Leveraging Exosomes as the Next-Generation Bio-Shuttles: The Next Biggest Approach against Th17 Cell Catastrophe
Abstract
:1. Introduction
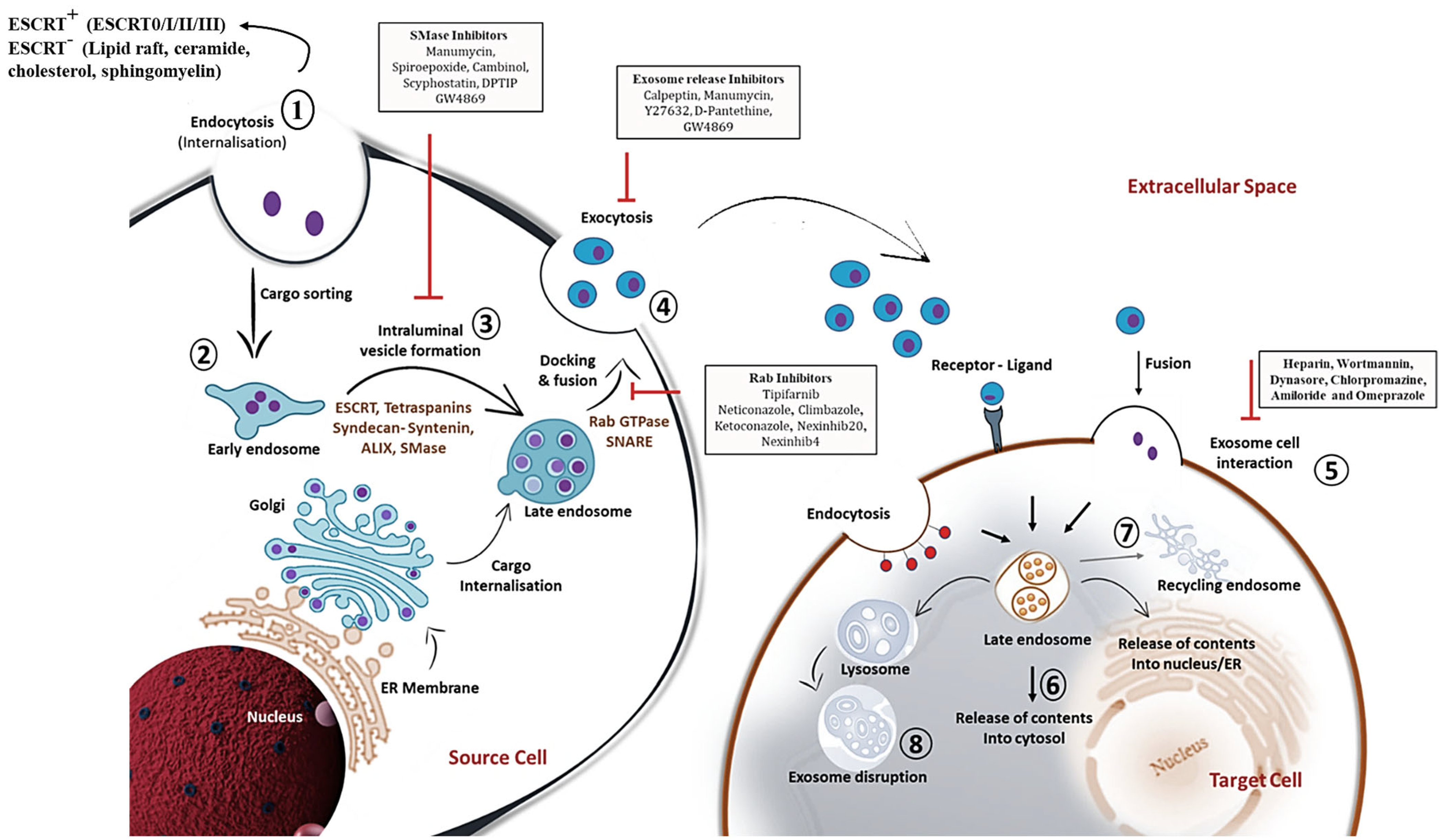
2. The Structure of Exosomes and Its Composition
3. Mechanistic Insights into Exosome Biogenesis and Release
4. Exosome Classification and Its Biodistribution: Current State-of-the-Art
5. Exosome in Diseases: The Boon against Evil
6. T Cell Exosomes: Its Biogenesis and Applicability
7. Exosome Shedding at the Crossroads of Immune Synapse
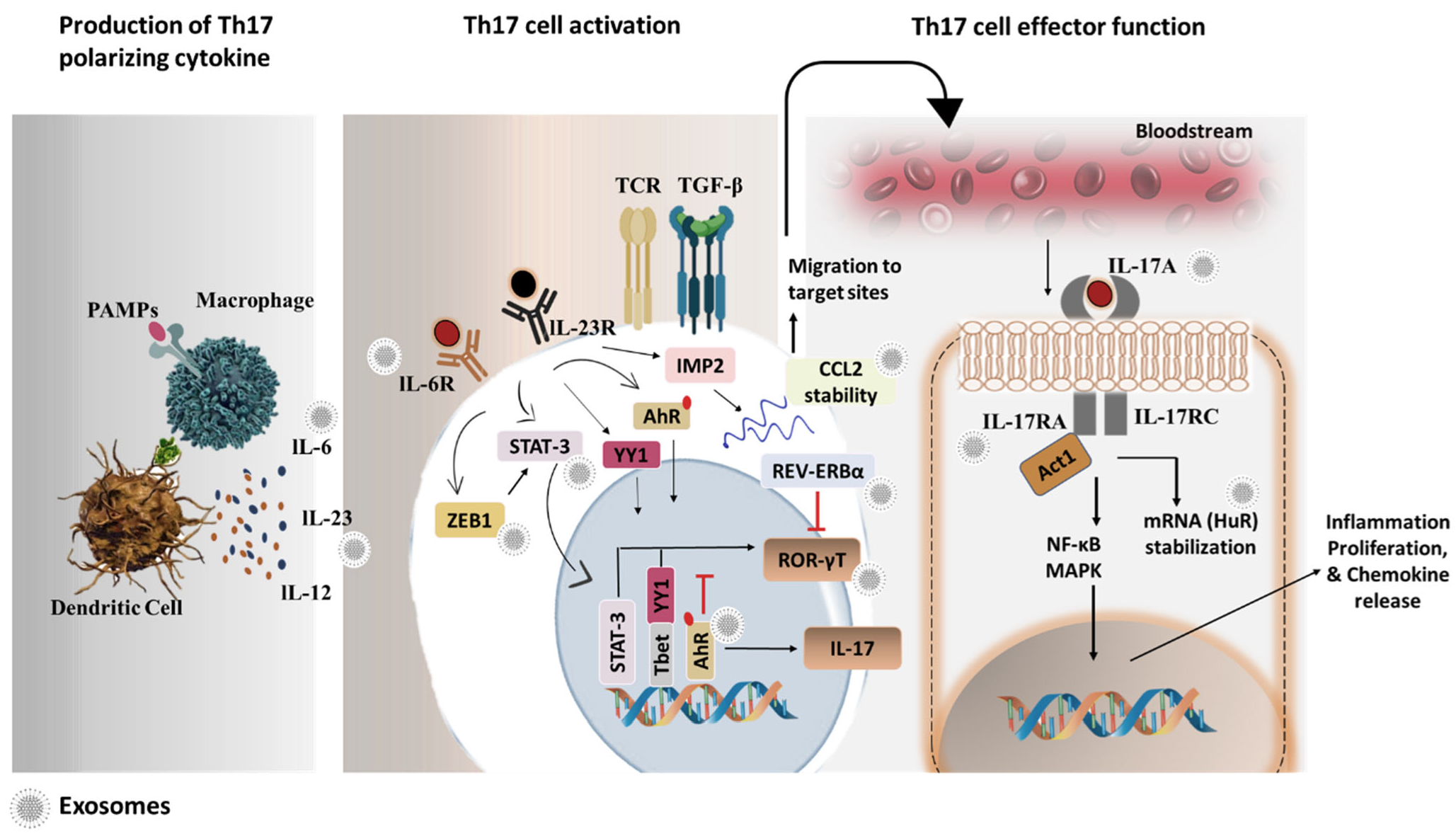
8. Th17 Cell Biology
9. Th17 Cell Pathologies: A Snap on Th17 Exosomes
10. Th17 Cell-Derived Exosomes in Disease Pathologies
11. Can Exosomes Potentiate as a Bona Fide Therapeutic Candidate against Th17 Biology?
12. Exosomes as a Budding Bio-Shuttle: A Perspective in the Landscape of Th17 Cell
13. Packaging of Small Molecules
14. Packaging of Proteins
15. Packaging of Genetic Substances
16. Strategies to Engineer Exosomes for Specific Targeting of Th17 Cells
17. Present Challenges and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agamah, F.E.; Mazandu, G.K.; Hassan, R.; Bope, C.D.; Thomford, N.E.; Ghansah, A.; Chimusa, E.R. Computational/in silico methods in drug target and lead prediction. Brief. Bioinform. 2020, 21, 1663–1675. [Google Scholar] [CrossRef]
- Lin, A.; Giuliano, C.J.; Palladino, A.; John, K.M.; Abramowicz, C.; Yuan, M.L.; Sausville, E.L.; Lukow, D.A.; Liu, L.; Chait, A.R.; et al. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci. Transl. Med. 2019, 11, eaaw8412. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteom. Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2021, 17, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lu, Y.; Li, X. Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. J. Drug Target. 2019, 28, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Kalluri, R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J. 2020, 288, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Anel, A.; Gallego-Lleyda, A.; de Miguel, D.; Naval, J.; Martínez-Lostao, L. Role of Exosomes in the Regulation of T-Cell Mediated Immune Responses and in Autoimmune Disease. Cells 2019, 8, 154. [Google Scholar] [CrossRef]
- Ahmadi, M.; Rezaie, J. Tumor cells derived-exosomes as angiogenenic agents: Possible therapeutic implications. J. Transl. Med. 2020, 18, 1–17. [Google Scholar] [CrossRef]
- Maybruck, B.T.; Pfannenstiel, L.W.; Diaz-Montero, M.; Gastman, B.R. Tumor-derived exosomes induce CD8+ T cell suppressors. J. Immunother. Cancer 2017, 5, 65. [Google Scholar] [CrossRef]
- Ye, L.; Zhu, Z.; Chen, X.; Zhang, H.; Huang, J.; Gu, S.; Zhao, X. The Importance of Exosomal PD-L1 in Cancer Progression and Its Potential as a Therapeutic Target. Cells 2021, 10, 3247. [Google Scholar] [CrossRef] [PubMed]
- Skriner, K.; Adolph, K.; Jungblut, P.R.; Burmester, G.R. Association of citrullinated proteins with synovial exosomes. Arthr. Rheum. 2006, 54, 3809–3814. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Bai, O.; Yuan, J.; Qureshi, M.; Xiang, J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and anti-tumor immunity than tumor cell-derived exosomes. Cell Mol. Immunol. 2006, 3, 205–211. [Google Scholar] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Wilke, C.M.; Kryczek, I.; Wei, S.; Zhao, E.; Wu, K.; Wang, G.; Zou, W. Th17 cells in cancer: Help or hindrance? Carcinog 2011, 32, 643–649. [Google Scholar] [CrossRef]
- Yang, J.; Sundrud, M.S.; Skepner, J.; Yamagata, T. Targeting Th17 cells in autoimmune diseases. Trends Pharmacol. Sci. 2014, 35, 493–500. [Google Scholar] [CrossRef]
- Zou, J.Y.; Su, C.H.; Luo, H.H.; Lei, Y.Y.; Zeng, B.; Zhu, H.S.; Chen, Z.G. Curcumin converts Foxp3+ regulatory T cells to T helper 1 cells in patients with lung cancer. J. Cell Biochem. 2017, 119, 1420–1428. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular sig-nalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Gheinani, A.H.; Vögeli, M.; Baumgartner, U.; Vassella, E.; Draeger, A.; Burkhard, F.C.; Monastyrskaya, K. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 2018, 8, 3945. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Nanda, H.S.; Peng, X.; Zhou, Y. Exosomes, a new star for targeted delivery. Front. Cell Dev. Biol. 2021, 9, 2827. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Wubbolts, R.; Leckie, R.S.; Veenhuizen, P.T.; Schwarzmann, G.; Mȯbius, W.; Hoernschemeyer, J.; Slot, J.W.; Geuze, H.J.; Stoorvogel, W. Proteomic and biochemical analyses of human B cell-derived exosomes: Potential implications for their function and mul-tivesicular body formation. J. Biol. Chem. 2003, 278, 10963–10972. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, X.; Yu, J. Exosomes and organ-specific metastasis. Mol. Therapy-Methods Clin. Dev. 2021, 22, 133–147. [Google Scholar] [CrossRef]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered exosomes: Desirable target-tracking characteristics for cere-brovascular and neurodegenerative disease therapies. Theranostics 2021, 11, 8926. [Google Scholar] [CrossRef]
- Shen, A.-R.; Zhong, X.; Tang, T.-T.; Wang, C.; Jing, J.; Liu, B.-C.; Lv, L.-L. Integrin, Exosome and Kidney Disease. Front. Physiol. 2021, 11, 627800. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Wahlund, C.J.E.; Güclüler, G.; Hiltbrunner, S.; Veerman, R.E.; Näslund, T.I.; Gabrielsson, S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Fu, W.; Lei, C.; Liu, S.; Cui, Y.; Wang, C.; Qian, K.; Li, T.; Shen, Y.; Fan, X.; Lin, F.; et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 2019, 10, 4355. [Google Scholar] [CrossRef]
