Polylactic-Containing Hyperbranched Polymers through the CuAAC Polymerization of Aromatic AB2 Monomers
Abstract
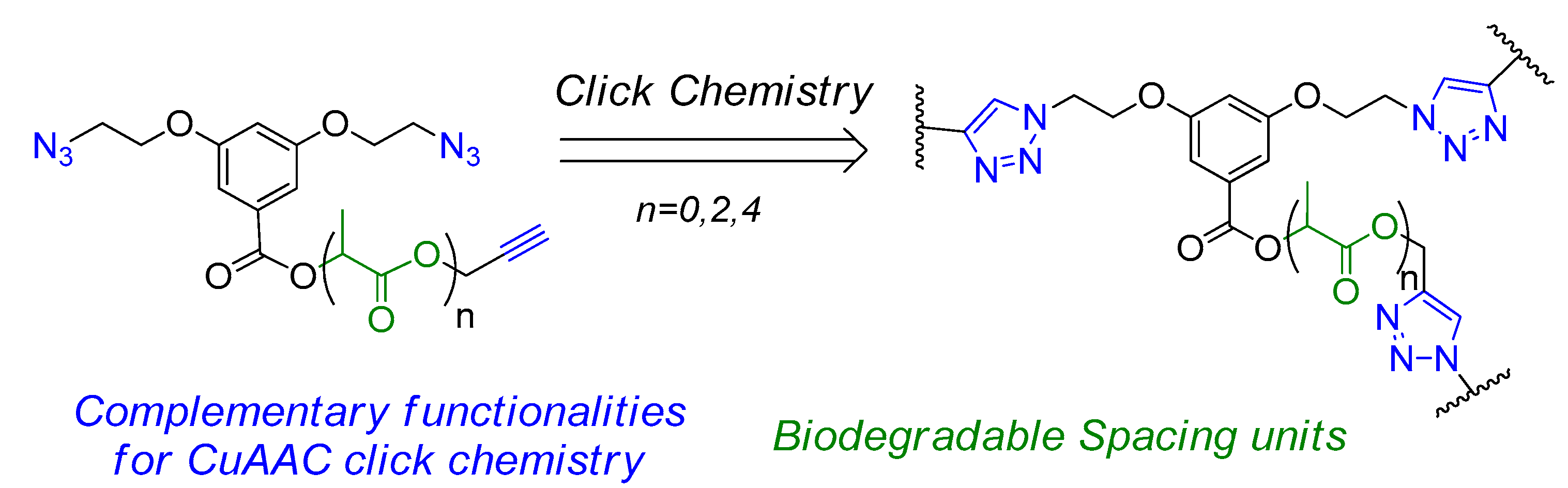
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voit, B.I.; Lederer, A. Hyperbranched and Highly Branched Polymer Architectures—Synthetic Strategies and Major Characterization Aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, T.; Zhu, X.; Yan, D.; Wang, W. Bioapplications of Hyperbranched Polymers. Chem. Soc. Rev. 2015, 44, 4023–4071. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched Polymers: Advances from Synthesis to Applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jin, Y.; Zhu, X.; Yan, D. Synthesis and applications of stimuli-responsive hyperbranched polymers. Progr. Polym. Sci. 2017, 64, 114–153. [Google Scholar] [CrossRef]
- Kapil, K.; Szczepaniak, G.; Martinez, M.R.; Murata, H.; Moini Jazani, A.; Jeong, J.; Das, S.R.; Matyjaszewski, K. Visible-Light-Mediated Controlled Radical Branching Polymerization in Water. Angew. Chem. Int. Ed. 2023, 62, e202217658. [Google Scholar] [CrossRef]
- Kurniasih, I.R.; Keilitz, J.; Haag, R. Dendritic nanocarriers based on hyperbranched polymers. Chem. Soc. Rev. 2015, 44, 4145–4164. [Google Scholar] [CrossRef]
- Sunder, A.; Heinemann, J.; Frey, H. Controlling the Growth of Polymer Trees: Concepts and Perspectives for Hyperbranched Polymers. Chem. Eur. J. 2000, 6, 2499–2506. [Google Scholar] [CrossRef]
- Kavand, A.; Anton, N.; Vandamme, T.; Serra, C.A.; Chan-Seng, D. Synthesis and functionalization of hyperbranched polymers for targeted drug delivery. J. Control. Release 2020, 321, 285–311. [Google Scholar] [CrossRef]
- Lin, X.; Chen, S.; Lu, W.; Liu, M.; Zhang, Z.; Zhu, J.; Pan, X. Diselenide–yne polymerization for multifunctional selenium-containing hyperbranched polymers. Polym. Chem. 2021, 12, 3383–3390. [Google Scholar] [CrossRef]
- Heckert, B.; Banerjee, T.; Sulthana, S.; Naz, S.; Alnasser, R.; Thompson, D.; Normand, G.; Grimm, J.; Perez, J.M.; Santra, S. Design and Synthesis of New Sulfur-Containing Hyperbranched Polymer and Theranostic Nanomaterials for Bimodal Imaging and Treatment of Cancer. ACS Macro Lett. 2017, 6, 235–240. [Google Scholar] [CrossRef]
- Yang, D.; Kong, J. 100% hyperbranched polymers via the acid-catalyzed Friedel–Crafts aromatic substitution reaction. Polym. Chem. 2016, 7, 5226–5232. [Google Scholar] [CrossRef]
- Rauschenbach, M.; Lawrenson, S.B.; Taresco, V.; Pearce, A.K.; O’Reilly, R.K. Antimicrobial Hyperbranched Polymer–Usnic Acid Complexes through a Combined ROP-RAFT Strategy. Macromol. Rapid Commun. 2020, 41, 2000190. [Google Scholar] [CrossRef] [PubMed]
