Nifedipine Ameliorates Cellular Differentiation Defects of Smn-Deficient Motor Neurons and Enhances Neuromuscular Transmission in SMA Mice
Abstract
1. Introduction
2. Results
2.1. Nifedipine Treatment Leads to Proper Cellular Differentiation of SMN-Deficient Motor Neurons by Restoring Altered Cav2.2 Cluster Formation and Spontaneous Ca2+ Transients at Growth Cones
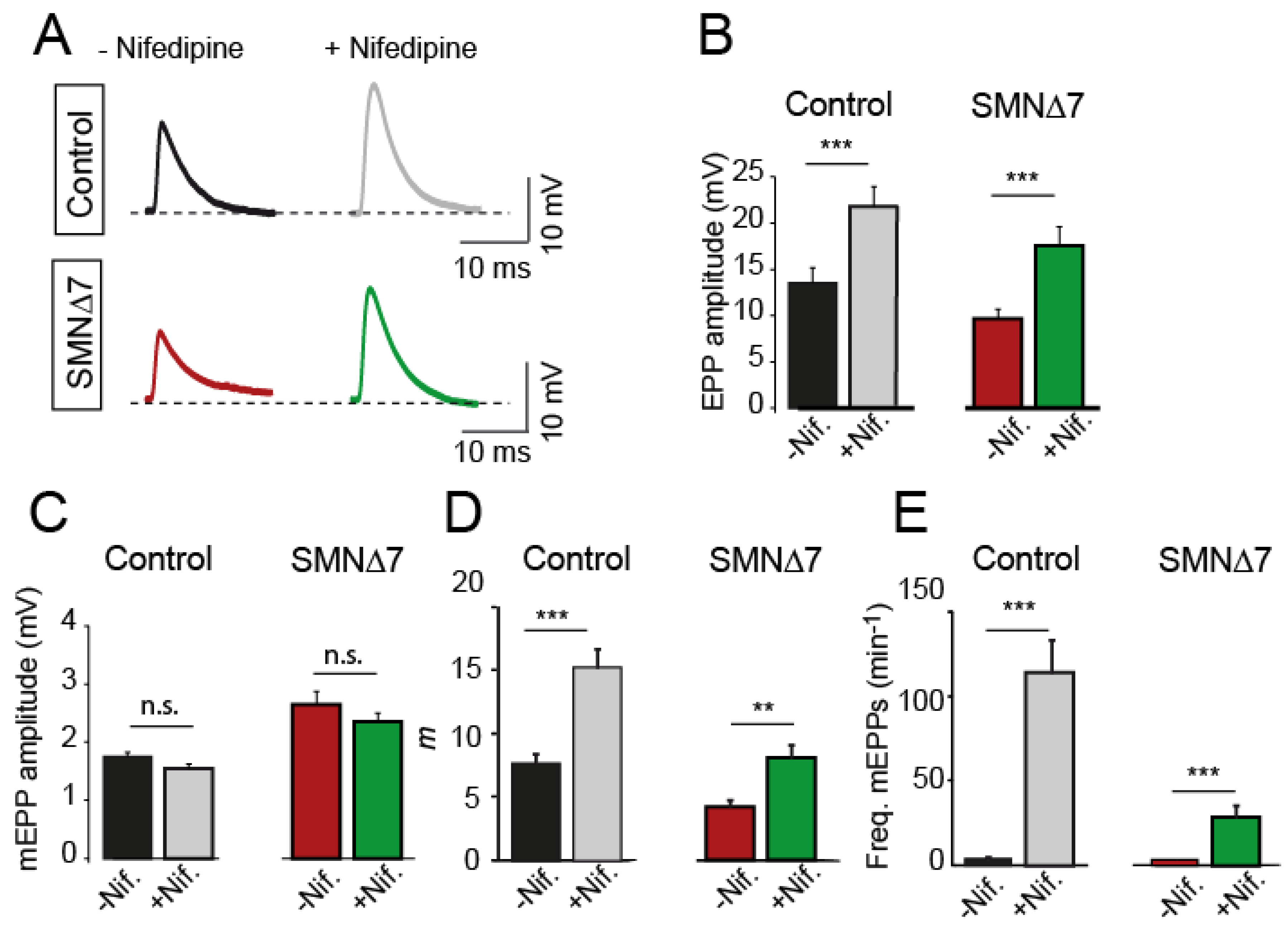