- Enqvist, M.; Ask, E.H.; Forslund, E.; Carlsten, M.; Abrahamsen, G.; Béziat, V.; Andersson, S.; Schaffer, M.; Spurkland, A.; Bryceson, Y.; et al. Coordinated Expression of DNAM-1 and LFA-1 in Educated NK Cells. J. Immunol. 2015, 194, 4518–4527. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y. Exosomes Derived from Immune Cells: The New Role of Tumor Immune Microenvironment and Tumor Therapy. Int. J. Nanomed. 2022, 17, 6527–6550. [Google Scholar] [CrossRef] [PubMed]
- Larios, J.; Mercier, V.; Roux, A.; Gruenberg, J. ALIX- and ESCRT-III–dependent sorting of tetraspanins to exosomes. J. Cell Biol. 2020, 219, e201904113. [Google Scholar] [CrossRef] [PubMed]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The tetraspanin CD63 regulates ESCRT-independent and-dependent endosomal sorting during melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.; Colas, L.; Magnan, A.; Brouard, S. CD9 Tetraspanin: A New Pathway for the Regulation of Inflammation? Front. Immunol. 2018, 9, 2316. [Google Scholar] [CrossRef] [PubMed]
- Albacete-Albacete, L.; Navarro-Lérida, I.; López, J.A.; Martín-Padura, I.; Astudillo, A.M.; Ferrarini, A.; Van-Der-Heyden, M.; Balsinde, J.; Orend, G.; Vázquez, J.; et al. ECM deposition is driven by caveolin-1–dependent regulation of exosomal biogenesis and cargo sorting. J. Cell Biol. 2020, 219, e202006178. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.; Wijaya, C.H. Plant-derived exosome-like nanoparticles: A concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J. Food Sci. 2021, 86, 2350–2838. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bae, K.; Baek, A.-R.; Kwon, E.-B.; Kim, Y.-H.; Nam, S.-W.; Lee, G.H.; Chang, Y. Glioblastoma-Derived Exosomes as Nanopharmaceutics for Improved Glioma Treatment. Pharmaceutics 2022, 14, 1002. [Google Scholar] [CrossRef]
- Cao, Q.; Huang, C.; Chen, X.-M.; Pollock, C.A. Mesenchymal Stem Cell-Derived Exosomes: Toward Cell-Free Therapeutic Strategies in Chronic Kidney Disease. Front. Med. 2022, 9, 816656. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of extracellular vesicles following administration into animals: A systematic review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, E.; Bilousova, T.; Melnik, M.; Fakhrutdinov, D.; Poon, W.W.; Vinters, H.V.; Miller, C.A.; Corrada, M.; Kawas, C.; Bohannan, R.; et al. Exosomal tau with seeding activity is released from Alzheimer’s disease synapses, and seeding potential is associ-ated with amyloid beta. Lab. Investig. 2021, 101, 1605–1617. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.K.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Circulating exosomes from patients with systemic lupus erythematosus induce a proinflammatory immune response. Arthritis Res. Ther. 2016, 18, 264. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, L.; Wu, H.; Zhao, Q.; Wu, S. Exosomes derived from synovial fibroblasts under hypoxia aggravate rheumatoid arthritis by regulating Treg/Th17 balance. Exp. Biol. Med. 2020, 245, 1177–1186. [Google Scholar] [CrossRef]
- Ding, H.; Li, L.X.; Harris, P.C.; Yang, J.; Li, X. Extracellular vesicles and exosomes generated from cystic renal epithelial cells promote cyst growth in autosomal dominant polycystic kidney disease. Nat. Commun. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yue, B.-L.; Huang, Y.-Z.; Lan, X.-Y.; Liu, W.-J.; Chen, H. Exosomal RNAs: Novel Potential Biomarkers for Diseases—A Review. Int. J. Mol. Sci. 2022, 23, 2461. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, B.; Chen, Q.; Wang, M.; Fei, X.; Zhao, N. Serum exosomes from diabetic kidney disease patients promote pyroptosis and oxidative stress through the miR-4449/HIC1 pathway. Nutr. Diabet. 2021, 11, 33. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Wei, S.; He, Z.; Huang, X.; Wang, L.; Xia, Y.; Xu, Z.; Li, Z.; Wang, W.; et al. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018, 9, 854. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Z.; Fan, J.; Zhang, S.; Yang, W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct. Target. Ther. 2019, 4, 47. [Google Scholar] [CrossRef]
- Garcia, G.; Pinto, S.; Ferreira, S.; Lopes, D.; Serrador, M.J.; Fernandes, A.; Vaz, A.R.; de Mendonça, A.; Edenhofer, F.; Malm, T.; et al. Emerging Role of miR-21-5p in Neuron–Glia Dysregulation and Exosome Transfer Using Multiple Models of Alzheimer’s Disease. Cells 2022, 11, 3377. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hong, G.; Wang, S.; Gao, W.; Wang, P. Tumor-derived exosomal miRNA-141 promote angiogenesis and malignant pro-gression of lung cancer by targeting growth arrest-specific homeobox gene (GAX). Bioengineered 2021, 12, 821–831. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, X.; Li, W.; Feng, Q.; Feng, H.; Tong, Y.; Rong, H.; Wang, W.; Zhang, D.; Zhang, Z.; et al. Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer Manag. Res. 2019, 11, 4023–4040. [Google Scholar] [CrossRef]
- Mao, S.; Zheng, S.; Lu, Z.; Wang, X.; Wang, Y.; Zhang, G.; Xu, H.; Huang, J.; Lei, Y.; Liu, C.; et al. Exosomal miR-375-3p breaks vascular barrier and promotes small cell lung cancer metastasis by targeting claudin-1. Transl. Lung Cancer Res. 2021, 10, 3155–3172. [Google Scholar] [CrossRef]
- Yu, Y.; Bai, F.; Qin, N.; Liu, W.; Sun, Q.; Zhou, Y.; Yang, J. Non-proximal renal tubule-derived urinary exosomal miR-200b as a biomarker of renal fibrosis. Nephron 2018, 139, 269–282. [Google Scholar] [CrossRef]
- Lang, H.L.; Hu, G.W.; Chen, Y.; Liu, Y.; Tu, W.; Lu, Y.M.; Wu, L.; Xu, G.H. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 959–972. [Google Scholar]
- Sun, Y.; Hao, G.; Zhuang, M.; Lv, H.; Liu, C.; Su, K. MEG3 LncRNA from Exosomes Released from Cancer-Associated Fibroblasts Enhances Cisplatin Chemoresistance in SCLC via a MiR-15a-5p/CCNE1 Axis. Yonsei Med. J. 2022, 63, 229–240. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, H.; Lu, K.; Lu, Y.; Wang, Y.; Feng, T. Exosome-mediated delivery of MALAT1 induces cell proliferation in breast cancer. OncoTargets Ther. 2018, 11, 291–299. [Google Scholar] [CrossRef]
