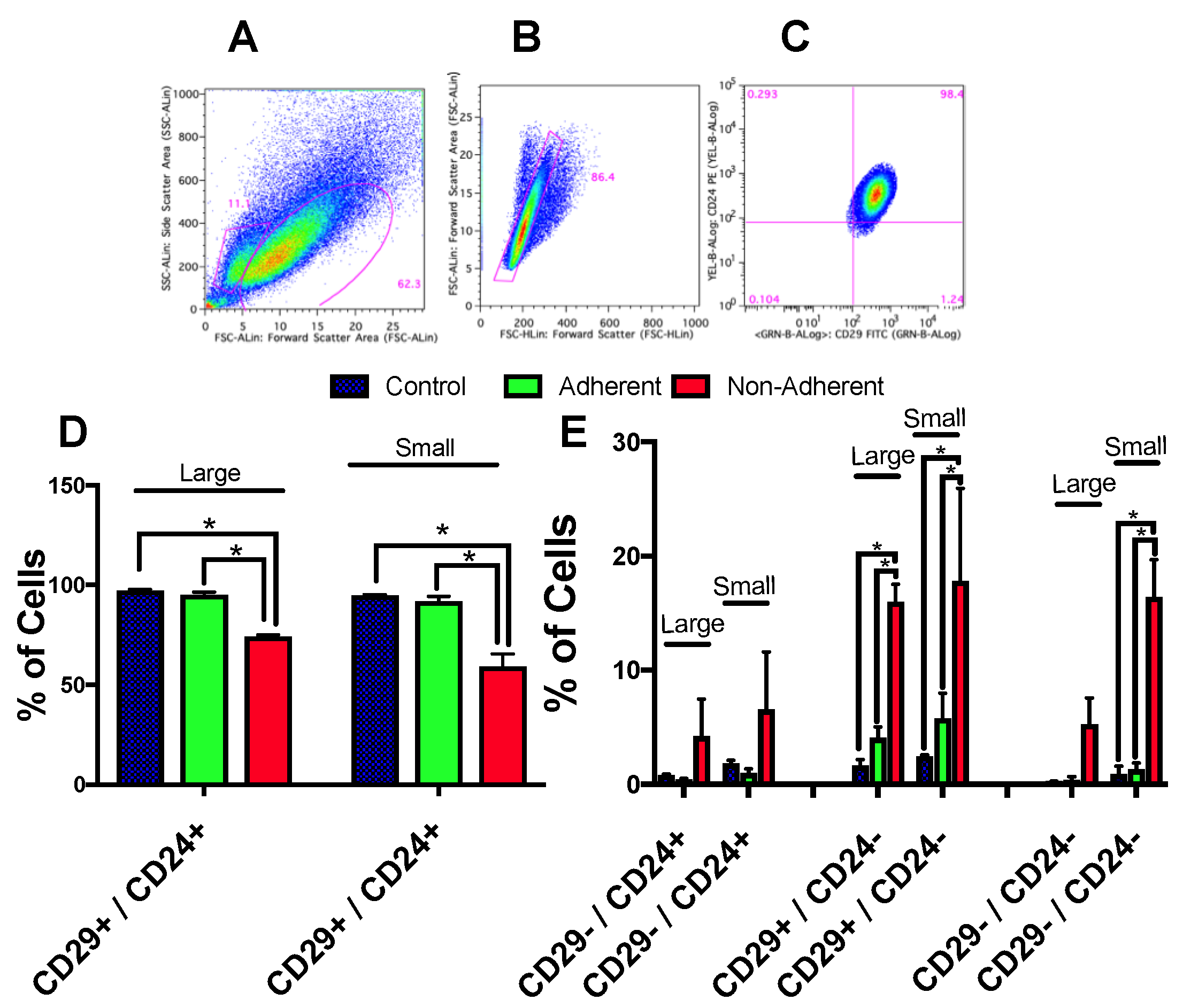
2.5. Growth under Simulated Microgravity Results in an Increase in a Small Sub-Population of Cells
2.6. Stem Cell Populations Are Altered following Simulated Microgravity Exposure
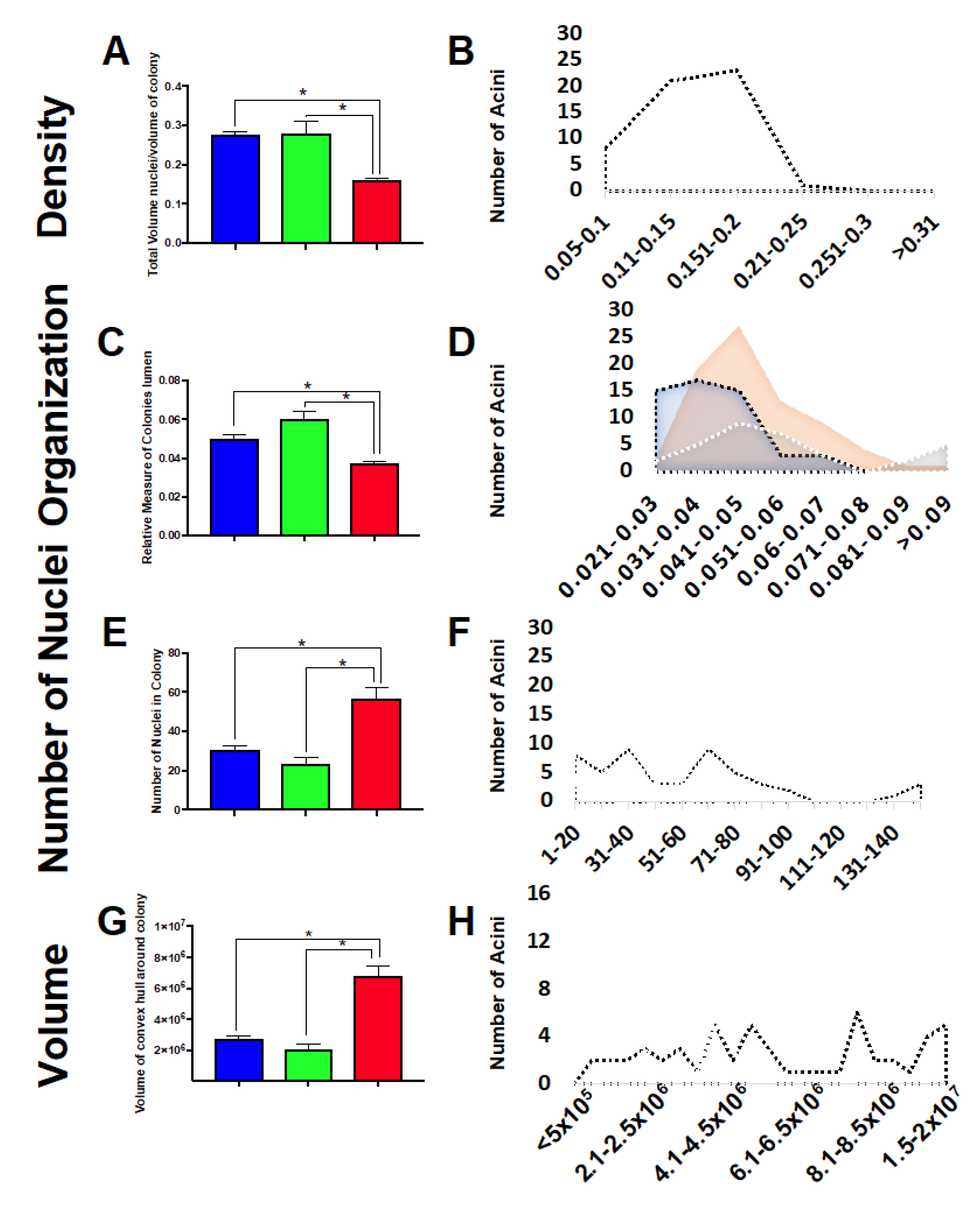
2.7. Three-Dimensional Acini Growth Is Altered with Simulated Microgravity Exposure
3. Discussion
4. Materials and Methods
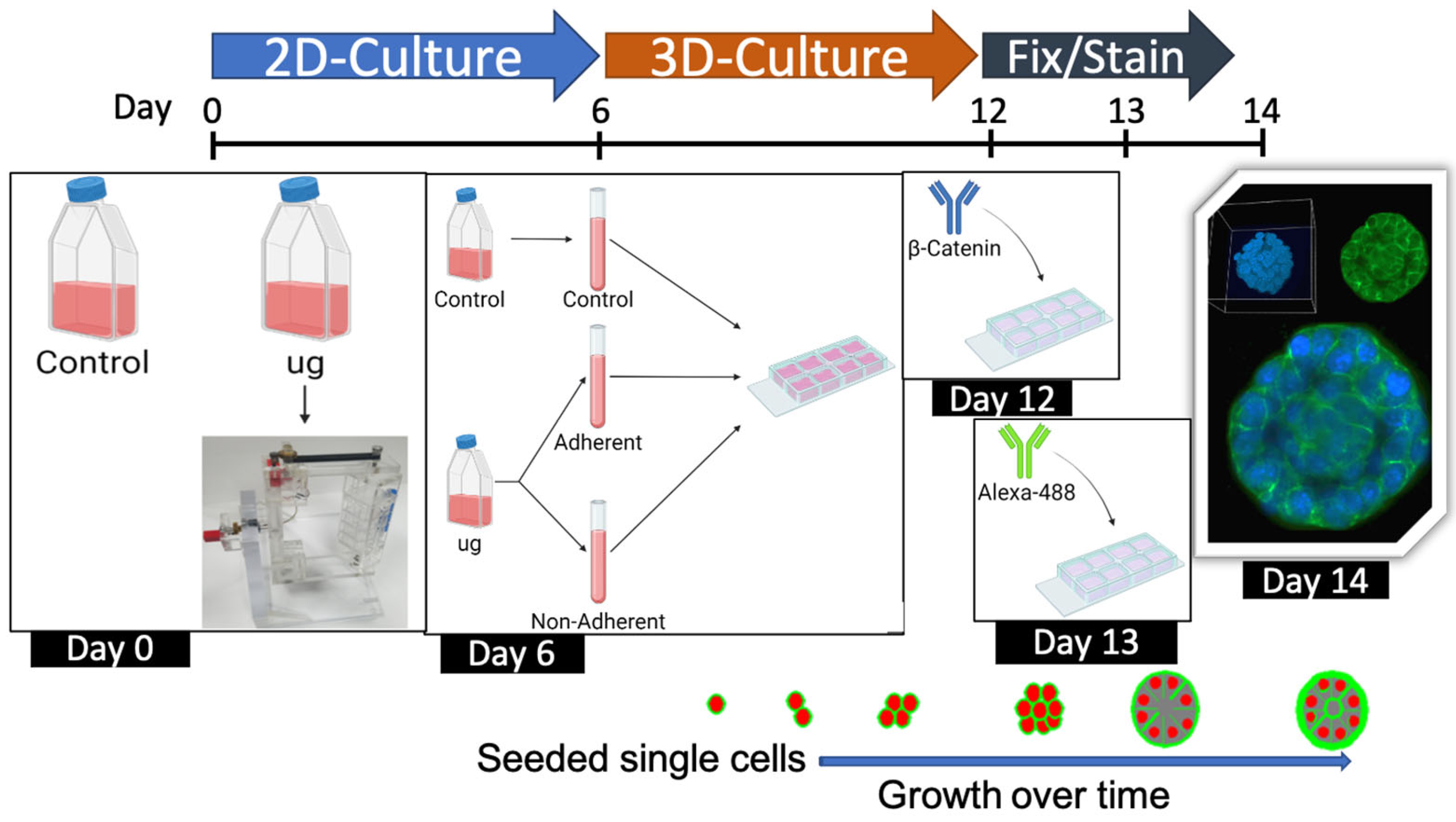
4.1. Cell Culture
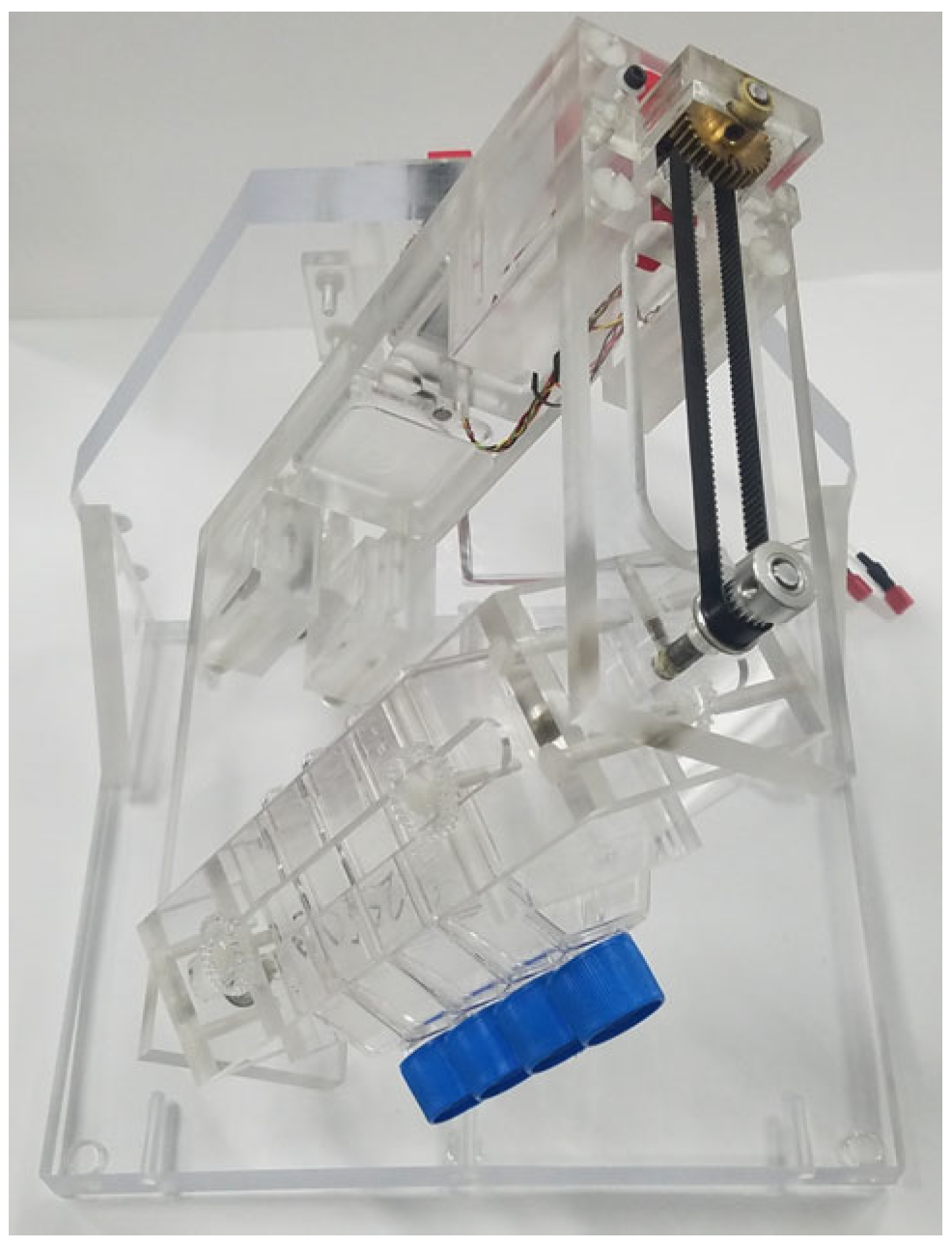
4.2. Three-Dimensional Clinostat (RPM)
4.3. Cell Cycle and Sub-G1 Analysis
4.4. Cell Diameter
4.5. γH2AX Levels
4.6. Apoptosis Assay
4.7. CD24/CD29 Stem Cell Marker Profiling: Cell Labeling, Flow Cytometry, and Analysis
4.8. Three-Dimensional Acini Growth and Culture
4.9. Imaging and Analysis of 3D Acini Structure and Organization
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferranti, F.; Caruso, M.; Cammarota, M.; Masiello, M.G.; Corano Scheri, K.; Fabrizi, C.; Fumagalli, L.; Schiraldi, C.; Cucina, A.; Catizone, A.; et al. Cytoskeleton modifications and autophagy induction in TCam-2 seminoma cells exposed to simulated microgravity. BioMed Res. Int. 2014, 2014, 904396. [Google Scholar] [CrossRef] [PubMed]
- Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Kruger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schutte, A.; et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3156. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Ambesi-Impiombato, F.S.; Peverini, M.; Damaskopoulou, E.; Fontanini, E.; Lazzarini, R.; Curcio, F.; Perrella, G. Thyrotropin receptor and membrane interactions in FRTL-5 thyroid cell strain in microgravity. Astrobiology 2011, 11, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Vassy, J.; Portet, S.; Beil, M.; Millot, G.; Fauvel-Lafeve, F.; Karniguian, A.; Gasset, G.; Irinopoulou, T.; Calvo, F.; Rigaut, J.P.; et al. The effect of weightlessness on cytoskeleton architecture and proliferation of human breast cancer cell line MCF-7. FASEB J. 2001, 15, 1104–1106. [Google Scholar]
- Nassef, M.Z.; Kopp, S.; Melnik, D.; Corydon, T.J.; Sahana, J.; Kruger, M.; Wehland, M.; Bauer, T.J.; Liemersdorf, C.; Hemmersbach, R.; et al. Short-Term Microgravity Influences Cell Adhesion in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5730. [Google Scholar] [CrossRef]
- Coinu, R.; Chiaviello, A.; Galleri, G.; Franconi, F.; Crescenzi, E.; Palumbo, G. Exposure to modeled microgravity induces metabolic idleness in malignant human MCF-7 and normal murine VSMC cells. FEBS Lett. 2006, 580, 2465–2470. [Google Scholar] [CrossRef]
- Jian, N.; Chen, Z.; Li, B.; Guo, S.; Li, A.; Zhang, T.; Fu, X.; Si, S.; Cui, Y. Effects of rotary cell culture system-simulated microgravity on the ultrstructure and biological behavior of human MDA-MB-231 breast cancer cells. Precis. Radiat. Oncol. 2019, 3, 87–93. [Google Scholar] [CrossRef]
- Kopp, S.; Sahana, J.; Islam, T.; Petersen, A.G.; Bauer, J.; Corydon, T.J.; Schulz, H.; Saar, K.; Huebner, N.; Slumstrup, L.; et al. The role of NFkappaB in spheroid formation of human breast cancer cells cultured on the Random Positioning Machine. Sci. Rep. 2018, 8, 921. [Google Scholar] [CrossRef]
- Cao, D.; Song, J.; Ling, S.; Niu, S.; Lu, L.; Cui, Z.; Li, Y.; Hao, S.; Zhong, G.; Qi, Z.; et al. Hematopoietic stem cells and lineage cells undergo dynamic alterations under microgravity and recovery conditions. FASEB J. 2019, 33, 6904–6918. [Google Scholar] [CrossRef]