- Mou, Q.; Ma, Y.; Jin, X.; Zhu, X. Designing hyperbranched polymers for gene delivery. Mol. Syst. Des. Eng. 2016, 1, 25–39. [Google Scholar] [CrossRef]
- Thi Ngoc Doan, A.; Thi Hong Doan, V.; Katsuki, J.; Fujii, S.; Kono, H.; Sakurai, K. Dramatically Increased Binding Constant of Water-Soluble Cyclodextrin Hyperbranched Polymers: Explored with Diffusion Ordered NMR Spectroscopy (DOSY). ACS Omega 2022, 7, 10890–10900. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Hyperbranched Polymers for Coating Applications: A Review Polym. -Plast. Tech. Mater. 2016, 55, 92–117. [Google Scholar] [CrossRef]
- Seo, S.E.; Hawker, C.J. The beauty of branching in polymer science. Macromolecules 2020, 53, 3257–3261. [Google Scholar] [CrossRef]
- Jikey, M.; Kakimoto, M. Hyperbranched polymers: A promising new class of materials Prog. Polym. Sci. 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Hawker, C.J.; Lee, R.; Frechet, J.M.J. One-Step Synthesis of Hyperbranched Dendritic Polyesters. J. Am. Chem. Soc. 1991, 113, 4583–4588. [Google Scholar] [CrossRef]
- Turner, S.R.; Voit, B.I.; Mourey, T.H. All-Aromatic Hyperbranched Polyesters with Phenol and Acetate End Groups: Synthesis and Characterization. Macromolecules 1993, 26, 4617–4623. [Google Scholar] [CrossRef]
- Turner, S.R.; Walter, F.; Voit, B.I.; Mourey, T.H. Hyperbranched Aromatic Polyesters with Carboxylic Acid Terminal Groups. Macromolecules 1994, 27, 1611–1616. [Google Scholar] [CrossRef]
- Uhrich, K.E.; Hawker, C.J.; Frechet, J.M.J.; Turner, S.R. One-Pot Synthesis of Hyperbranched Polyethers. Macromolecules 1992, 25, 4583–4587. [Google Scholar] [CrossRef]
- Miao, X.; Xing, A.; He, L.; Meng, Y.; Li, X. One-Step Preparation of Hyperbranched Polyether Functionalized Graphene Oxide for Improved Corrosion Resistance of Epoxy Coatings. Coatings 2019, 9, 844. [Google Scholar] [CrossRef]
- Klopp, J.M.; Pasini, D.; Frechet, J.M.J.; Willson, C.G.; Byers, J.D. Microlithographic Assessment of a Novel Family of Transparent and Etch Resistant Chemically Amplified 193 nm Resists Based on Cyclopolymers, Chem. Mater. 2001, 13, 4147–4153. [Google Scholar]
- Saha, A.; Ramakrishnan, S. Single Step Synthesis of Peripherally “Clickable” Hyperbranched Polyethers. Macromolecules 2009, 42, 4956–4959. [Google Scholar] [CrossRef]
- Pasini, D.; Klopp, J.M.; Frechet, J.M.J. Design, Synthesis, and Characterization of Carbon-Rich Cyclopolymers for 193nm Microlithography. Chem. Mater. 2001, 13, 4136–4146. [Google Scholar] [CrossRef]
- Karpov, S.V.; Iakunkov, A.; Akkuratov, A.V.; Petrov, A.O.; Perepelitsina, E.O.; Malkov, G.V.; Badamshina, E.R. One-Pot Synthesis of Hyperbranched Polyurethane-Triazoles with Controlled Structural, Molecular Weight and Hydrodynamic Characteristics. Polymers 2022, 14, 4514. [Google Scholar] [CrossRef]