- Li, Z.; Yanfang, W.U.; Li, J.; Jiang, P.; Peng, T.; Chen, K.; Zhao, X.; Zhang, Y.; Zhen, P.; Zhu, J.; et al. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018, 432, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Chen, C.; Yang, Q.; Xue, J.; Chen, X.; Sun, B.; Luo, F.; Liu, X.; Xiao, T.; Xu, H.; et al. Exosomal circRNA_100284 from arsenite-transformed cells, via microRNA-217 regulation of EZH2, is involved in the malignant transformation of human hepatic cells by accelerating the cell cycle and promoting cell proliferation. Cell Death Dis. 2018, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, S.; Zheng, X.; Zheng, P.; Fu, Y.; Wu, C.; Lu, B.; Ju, J.; Jiang, J. Immune suppressed tumor microenvironment by exosomes derived from gastric cancer cells via modulating immune functions. Sci. Rep. 2020, 10, 14749. [Google Scholar] [CrossRef] [PubMed]
- Jafari, N.; Kolla, M.; Meshulam, T.; Shafran, J.S.; Qiu, Y.; Casey, A.N.; Pompa, I.R.; Ennis, C.S.; Mazzeo, C.S.; Rabhi, N.; et al. Adipo-cyte-derived exosomes may promote breast cancer progression in type 2 diabetes. Sci. Signal. 2021, 14, eabj2807. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, J.; Mao, Z.; Jiang, H.; Liu, W.; Shi, H.; Ji, R.; Xu, W.; Qian, H.; Zhang, X. Extracellular Vesicles from Gastric Cancer Cells Induce PD-L1 Expression on Neutrophils to Suppress T-Cell Immunity. Front. Oncol. 2020, 10, 629. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Lee, J.-E.; Sung, K.J.; Sung, Y.H.; Pack, C.-G.; Jung, M.-K.; Han, B.; et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Poggio, M.; Hu, T.; Pai, C.-C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019, 177, 414–427.e413. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Qu, J.; Che, X.; Fan, Y.; Hou, K.; Guo, T.; Deng, G.; Song, N.; Li, C.; et al. Exosomes promote cetuximab resistance via the PTEN/Akt pathway in colon cancer cells. Braz. J. Med. Biol. Res. 2018, 51, e6472. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Li, X.; Ma, J.; Fang, L.; Wu, N.; Li, F.; Dhaliwal, P.; Yang, W.; Yee, A.J.; Yang, B.B. Promotion of tumor progression by exosome transmission of circular RNA circSKA3. Mol. Ther. Nucleic Acids 2022, 27, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Escara-Wilke, J.; Keller, J.M.; Jung, Y.; Taichman, R.S.; Pienta, K.J.; Keller, E.T. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J. Exp. Med. 2019, 216, 2883–2899. [Google Scholar] [CrossRef]
- Yu, C.; Xue, B.; Li, J.; Zhang, Q. Tumor cell-derived exosome RNF126 affects the immune microenvironment and promotes naso-pharyngeal carcinoma progression by regulating PTEN ubiquitination. Apoptosis 2022, 27, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Gyukity-Sebestyén, E.; Harmati, M.; Dobra, G.; Németh, I.B.; Mihály, J.; Zvara, Á.; Hunyadi-Gulyás, É.; Katona, R.; Nagy, I.; Horváth, P.; et al. Melanoma-derived exosomes induce PD-1 overexpression and tumor progression via mesenchymal stem cell onco-genic reprogramming. Front. Immunol. 2019, 10, 2459. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Z.; Hackert, T.; Pitzer, C.; Zöller, M. Tspan8 and Tspan8/CD151 knockout mice unravel the contribution of tumor and host exosomes to tumor progression. J. Exp. Clin. Cancer Res. 2018, 37, 312. [Google Scholar] [CrossRef]
- Wang, B.; Tan, Z.; Guan, F. Tumor-Derived Exosomes Mediate the Instability of Cadherins and Promote Tumor Progression. Int. J. Mol. Sci. 2019, 20, 3652. [Google Scholar] [CrossRef]
- Jeong, K.; Yu, Y.J.; You, J.Y.; Rhee, W.J.; Kim, J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab Chip 2020, 20, 548–557. [Google Scholar] [CrossRef]
- Wu, X.; Jin, S.; Ding, C.; Wang, Y.; He, D.; Liu, Y. Mesenchymal Stem Cell-Derived Exosome Therapy of Microbial Diseases: From Bench to Bed. Front. Microbiol. 2022, 12, 4007. [Google Scholar] [CrossRef]
- Kaspi, H.; Semo, J.; Abramov, N.; Dekel, C.; Lindborg, S.; Kern, R.; Lebovits, C.; Aricha, R. MSC-NTF (NurOwn®) exosomes: A novel therapeutic modality in the mouse LPS-induced ARDS model. Stem Cell Res. Ther. 2021, 12, 72. [Google Scholar] [CrossRef]
- Wan, T.; Zhong, J.; Pan, Q.; Zhou, T.; Ping, Y.; Liu, X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tis-sue-specific gene therapy of liver diseases. Sci. Adv. 2022, 8, eabp9435. [Google Scholar] [CrossRef]
- Melzer, C.; Rehn, V.; Yang, Y.; Bähre, H.; von der Ohe, J.; Hass, R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers 2019, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs from the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, J.; Zeng, C.; Wu, D.; Mu, Z.; Chen, B.; Xie, Y.; Ye, Y.; Liu, J. Curcumin increases exosomal TCF21 thus suppressing exo-some-induced lung cancer. Oncotarget 2016, 7, 87081. [Google Scholar] [CrossRef]
- Toffoli, G.; Hadla, M.; Corona, G.; Caligiuri, I.; Palazzolo, S.; Semeraro, S.; Gamini, A.; Canzonieri, V.; Rizzolio, F. Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine 2015, 10, 2963–2971. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, Y.; Zhao, M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2017, 7, 533. [Google Scholar] [CrossRef]
- Shen, L.-M.; Quan, L.; Liu, J. Tracking Exosomes in Vitro and in Vivo To Elucidate Their Physiological Functions: Implications for Diagnostic and Therapeutic Nanocarriers. ACS Appl. Nano Mater. 2018, 1, 2438–2448. [Google Scholar] [CrossRef]
- Hikita, T.; Miyata, M.; Watanabe, R.; Oneyama, C. In vivo imaging of long-term accumulation of cancer-derived exosomes using a BRET-based reporter. Sci. Rep. 2020, 10, 16616. [Google Scholar] [CrossRef]
- Ventimiglia, L.N.; Alonso, M.A. Biogenesis and function of T cell-derived exosomes. Front. Cell Dev. Biol. 2016, 4, 84. [Google Scholar] [CrossRef]