- Ulbrich, C.; Wehland, M.; Pietsch, J.; Aleshcheva, G.; Wise, P.; van Loon, J.; Magnusson, N.; Infanger, M.; Grosse, J.; Eilles, C.; et al. The impact of simulated and real microgravity on bone cells and mesenchymal stem cells. BioMed Res. Int. 2014, 2014, 928507. [Google Scholar] [CrossRef]
- Arun, R.P.; Sivanesan, D.; Patra, B.; Varadaraj, S.; Verma, R.S. Simulated microgravity increases polyploid giant cancer cells and nuclear localization of YAP. Sci. Rep. 2019, 9, 10684. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, M.; Vaillant, F.; Simpson, K.J.; Stingl, J.; Smyth, G.K.; Asselin-Labat, M.L.; Wu, L.; Lindeman, G.J.; Visvader, J.E. Generation of a functional mammary gland from a single stem cell. Nature 2006, 439, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Rallis, C. The TOR Signaling Pathway in Spatial and Temporal Control of Cell Size and Growth. Front. Cell Dev. Biol. 2017, 5, 61. [Google Scholar] [CrossRef]
- Li, Q.; Rycaj, K.; Chen, X.; Tang, D.G. Cancer stem cells and cell size: A causal link? Semin. Cancer Biol. 2015, 35, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Rajput, M.; Singh, R.P. Simulated microgravity triggers DNA damage and mitochondria-mediated apoptosis through ROS