- Fonseca, L.P.; Zanata, D.D.M.; Gauche, C.; Felisberti, M.I. A One-Pot, Solvent-Free, and Controlled Synthetic Route for Thermoresponsive Hyperbranched Polyurethanes. Polym. Chem. 2020, 11, 6295–6307. [Google Scholar] [CrossRef]
- Spindler, R.; Frechet, J.M.J. Synthesis and Characterization of Hyperbranched Polyurethanes Prepared from Blocked Isocyanate Monomers by Step-Growth Polymerization. Macromolecules 1993, 26, 4809–4813. [Google Scholar] [CrossRef]
- Si, Q.-F.; Fan, X.-D.; Liu, Y.-Y.; Kong, J.; Wang, S.-J.; Qiao, W.-Q. Synthesis and Characterization of Hyperbranched-Poly(Siloxysilane)-Based Polymeric Photoinitiators. J. Polym. Sci. Part Polym. Chem. 2006, 44, 3261–3270. [Google Scholar] [CrossRef]
- Ferri, N.; Ozaydin, G.B.; Zeffiro, A.; Nitti, A.; Aviyente, V.; Pasini, D. The efficient cyclopolymerization of silyl-tethered styrenic difunctional monomers. J. Polym. Sci. Part A 2018, 56, 1593–1599. [Google Scholar] [CrossRef]
- Mathias, L.J.; Carothers, T.W. Hyperbranched Poly(Siloxysilanes). J. Am. Chem. Soc. 1991, 113, 4043–4044. [Google Scholar] [CrossRef]
- Miravet, J.F.; Fréchet, J.M.J. New Hyperbranched Poly(Siloxysilanes): Variation of the Branching Pattern and End-Functionalization. Macromolecules 1998, 31, 3461–3468. [Google Scholar] [CrossRef]
- Havard, J.M.; Yoshida, M.; Pasini, D.; Vladimirov, N.; Frechet, J.M.J.; Medeiros, D.R.; Patterson, K.; Yamada, S.; Willson, C.G.; Byers, J.D. Design of Photoresists with Reduced Environmental Impact. 2. Water-soluble Resists Based on Photocrosslinking of Poly(2-isopropenyl-2-oxazoline). J. Polym. Sci. A Polym. Chem. 1999, 37, 1225–1236. [Google Scholar] [CrossRef]
- Kim, Y.H.; Webster, O.W. Hyperbranched Polyphenylenes. Macromolecules 1992, 25, 5561–5572. [Google Scholar] [CrossRef]
- Kim, Y.H. Lyotropic Liquid Crystalline Hyperbranched Aromatic Polyamides. J. Am. Chem. Soc. 1992, 114, 4947–4948. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Zarafshani, Z. Efficient Construction of Therapeutics, Bioconjugates, Biomaterials and Bioactive Surfaces Using Azide–Alkyne “Click” Chemistry. Adv. Drug Deliv. Rev. 2008, 60, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Hong, V.; Presolski, S.I.; Ma, C.; Finn, M.G. Analysis and Optimization of Copper-Catalyzed Azide–Alkyne Cycloaddition for Bioconjugation. Angew. Chem. Int. Ed. 2009, 48, 9879–9883. [Google Scholar] [CrossRef]
- Pasini, D. The Click Reaction as an Efficient Tool for the Construction of Macrocyclic Structures. Molecules 2013, 18, 9512–9530. [Google Scholar] [CrossRef]