- Alonso, R.; Mazzeo, C.; Rodríguez, M.C.; Marsh, M.; Fraile-Ramos, A.; Calvo, V.; Avila-Flores, A.; Merida, I.; Izquierdo, M. Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas lig-and-containing exosomes in T lymphocytes. Cell Death Differ. 2011, 18, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Vazquez, C.; Villarroya-Beltri, C.; Mittelbrunn, M.; Sánchez-Madrid, F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol. Rev. 2012, 251, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.J.; McMillan, L.E.; Tan, S.L.; Bellomo, G.; Massoue, C.; Thompson, H.; Mykhaylechko, L.; Alibhai, D.; Ruan, X.; Singleton, K.L.; et al. Transient protein accumulation at the center of the T cell antigen-presenting cell interface drives efficient IL-2 secretion. Elife 2019, 8, e45789. [Google Scholar] [CrossRef]
- Abusamra, A.J.; Zhong, Z.; Zheng, X.; Li, M.; Ichim, T.E.; Chin, J.L.; Min, W.-P. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 2005, 35, 169–173. [Google Scholar] [CrossRef]
- Wahlgren, J.; Karlson, T.D.; Glader, P.; Telemo, E.; Valadi, H. Activated human T cells secrete exosomes that participate in IL-2 mediated immune response signaling. PLoS ONE 2012, 7, e49723. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.A.; Ratnasothy, K.; Tsang, J.Y.; Boardman, D.; Warley, A.; Lechler, R.; Lombardi, G. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur. J. Immunol. 2013, 43, 2430–2440. [Google Scholar] [CrossRef]
- Okoye, I.S.; Coomes, S.M.; Pelly, V.S.; Czieso, S.; Papayannopoulos, V.; Tolmachova, T.; Seabra, M.C.; Wilson, M.S. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014, 41, 89–103. [Google Scholar] [CrossRef]
- Lin, C.; Guo, J.; Jia, R. Roles of Regulatory T Cell-Derived Extracellular Vesicles in Human Diseases. Int. J. Mol. Sci. 2022, 23, 11206. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Y.; Peng, J.; Thakur, A.; Bai, N.; Yang, K.; Xu, Z. Decoding Roles of Exosomal lncRNAs in Tumor-Immune Regulation and Therapeutic Potential. Cancers 2022, 15, 286. [Google Scholar] [CrossRef]
- Bettelli, E.; Korn, T.; Oukka, M.; Kuchroo, V.K. Induction and effector functions of TH17 cells. Nature 2008, 453, 1051–1057. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of Effector CD4 T Cell Populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.M.; Way, S.S. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 2009, 126, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, N.; Feng, C.G. Regulation of T Helper Cell Fate by TCR Signal Strength. Front. Immunol. 2020, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.; Zinser, E.; Holzinger, C.; Sandrock, L.; Koch, S.; Finotto, S. NFATc1 deficiency in T cells protects mice from experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2015, 45, 1426–1440. [Google Scholar] [CrossRef]
- Bhaumik, S.; Basu, R. Cellular and Molecular Dynamics of Th17 Differentiation and its Developmental Plasticity in the Intestinal Immune Response. Front. Immunol. 2017, 8, 254. [Google Scholar] [CrossRef]
- Revu, S.; Wu, J.; Henkel, M.; Rittenhouse, N.; Menk, A.; Delgoffe, G.M.; Poholek, A.C.; McGeachy, M.J. IL-23 and IL-1β Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation. Cell Rep. 2018, 22, 2642–2653. [Google Scholar] [CrossRef]
- Foley, J.F. STAT3 Regulates the Generation of Th17 Cells. Sci. STKE 2007, 2007, tw113. [Google Scholar] [CrossRef]
- Baricza, E.; Tamási, V.; Marton, N.; Buzás, E.I.; Nagy, G. The emerging role of aryl hydrocarbon receptor in the activation and differentiation of Th17 cells. Cell Mol. Life Sci. 2015, 73, 95–117. [Google Scholar] [CrossRef]
- Mudter, J.; Yu, J.; Zufferey, C.; Brüstle, A.; Wirtz, S.; Weigmann, B.; Hoffman, A.; Schenk, M.; Galle, P.R.; Lehr, H.A.; et al. IRF4 reg-ulates IL-17A promoter activity and controls RORγt-dependent Th17 colitis in vivo. Inflamm. Bowel Dis. 2011, 17, 1343–1358. [Google Scholar] [CrossRef]
- Ciofani, M.; Madar, A.; Galan, C.; Sellars, M.; Mace, K.; Pauli, F.; Agarwal, A.; Huang, W.; Parkurst, C.N.; Muratet, M.; et al. A Validated Regulatory Network for Th17 Cell Specification. Cell 2012, 151, 289–303. [Google Scholar] [CrossRef]
- Lin, J.; Tang, J.; Lin, J.; He, Y.; Yu, Z.; Jiang, R.; Yang, B.; Ou, Q. YY1 regulation by miR-124-3p promotes Th17 cell pathogenicity through interaction with T-bet in rheumatoid arthritis. J. Clin. Investig. 2021, 6, e149985. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Arellano, G.; Ifergan, I.; Lin, J.; Snowden, C.; Kim, T.; Thomas, J.J.; Law, C.; Guan, T.; Balabanov, R.D.; et al. ZEB1 promotes pathogenic Th1 and Th17 cell differentiation in multiple sclerosis. Cell Rep. 2021, 36, 109602. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Martindale, J.L.; Abdelmohsen, K.; Kumar, G.; Fortina, P.M.; Gorospe, M.; Rostami, A.; Yu, S. RNA-binding protein HuR pro-motes Th17 cell differentiation and can be targeted to reduce autoimmune neuroinflammation. J. Immunol. 2020, 204, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Bechara, R.; Amatya, N.; Majumder, S.; Zhou, C.; Li, Y.; Liu, Q.; McGeachy, M.J.; Gaffen, S.L. The RNA-binding protein IMP2 drives a stromal-Th17 cell circuit in autoimmune neuroinflammation. J. Clin. Investig. 2022, 7, e152766. [Google Scholar] [CrossRef]
- Avdeev, S.N.; Trushenko, N.V.; Tsareva, N.A.; Yaroshetskiy, A.I.; Merzhoeva, Z.M.; Nuralieva, G.S.; Nekludova, G.V.; Chikina, S.Y.; Gneusheva, T.Y.; Suvorova, O.A.; et al. Anti-IL-17 monoclonal antibodies in hospitalized patients with severe COVID-19: A pilot study. Cytokine 2021, 146, 155627. [Google Scholar] [CrossRef]
- Omokanye, A.; Ong, L.C.; Lebrero-Fernandez, C.; Bernasconi, V.; Schön, K.; Strömberg, A.; Bemark, M.; Saelens, X.; Czarnewski, P.; Lycke, N. Clonotypic analysis of protective influenza M2e-specific lung resident Th17 memory cells reveals extensive functional diversity. Mucosal Immunol. 2022, 15, 717–729. [Google Scholar] [CrossRef]