generation in human promyelocytic leukemic cells. Mitochondrion 2021, 61, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.; An, L.; Fan, Y.; Hang, H.; Wang, S. Simulated microgravity potentiates generation of reactive oxygen species in cells. Biophys. Rep. 2016, 2, 100–105. [Google Scholar] [CrossRef]
- Kaanta, A.S.; Virtanen, C.; Selfors, L.M.; Brugge, J.S.; Neel, B.G. Evidence for a multipotent mammary progenitor with pregnancy-specific activity. Breast Cancer Res. BCR 2013, 15, R65. [Google Scholar] [CrossRef]
- Ball, R.K.; Friis, R.R.; Schoenenberger, C.A.; Doppler, W.; Groner, B. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988, 7, 2089–2095. [Google Scholar] [CrossRef]
- Whalen, M.K.; Gurai, S.K.; Zahed-Kargaran, H.; Pluth, J.M. Specific ATM-mediated phosphorylation dependent on radiation quality. Radiat. Res. 2008, 170, 353–364. [Google Scholar] [CrossRef]
- Vidi, P.A.; Bissell, M.J.; Lelievre, S.A. Three-dimensional culture of human breast epithelial cells: The how and the why. Methods Mol. Biol. 2013, 945, 193–219. [Google Scholar]
- Winkelmaier, G.; Parvin, B. An enhanced loss function simplifies the deep learning model for characterizing the 3D organoid models. Bioinformatics 2021, 37, 3084–3085. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, C.C.; Fontenay, G.; Cheng, Q.; Chang, H.; Han, J.; Parvin, B. BioSig3D: High Content Screening of Three-Dimensional Cell Culture Models. PLoS ONE 2016, 11, e0148379. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkelmaier, G.; Jabbari, K.; Chien, L.-C.; Grabham, P.; Parvin, B.; Pluth, J. Influence of Simulated Microgravity on Mammary Epithelial Cells Grown as 2D and 3D Cultures. Int. J. Mol. Sci. 2023, 24, 7615. https://doi.org/10.3390/ijms24087615
Winkelmaier G, Jabbari K, Chien L-C, Grabham P, Parvin B, Pluth J. Influence of Simulated Microgravity on Mammary Epithelial Cells Grown as 2D and 3D Cultures. International Journal of Molecular Sciences. 2023; 24(8):7615. https://doi.org/10.3390/ijms24087615
Chicago/Turabian StyleWinkelmaier, Garrett, Kosar Jabbari, Lung-Chang Chien, Peter Grabham, Bahram Parvin, and Janice Pluth. 2023. "Influence of Simulated Microgravity on Mammary Epithelial Cells Grown as 2D and 3D Cultures" International Journal of Molecular Sciences 24, no. 8: 7615. https://doi.org/10.3390/ijms24087615