- Caricato, M.; Olmo, A.; Gargiulli, C.; Gattuso, G.; Pasini, D. A “clicked” macrocyclic probe incorporating Binol as the signalling unit for the chiroptical sensing of anions. Tetrahedron 2012, 68, 7861–7866. [Google Scholar] [CrossRef]
- Cedrati, V.; Pacini, A.; Nitti, A.; de Ilarduya, A.M.; Muñoz-Guerra, S.; Sanyal, A.; Pasini, D. “Clickable” Bacterial Poly(γ-Glutamic Acid). Polym. Chem. 2020, 11, 5582–5589. [Google Scholar] [CrossRef]
- Pacini, A.; Caricato, M.; Ferrari, S.; Capsoni, D.; Martínez De Ilarduya, A.; Muñoz-Guerra, S. Poly(γ-glutamic acid) esters with reactive functional groups suitable for orthogonal conjugation strategies. J. Polym. Sci. A Polym. Chem. 2012, 50, 4790–4799. [Google Scholar] [CrossRef]
- Gan, W.; Shi, Y.; Jing, B.; Cao, X.; Zhu, Y.; Gao, H. Produce Molecular Brushes with Ultrahigh Grafting Density Using Accelerated CuAAC Grafting-Onto Strategy. Macromolecules 2017, 50, 215–222. [Google Scholar] [CrossRef]
- Scheel, A.J.; Komber, H.; Voit, B.I. Novel Hyperbranched Poly([1,2,3]-Triazole)s Derived from AB2 Monomers by a 1,3-Dipolar Cycloaddition. Macromol. Rapid Commun. 2004, 25, 1175–1180. [Google Scholar] [CrossRef]
- Li, Z.; Yu, G.; Hu, P.; Ye, C.; Liu, Y.; Qin, J.; Li, Z. New Azo-Chromophore-Containing Hyperbranched Polytriazoles Derived from AB2 Monomers via Click Chemistry under Copper(I) Catalysis. Macromolecules 2009, 42, 1589–1596. [Google Scholar] [CrossRef]
- Shi, Y.; Graff, R.W.; Cao, X.; Wang, X.; Gao, H. Chain-Growth Click Polymerization of AB2 Monomers for the Formation of Hyperbranched Polymers with Low Polydispersities in a One-Pot Process. Angew. Chem. Int. Ed. 2015, 127, 7741–7745. [Google Scholar] [CrossRef]
- Cao, X.; Shi, Y.; Gao, H. A Novel Chain-Growth CuAAC Polymerization: One-Pot Synthesis of Dendritic Hyperbranched Polymers with Well-Defined Structures. Synlett 2017, 28, 391–396. [Google Scholar] [CrossRef]
- Cao, X.; Shi, Y.; Gan, W.; Naguib, H.; Wang, X.; Graff, R.W.; Gao, H. Effect of Monomer Structure on the CuAAC Polymerization To Produce Hyperbranched Polymers. Macromolecules 2016, 49, 5342–5349. [Google Scholar] [CrossRef]
- Pacini, A.; Nitti, A.; Sangiovanni, G.; Vitale, M.; Pasini, D. Clickable 2,2-Bis(Hydroxymethyl)Propionic Acid-Derived AB2 Monomers: Hyperbranched Polyesters through the CuAAC Cycloaddition (Click) Reaction. J. Polym. Sci. 2021, 59, 2014–2022. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Zhang, M.; Shao, W.; Zhao, Y. Facile Synthesis of ABCDE-Type H-Shaped Quintopolymers by Combination of ATRP, ROP, and Click Chemistry and Their Potential Applications as Drug Carriers. J. Polym. Sci. A Polym. Chem. 2012, 50, 4705–4716. [Google Scholar] [CrossRef]