- Tan, Z.; Qian, X.; Jiang, R.; Liu, Q.; Wang, Y.; Chen, C.; Wang, X.; Ryffel, B.; Sun, B. IL-17A Plays a Critical Role in the Pathogenesis of Liver Fibrosis through Hepatic Stellate Cell Activation. J. Immunol. 2013, 191, 1835–1844. [Google Scholar] [CrossRef]
- Molesworth-Kenyon, S.J.; Yin, R.; Oakes, J.E.; Lausch, R.N. IL-17 receptor signaling influences virus-induced corneal inflammation. J. Leukoc. Biol. 2007, 83, 401–408. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, M.; Lin, Q.W.; Cao, A.L.; Yu, X.; Dong, J.H.; Wang, J.P.; Zhang, J.H.; Wang, M.; Guo, H.P.; et al. Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J. Immunol. 2010, 185, 4004–4010. [Google Scholar] [CrossRef]
- Li, B.; Huang, L.; Lv, P.; Li, X.; Liu, G.; Chen, Y.; Wang, Z.; Qian, X.; Shen, Y.; Li, Y.; et al. The role of Th17 cells in psoriasis. Immunol. Res. 2020, 68, 296–309. [Google Scholar] [CrossRef]
- Nishimoto, S.; Kotani, H.; Tsuruta, S.; Shimizu, N.; Ito, M.; Shichita, T.; Morita, R.; Takahashi, H.; Amagai, M.; Yoshimura, A. Th17 Cells Carrying TCR Recognizing Epidermal Autoantigen Induce Psoriasis-like Skin Inflammation. J. Immunol. 2013, 191, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Kadono, T. The contribution of IL-17 to the development of autoimmunity in psoriasis. J. Endotoxin Res. 2019, 25, 337–343. [Google Scholar] [CrossRef]
- Zhao, L.; Ghetie, D.; Jiang, Z.; Chu, C.Q. How Can We Manipulate the IL-23/IL-17 Axis? Curr. Treat. Options Rheumatol. 2015, 1, 182–196. [Google Scholar] [CrossRef]
- Balasa, R.; Barcutean, L.; Balasa, A.; Motataianu, A.; Roman-Filip, C.; Manu, D. The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum. Immunol. 2020, 81, 237–243. [Google Scholar] [CrossRef]
- Hu, W. Huntington’s disease is a TH17 related autoimmune disorder against mutant Huntingtin coded by multiple CAG triplets. Nat. Précéd. 2011, 219. [Google Scholar] [CrossRef]
- Da Silva, R.R.; Pereira, M.C.; Rêgo, M.J.; Hatzlhofer, B.L.; da Silva Araújo, A.; Bezerra, M.A.; da Rocha Pitta, I.; da Rocha Pitta, M.G. Evaluation of Th17 related cytokines associated with clinical and laboratorial parameters in sickle cell anemia patients with leg ulcers. Cytokine 2014, 65, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Adabavazeh, R.; Nikoueinejad, H.; Sharif, M.R.; Faraji, S.; Shahreza, B.O.; Akbari, H.; Einollahi, B. T helper 17 lymphocyte pathway in the diagnosis of autosomal dominant polycystic kidney disease. Iran. J. Kidney Dis. 2015, 9, 105–112. [Google Scholar] [PubMed]
- Zhang, M.; Yu, Q.; Tang, W.; Wu, Y.; Lv, J.; Sun, L.; Shi, G.; Wu, M.; Qu, J.; Di, C.; et al. Epithelial exosomal contactin-1 promotes monocyte-derived dendritic cell-dominant T-cell responses in asthma. J. Allergy Clin. Immunol. 2021, 148, 1458–1545. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jia, H.; Bao, X.; Wu, Y.; Zhu, T.; Li, R.; Zhao, H. Tumor exosome promotes Th17 cell differentiation by transmitting the lncRNA CRNDE-h in colorectal cancer. Cell Death Dis. 2021, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, J.; Sun, L.; Sun, Y.; Zhao, F.; Liu, P.; Peng, X.; Xuan, X.; Li, Y.; Wang, P.; et al. Exosomes derived from LPS-stimulated human thymic mesenchymal stromal cells enhance inflammation via thrombospondin-1. Biosci. Rep. 2021, 41, BSR20203573. [Google Scholar] [CrossRef]
- Pourakbari, R.; Parhizkar, F.; Soltani-Zangbar, M.S.; Samadi, P.; Zamani, M.; Aghebati-Maleki, L.; Motavalli, R.; Mahmoodpoor, A.; Jadidi-Niaragh, F.; Yousefi, B.; et al. Preeclampsia-Derived Exosomes Imbalance the Activity of Th17 and Treg in PBMCs from Healthy Pregnant Women. Reprod. Sci. 2022, 1–2. [Google Scholar] [CrossRef]
- Liu, F.; Bu, Z.; Zhao, F.; Xiao, D. Increased T-helper 17 cell differentiation mediated by exosome-mediated micro RNA-451 redistribution in gastric cancer infiltrated T cells. Cancer Sci. 2018, 109, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Tian, J.; Wu, X.; Li, M.; Tang, X.; Rui, K.; Guo, H.; Ma, J.; Xu, H.; Wang, S. G-MDSC-derived exosomes attenuate collagen-induced arthritis by impairing Th1 and Th17 cell responses. Biochim. Biophys. Acta Mol.-Basis Dis. 2019, 1865, 165540. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cheng, T.; Zhou, M.; Kang, F.; Liao, C. hBMSC-Derived Exosomes Mitigate Th17/Treg Homeostasis in Periodontitis via miR-1246; Research Square: Durham, NC, USA, 2022. [Google Scholar]
- Yang, R.; Huang, H.; Cui, S.; Zhou, Y.; Zhang, T.; Zhou, Y. IFN-γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death Dis. 2020, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Fu, H.; Kuang, S.; He, F.; Zhang, M.; Shen, Z.; Qin, W.; Lin, Z.; Huang, S. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int. J. Oral Sci. 2021, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhu, Q.; Zhang, Y.; Bian, Q.; Hong, Y.; Shen, Z.; Xu, H.; Rui, K.; Yin, K.; Wang, S. Olfactory ecto-mesenchymal stem cell-derived exosomes ameliorate experimental colitis via modulating Th1/Th17 and Treg cell responses. Front. Immunol. 2020, 11, 598322. [Google Scholar] [CrossRef]
- Mao, F.; Wu, Y.; Tang, X.; Kang, J.; Zhang, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. BioMed Res. Int. 2017, 2017, 5356760. [Google Scholar] [CrossRef]
- Tu, J.; Zheng, N.; Mao, C.; Liu, S.; Zhang, H.; Sun, L. UC-BSCs Exosomes Regulate Th17/Treg Balance in Patients with Systemic Lupus Erythematosus via miR-19b/KLF13. Cells 2022, 11, 4123. [Google Scholar] [CrossRef]
- Li, B.; Xing, Y.; Gan, Y.; He, J.; Hua, H. Labial gland-derived mesenchymal stem cells and their exosomes ameliorate murine Sjögren’s syndrome by modulating the balance of Treg and Th17 cells. Stem Cell Res. Ther. 2021, 12, 478. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Z.; Ling, W.; Zhu, D.; Feng, Z.; Kong, L. Exosomes Derived from Dendritic Cells Attenuate Liver Injury by Modulating the Balance of Treg and Th17 Cells After Ischemia Reperfusion. Cell Physiol. Biochem. 2018, 46, 740–756. [Google Scholar] [CrossRef]