2.2. Nifedipine Increases Neurotransmission in Motor Nerve Terminals of Control and SMA Mice
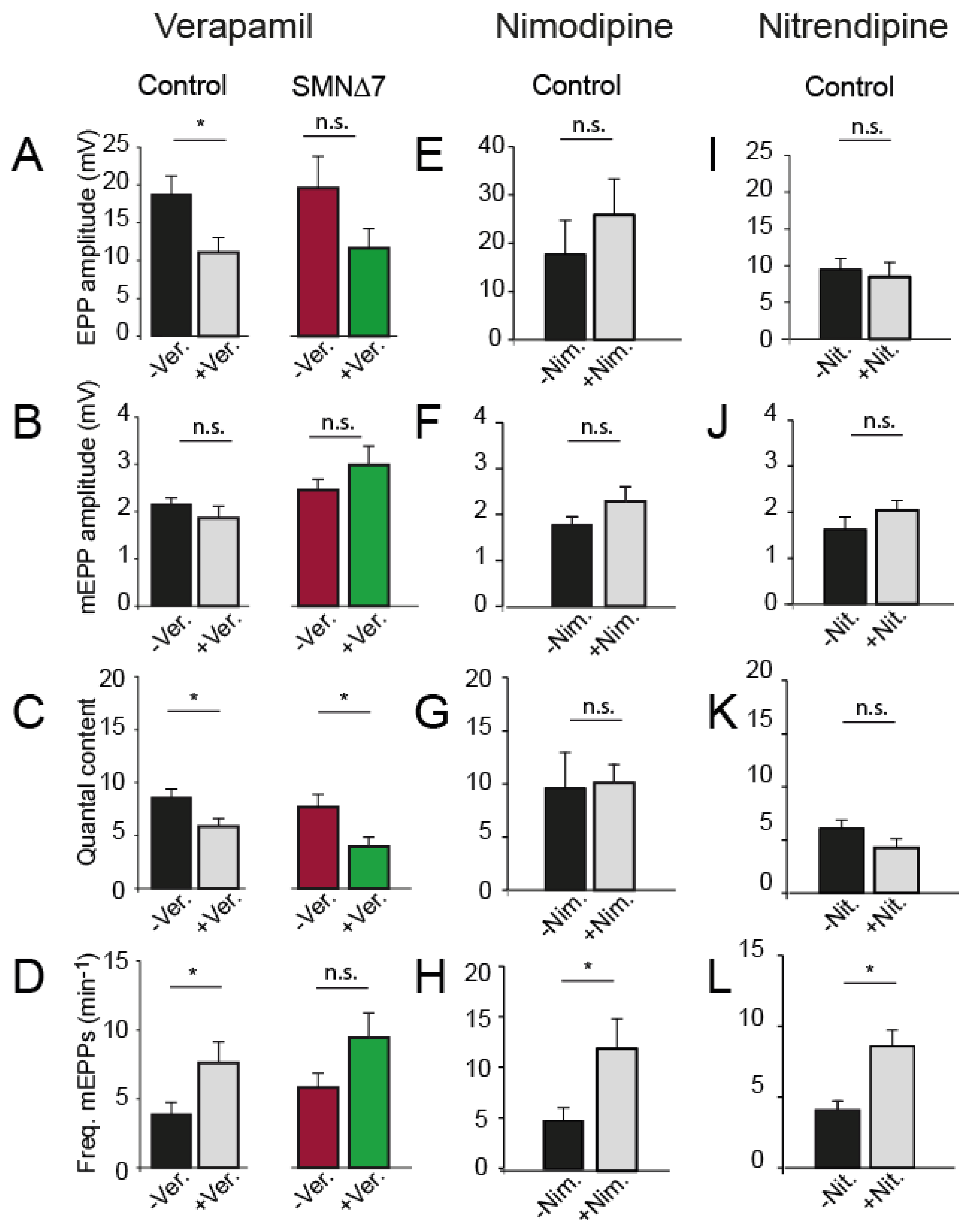
2.3. The Effect of Nifedipine on Neurotransmission Is Independent of L-Type Ca2+ Channels
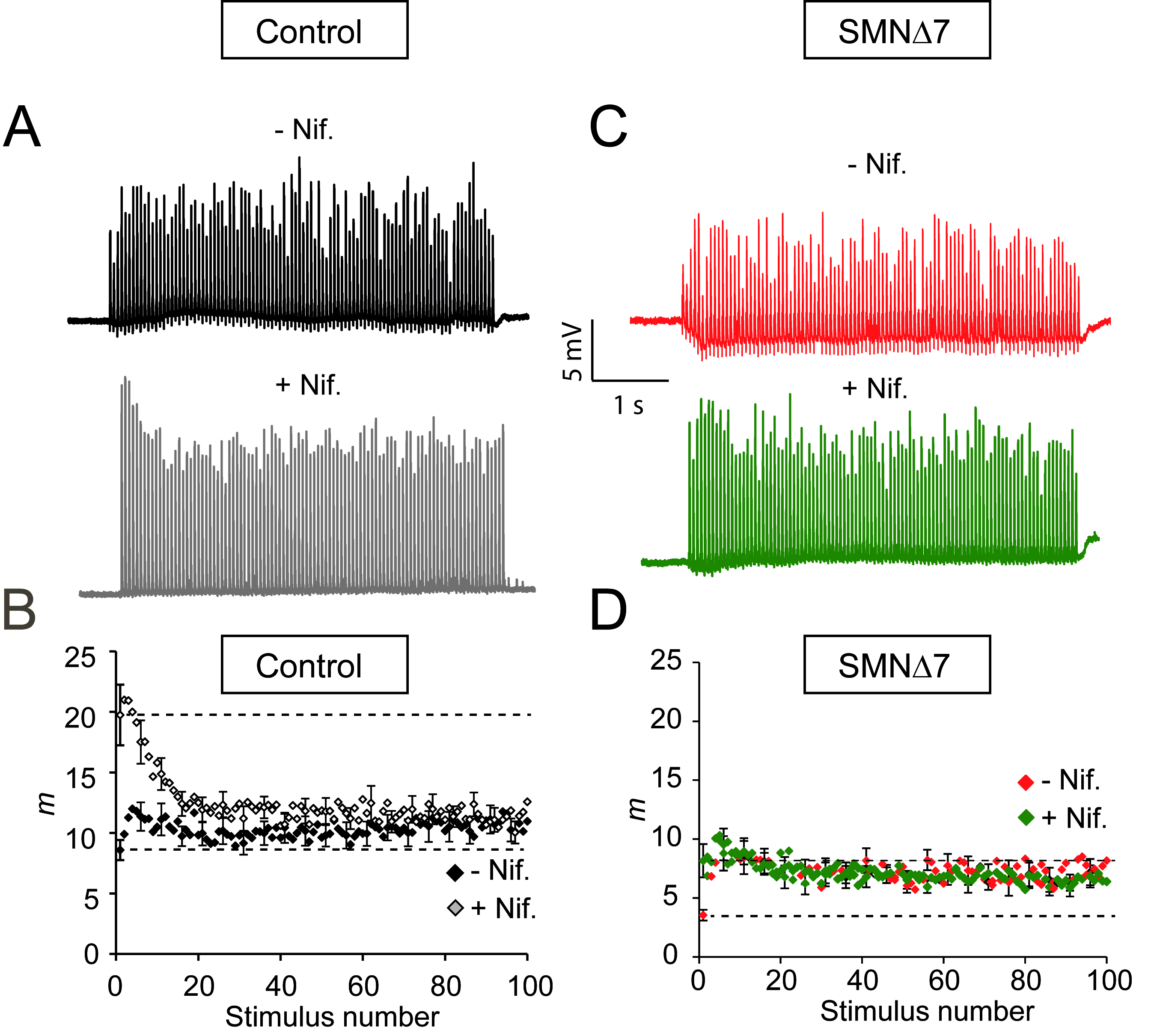