- Kumar, A.; Ramakrishnan, S. Structural Variants of Hyperbranched Polyesters. Macromolecules 1996, 29, 2524–2530. [Google Scholar] [CrossRef]
- Li, L.; He, C.; He, W.; Wu, C. Formation Kinetics and Scaling of “Defect-Free” Hyperbranched Polystyrene Chains with Uniform Subchains Prepared from Seesaw-Type Macromonomers. Macromolecules 2011, 44, 8195–8206. [Google Scholar] [CrossRef]
- Zou, L.; Shi, Y.; Cao, X.; Gan, W.; Wang, X.; Graff, R.; Hu, D.; Gao, H. Synthesis of Acid-Degradable Hyperbranched Polymers by Chain-Growth CuAAC Polymerization of an AB3 Monomer. Polym. Chem. 2016, 7, 5512–5517. [Google Scholar] [CrossRef]
- Hölter, D.; Burgath, A.; Frey, H. Degree of Branching in Hyperbranched Polymers. Acta Polym. 1997, 48, 30–35. [Google Scholar] [CrossRef]
- Asano, K.; Matsubara, S. Effects of a Flexible Alkyl Chain on a Ligand for CuAAC Reaction. Org. Lett. 2010, 12, 4988–4991. [Google Scholar] [CrossRef]
- Coluccini, C.; Dondi, D.; Caricato, M.; Taglietti, A.; Boiocchi, M.; Pasini, D. Structurally-Variable, Rigid and Optically-Active D2 and D3 Macrocycles Possessing Recognition Properties towards C60. Org. Biomol. Chem. 2010, 8, 1640–1649. [Google Scholar] [CrossRef]
- Ricci, M.; Pasini, D. Rigid Optically-Active D2 and D3 Macrocycles. Org. Biomol. Chem. 2003, 1, 3261–3262. [Google Scholar] [CrossRef]




| Entry | HP | Yield 2 | Mn | Mw | DP 3 | DB 4 |
|---|---|---|---|---|---|---|
| 1 | HP1 | 40 | 1700 5 | 2600 5 | 5 | 0.43 |
| 2 | HP2 | 38 | 3500 5 | 10,400 5 | 7 | 0.39 |
| 3 | HP3 | 56 | 4800 | 9500 | 8 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacini, A.; Nitti, A.; Vitale, M.; Pasini, D. Polylactic-Containing Hyperbranched Polymers through the CuAAC Polymerization of Aromatic AB2 Monomers. Int. J. Mol. Sci. 2023, 24, 7620. https://doi.org/10.3390/ijms24087620
Pacini A, Nitti A, Vitale M, Pasini D. Polylactic-Containing Hyperbranched Polymers through the CuAAC Polymerization of Aromatic AB2 Monomers. International Journal of Molecular Sciences. 2023; 24(8):7620. https://doi.org/10.3390/ijms24087620
Chicago/Turabian StylePacini, Aurora, Andrea Nitti, Marcello Vitale, and Dario Pasini. 2023. "Polylactic-Containing Hyperbranched Polymers through the CuAAC Polymerization of Aromatic AB2 Monomers" International Journal of Molecular Sciences 24, no. 8: 7620. https://doi.org/10.3390/ijms24087620
APA StylePacini, A., Nitti, A., Vitale, M., & Pasini, D. (2023). Polylactic-Containing Hyperbranched Polymers through the CuAAC Polymerization of Aromatic AB2 Monomers. International Journal of Molecular Sciences, 24(8), 7620. https://doi.org/10.3390/ijms24087620