- Shi, Q.-Z.; Yu, H.-M.; Chen, H.-M.; Liu, M.; Cheng, X. Exosomes derived from mesenchymal stem cells regulate Treg/Th17 balance in aplastic anemia by transferring miR-23a-3p. Clin. Exp. Med. 2021, 21, 429–437. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, J.; Li, B.; Yi, L.; Wu, Y.; Xing, J.; Wang, L.; Wang, J.; Guo, L. Exosomes with FOXP3 from gene-modified dendritic cells ameliorate the development of EAE by regulating the balance of Th/Treg. Int. J. Med. Sci. 2022, 19, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, W.; Yang, F.; Yu, L.; Yu, Z.; Pan, J.; Wang, L.; Cao, X.; Wang, J. Immunosuppressive exosomes from TGF-β1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res. 2011, 22, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Chen, Y.; Wang, S.; Yu, L.; Shen, Y.; Zhong, H.; Yang, Y. Exosomes from heat-stressed tumour cells inhibit tumour growth by converting regulatory T cells to Th17 cells via IL-6. Immunology 2018, 154, 132–143. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Yue, Z.; Liu, Y.; Chen, Q.; Hu, D.; Han, J. Adipose-Derived Stem Cell Exosomes Inhibit Hypertrophic Scaring Formation by Regulating Th17/Treg Cell Balance. BioMed Res. Int. 2022, 2022, 9899135. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.M.; Xia, S.J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Pharm. Int. J. Pharm. Sci. 2021, 76, 61–67. [Google Scholar]
- Sterzenbach, U.; Putz, U.; Low, L.H.; Silke, J.; Tan, S.S.; Howitt, J. Engineered exosomes as vehicles for biologically active proteins. Mol. Ther. 2017, 25, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Noguchi, K.; Aoki, A.; Takatani-Nakase, T.; Fujii, I.; Futaki, S. Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery. Sci. Rep. 2017, 7, 1991. [Google Scholar] [CrossRef]
- Shimaoka, M.; Kawamoto, E.; Gaowa, A.; Okamoto, T.; Park, E. Connexins and Integrins in Exosomes. Cancers 2019, 11, 106. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Elashiry, M.; Elsayed, R.; Cutler, C.W. Exogenous and endogenous dendritic cell-derived exosomes: Lessons learned for immu-notherapy and disease pathogenesis. Cells 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell–Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharmacol. 2021, 11, 590470. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Pandrangi, S.; Kumari, S.; Gavara, M.M.; Badana, A.K. Exosomal tetraspanins as regulators of cancer progression and metastasis and novel diagnostic markers. Asia-Pacific J. Clin. Oncol. 2018, 14, 383–391. [Google Scholar] [CrossRef]
- Revenfeld, A.L.S.; Bæk, R.; Nielsen, M.H.; Stensballe, A.; Varming, K.; Jørgensen, M. Diagnostic and Prognostic Potential of Extracellular Vesicles in Peripheral Blood. Clin. Ther. 2014, 36, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.R.; Littman, D.R. Small molecule inhibitors of ROR γt: Targeting T h17 cells and other applications. Eur. J. Immunol. 2012, 42, 2232–2237. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; De Leon-Tabaldo, A.; Luna-Roman, R.; Castro, G.; Albers, M.; Schoetens, F.; DePrimo, S.; Devineni, D.; Wilde, T.; Goldberg, S.; et al. Preclinical and clinical characterization of the RORγt inhibitor JNJ-61803534. Sci. Rep. 2021, 11, 11066. [Google Scholar] [CrossRef] [PubMed]
- Cherney, R.J.; Cornelius, L.A.M.; Srivastava, A.; Weigelt, C.A.; Marcoux, D.; Duan, J.J.-W.; Shi, Q.; Batt, D.G.; Liu, Q.; Yip, S.; et al. Discovery of BMS-986251: A Clinically Viable, Potent, and Selective RORγt Inverse Agonist. ACS Med. Chem. Lett. 2020, 11, 1221–1227. [Google Scholar] [CrossRef]
- Cheung, K.; Lu, G.; Sharma, R.; Vincek, A.; Zhang, R.; Plotnikov, A.N.; Zhang, F.; Zhang, Q.; Ju, Y.; Hu, Y.; et al. BET N-terminal bromodomain inhibition selectively blocks Th17 cell differentiation and ameliorates colitis in mice. Proc. Nat. Acad. Sci. USA 2017, 114, 2952–2957. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Hu, X.; Schewitz-Bowers, L.P.; Stimpson, M.; Miao, L.; Ge, X.; Yang, L.; Li, Y.; Bible, P.W.; Wen, X.; et al. The DNA Methylation Inhibitor Zebularine Controls CD4+ T Cell Mediated Intraocular Inflammation. Front. Immunol. 2019, 10, 1950. [Google Scholar] [CrossRef]
- Hu, X.; Schewitz-Bowers, L.P.; Lait, P.J.P.; Copland, D.A.; Stimpson, M.L.; Li, J.J.; Liu, Y.; Dick, A.D.; Lee, R.W.J.; Wei, L. The Bromodomain and Extra-Terminal Protein Inhibitor OTX015 Suppresses T Helper Cell Proliferation and Differentiation. Curr. Mol. Med. 2019, 18, 594–601. [Google Scholar] [CrossRef]
- Aqel, S.I.; Kraus, E.E.; Jena, N.; Kumari, V.; Granitto, M.C.; Mao, L.; Yang, Y. Novel small molecule IL-6 inhibitor suppresses autoreactive Th17 development and promotes Treg development. Clin. Exp. Immunol. 2019, 196, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Gold, L.S.; Rubenstein, D.S.; Tallman, A.M.; Armstrong, A. Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor–modulating agent. J. Am. Acad. Dermatol. 2020, 84, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, H.J.; Heo, T.H. A directly GP130-targeting small molecule ameliorates collagen-induced arthritis (CIA) by inhibiting IL-6/GP130 signalling and Th17 differentiation. Clin. Exp. Pharmacol. Physiol. 2020, 47, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Yang, Y.J.; Ma, X.Q.; Cao, Q.; Wei, S.S.; Pan, R.R.; Nan, L.H.; Liu, Y.J.; Cao, Y.; Tian, X.Y.; et al. Neobaicalein Inhibits Th17 Cell Differentiation Resulting in Recovery of Th17/Treg Ratio through Blocking STAT3 Signaling Activation. Molecules 2022, 28, 18. [Google Scholar] [CrossRef]
- Hong, S.S.; Choi, J.H.; Lee, S.Y.; Park, Y.H.; Park, K.Y.; Lee, J.Y.; Kim, J.; Gajulapati, V.; Goo, J.I.; Singh, S.; et al. A novel small-molecule inhibitor targeting the IL-6 receptor β subunit, glycoprotein 130. J. Immunol. 2015, 195, 237–245. [Google Scholar] [CrossRef]
- Xiao, S.; Yosef, N.; Yang, J.; Wang, Y.; Zhou, L.; Zhu, C.; Wu, C.; Baloglu, E.; Schmidt, D.; Ramesh, R.; et al. Small-Molecule RORγt Antagonists Inhibit T Helper 17 Cell Transcriptional Network by Divergent Mechanisms. Immunity 2014, 40, 477–489. [Google Scholar] [CrossRef]
- Hu, X.; Zou, Y.; Copland, D.A.; Schewitz-Bowers, L.P.; Li, Y.; Lait, P.J.; Stimpson, M.; Zhang, Z.; Guo, S.; Liang, J.; et al. Epigenetic drug screen identified IOX1 as an inhibitor of Th17-mediated inflammation through targeting TET2. Ebiomedicine 2022, 86, 104333. [Google Scholar] [CrossRef]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 2022, 12, 2385–2402. [Google Scholar] [CrossRef]
- Kang, H.-K.; Chiang, M.-Y.; Liu, M.; Ecklund, D.; Datta, S.K. The Histone Peptide H471–94 Alone Is More Effective than a Cocktail of Peptide Epitopes in Controlling Lupus: Immunoregulatory Mechanisms. J. Clin. Immunol. 2011, 31, 379–394. [Google Scholar] [CrossRef]
- Niu, T.; Cheng, L.; Wang, H.; Zhu, S.; Yang, X.; Liu, K.; Jin, H.; Xu, X. KS23, a novel peptide derived from adiponectin, inhibits retinal inflammation and downregulates the proportions of Th1 and Th17 cells during experimental autoimmune uveitis. J. Neuroinflamm. 2019, 16, 278. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, R.; Xie, A.; Shi, W.; Huang, H.; Zhong, Y. Antagonistic peptides that specifically bind to the first and second ex-tracellular loops of CCR5 and anti-IL-23p19 antibody reduce airway inflammation by suppressing the IL-23/Th17 signaling pathway. Med. Inflamm. 2020, 2020, 1719467. [Google Scholar] [CrossRef]
- Min, H.K.; Choi, J.; Lee, S.Y.; Lee, A.R.; Min, B.M.; Cho, M.L.; Park, S.H. Vitronectin-derived bioactive peptide prevents spondyloarthritis by modulating Th17/Treg imbalance in mice with curdlan-induced spondyloarthritis. PLoS ONE 2022, 17, e0262183. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Park, B.; Lee, M.; Jeong, Y.S.; Lee, H.Y.; Sohn, D.H.; Song, J.J.; Lee, J.H.; Hwang, J.S.; Bae, Y.-S. A novel antimicrobial peptide acting via formyl peptide receptor 2 shows therapeutic effects against rheumatoid arthritis. Sci. Rep. 2018, 8, 14664. [Google Scholar] [CrossRef]
- Franke, M.; Schröder, J.; Monhasery, N.; Ackfeld, T.; Hummel, T.M.; Rabe, B.; Garbers, C.; Becker-Pauly, C.; Floss, D.M.; Scheller, J. Human and Murine Interleukin 23 Receptors Are Novel Substrates for A Disintegrin and Metalloproteases ADAM10 and ADAM17. J. Biol. Chem. 2016, 291, 10551–10561. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V. New Developments in Celiac Disease Treatment. Int. J. Mol. Sci. 2023, 24, 945. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiu, R.; Zhang, Z.; Han, Z.; Yao, C.; Hou, G.; Dai, D.; Jin, W.; Tang, Y.; Yu, X.; et al. The MicroRNA miR-22 Represses Th17 Cell Pathogenicity by Targeting PTEN-Regulated Pathways. Immunohorizons 2020, 4, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Ma, Y.; Tang, Y.; Liu, X.; Zhao, Y.; An, L. MiRNA-221-5p suppressed the Th17/Treg ratio in asthma via RORγt/Foxp3 by targeting SOCS1. Allergy Asthma Clin. Immunol. 2021, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Liu, R.; Fan, T.; Liao, L.; Wang, S.; Geng, W.; Wang, T.; Shi, W.; Ruan, Q. miR-340 Alleviates Psoriasis in Mice through Direct Targeting of IL-17A. J. Immunol. 2018, 201, 1412–1420. [Google Scholar] [CrossRef]
- Raveney, B.J.E.; Oki, S.; Yamamura, T. Nuclear Receptor NR4A2 Orchestrates Th17 Cell-Mediated Autoimmune Inflammation via IL-21 Signalling. PLoS ONE 2013, 8, e56595. [Google Scholar] [CrossRef]
- Søndergaard, J.N.; Geng, K.; Sommerauer, C.; Atanasoai, I.; Yin, X.; Kutter, C. Successful delivery of large-size CRISPR/Cas9 vectors in hard-to-transfect human cells using small plasmids. Commun. Biol. 2020, 3, 319. [Google Scholar] [CrossRef]
- Duan, L.; Ouyang, K.; Wang, J.; Xu, L.; Xu, X.; Wen, C.; Xie, Y.; Liang, Y.; Xia, J. Exosomes as Targeted Delivery Platform of CRISPR/Cas9 for Therapeutic Genome Editing. Chembiochem 2021, 22, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hollinshead, K.E.; Hao, Y.; Au, C.; Kroehling, L.; Ng, C.; Lin, W.-Y.; Li, D.; Silva, H.M.; Shin, J.; et al. Niche-Selective Inhibition of Pathogenic Th17 Cells by Targeting Metabolic Redundancy. Cell 2020, 182, 641–654.e20. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, X.; Wei, M.; Gao, X.; Zhao, L.; Shi, R.; Sun, W.; Duan, Y.; Yang, G.; Yuan, L. In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2018, 19, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Petrova, K.; Payton, N.M.; Persaud, I.; Redzic, J.S.; Graner, M.W.; Smith-Jones, P.; Anchordoquy, T.J. Surface Functionalization of Exosomes Using Click Chemistry. Bioconj. Chem. 2014, 25, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chen, X.; Hao, Y.; Wang, J.; Xie, Q.; Wang, X. Nanoengineering facilitating the target mission: Targeted extracellular vesicles delivery systems design. J. Nanobiotechnol. 2022, 20, 1–23. [Google Scholar] [CrossRef]
- Shi, X.; Song, P.; Tao, S.; Zhang, X.; Chu, C.Q. silencing RoRγt in Human CD4+ T cells with CD30 aptamer-RORγt shRNA Chimera. Sci. Rep. 2019, 9, 10375. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Y.; Cho, E.; Kim, G.B.; Kim, I.-S. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J. Extracell. Vesicles 2018, 7, 1440131. [Google Scholar] [CrossRef]
- Choi, H.; Choi, Y.; Yim, H.Y.; Mirzaaghasi, A.; Yoo, J.-K.; Choi, C. Biodistribution of Exosomes and Engineering Strategies for Targeted Delivery of Therapeutic Exosomes. Tissue Eng. Regen. Med. 2021, 18, 499–511. [Google Scholar] [CrossRef]

| Exosome Source | Exosome Cargo | Disease | Disease Outcome | Reference |
|---|---|---|---|---|
| HCT116 and Serum | miR-25, miR-130b and miR-425 | Colorectal Cancer | Aggravates liver metastasis | [52] |
| Serum | miR-1247-3p | Liver cancer | Promotes lung metastasis | [52] |
| A2780 CCM | miR-223 | Epithelial ovarian cancer | Promotes chemoresistance | [52] |
| Variable | miR-21 | Multiple cancers | Promotes cancer | [52] |
| Serum | miR-7977 | Lung adenocarcinoma | Increase in proliferation, invasion and inhibits apoptosis | [52] |
| Pan02 CCM | miR-155-5p and miR-221-5p | Pancreatic ductal adenocarcinoma | Promotes metastasis | [52] |
| HT-29/SW480 | miR-375-3p | Colon cancer | Induces EMT | [52] |
| MSC | miR-21-5p | Breast cancer | Promotes chemoresistance | [52] |
| Plasma | miR-1-3p | Sepsis | Endothelial cell dysfunction | [52] |
| Serum | miR-4449 | Diabetic kidney | Promotes pro-inflammation & oxidative stress | [53] |
| MGC803, MKN45, HGC27, and SGC7901 CCM | miR-21-5p | Gastric cancer | Promotes peritoneal metastasis | [54] |
| Human bronchial epithelial cells | miR-21 and miR-210 | COPD | Promotes myofibroblast differentiation and hypoxia | [55] |
| Serum | miR-96, miR-222-3p, miR-499a-5p | Lung cancer | Promote cell migration and invasion | [55] |
| Induced pluripotent stem cells (IPSC)-derived astrocytes/microglia | miR-21-5p | Adenocarcinoma | Induce neurotoxic reactive astrocytosis, cognitive impairment | [56] |
| TDEs | miR-141 | Lung cancer | Induces angiogenesis and malignancy | [57] |
| TDEs | miR-107 | Gastric Cancer | Promote immunosuppression | [58] |
| SCLC | miR-375-3p | Lung Cancer | Disrupts vascular barrier | [59] |
| Urine | miR-200b | Renal fibrosis | Fibrosis progression | [60] |
| Plasma | lnc-MKRN2 | Parkinson Disease | Develops disease occurrence | [52] |
| Serum | lncRNA-UCA1 | Pancreatic Cancer | Promotes angiogenesis | [52] |
| Serum | HOXD-AS1 | Prostate Cancer | Promotes metastasis | [52] |
| Urine | lncBCYRN1, lncLNMAT2 | Bladder Cancer | Promotes lymphatic metastasis | [52] |
| CCM | LncPCGEM1 | Gastric cancer | Induces metastasis and migration | [52] |
| TGF-β A549 | lnc-MMP2-2 | Lung cancer | Promotes invasion and metastasis | [55] |
| A172 cells | POU3F3 | Glioma | Promotes angiogenesis | [61] |
| CAF | MEG3 | SCLC | Cisplatin resistance | [62] |
| MCF7 | MALAT1 | Breast cancer | Promotes proliferation | [63] |
| Plasma | circ-RanGAP1 | Gastric cancer | Promotes metastasis | [52] |
| HCC CCM | circ-RNA-100338 | HCC | Promotes angiogenesis and invasion | [52] |
| Serum | Circ-0006156 | Thyroid cancer | Promotes tumorigenesis | [52] |
| TDEs | PDE8A | Pancreatic cancer | Elevates invasive growth | [64] |
| L-02 CCM | circ-100284 | Hepatocarcinoma | Accelerates cell cycle and proliferation | [65] |
| Exosome Source | Exosome Cargo | Disease | Clinical Status | Reference |
|---|---|---|---|---|
| Plant (Grapes) | Curcumin | Colon cancer | NCT01294072 Phase I | - |
| Plant (Ginger) | Curcumin | Inflammatory Bowel Disease | NCT04879810 (Completed) | - |
| Dendritic cell | Dex2 | Non-small cell lung cancer | NCT01159288 (Completed) | - |
| Plant (Grapes) | Lortab | Oral mucositis | NCT01668849 | - |
| Mesenchymal stromal cells | KRASG12D siRNA | Pancreatic Ductal Adenocarcinoma | NCT03608631 (Phase I) | - |
| Blood | Anlotinib | Non-small cell lung cancer | NCT05218759 | - |
| Blood | Pembrolizumab | Head and neck cancer | NCT04453046 (Terminated) | - |
| Dendritic cell/macrophage | Chimeric exosomal tumor vaccines | Bladder cancer | NCT05559177 (Phase I) | - |
| Circulating lymphocytes and serum | Merck 3475 Pembrolizumab | Triple-negative breast cancer | NCT02977468 (Phase I) | - |
| Liquid biopsies | 18F-DCFPyL PET/CT | Prostatic neoplasms | NCT03824275 (Phase II/III) | - |
| Macrophage | CDK-004 | Gastric cancer, colorectal cancer | NCT05375604 (Phase I) | - |
| Human cell | miR-497 | Lung cancer | - | [79] |
| Dental pulp stem cell | Chitosan hydrogel | Experimental periodontitis | - | [80] |
| MSC-NTF | - | COVID-19-induced ARDS | - | [81] |
| LX-2 cells | Cas9 ribonucleoprotein | Liver diseases | - | [82] |
| Drug | Structure | Molecular Target | Reference |
|---|---|---|---|
| Digoxin |  | ROR-γT | [156] |
| JNJ-61803534 |  | ROR-γT | [157] |
| BMS-986251 |  | ROR-γT | [158] |
| Izumerogant (IMU)-935 | - | ROR-γT | Phase I clinical trial |
| MS402 |  | BET N-terminal bromodomain | [159] |
| Zebularine |  | IL-17 | [160] |
| Birabresib (OTX015) |  | BET inhibitor | [161] |
| MDL-101 |  | IL-6 | [162] |
| Tapinarof |  | AhR | [163] |
| LMT-28 |  | IL-6/GP130, STAT-3 | [164] |
| Neobaicalein |  | STAT-3 | [165] |
| Bazedoxifene |  | IL-6/GP130 | [166] |
| 2,20,40-Trihydroxychalcone |  | ROR-γT | [167] |
| Ursolic Acid |  | ROR-γT | [166] |
| IOX1 |  | TET2 | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samarpita, S.; Li, X. Leveraging Exosomes as the Next-Generation Bio-Shuttles: The Next Biggest Approach against Th17 Cell Catastrophe. Int. J. Mol. Sci. 2023, 24, 7647. https://doi.org/10.3390/ijms24087647
Samarpita S, Li X. Leveraging Exosomes as the Next-Generation Bio-Shuttles: The Next Biggest Approach against Th17 Cell Catastrophe. International Journal of Molecular Sciences. 2023; 24(8):7647. https://doi.org/10.3390/ijms24087647
Chicago/Turabian StyleSamarpita, Snigdha, and Xiaogang Li. 2023. "Leveraging Exosomes as the Next-Generation Bio-Shuttles: The Next Biggest Approach against Th17 Cell Catastrophe" International Journal of Molecular Sciences 24, no. 8: 7647. https://doi.org/10.3390/ijms24087647
APA StyleSamarpita, S., & Li, X. (2023). Leveraging Exosomes as the Next-Generation Bio-Shuttles: The Next Biggest Approach against Th17 Cell Catastrophe. International Journal of Molecular Sciences, 24(8), 7647. https://doi.org/10.3390/ijms24087647