2.4. Nifedipine Increases the RRP Size in Controls but Not in SMA Terminals
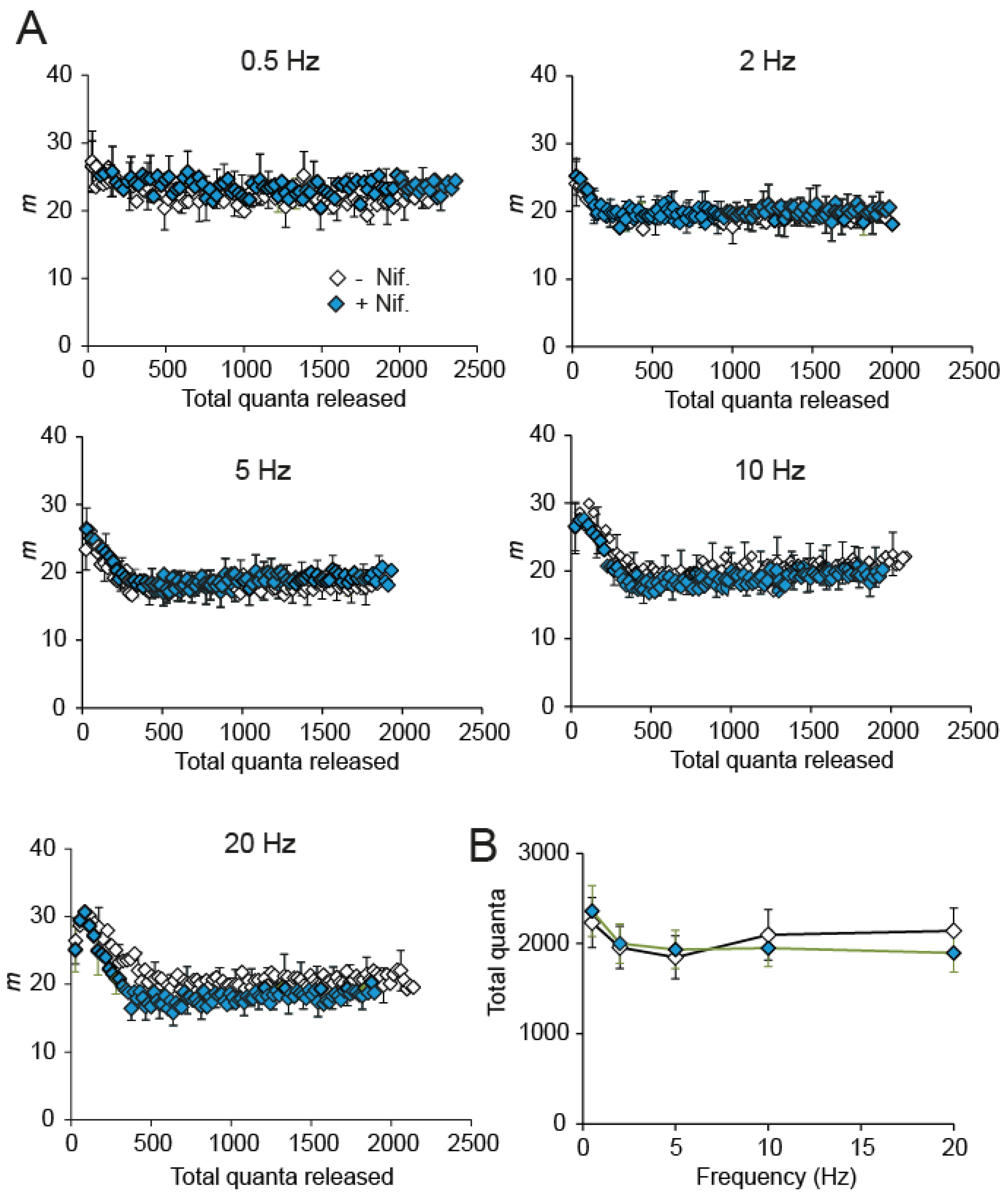
2.5. The RRP Pool Size Is Insensitive to Nifedipine in Adult Motor Nerve Terminals
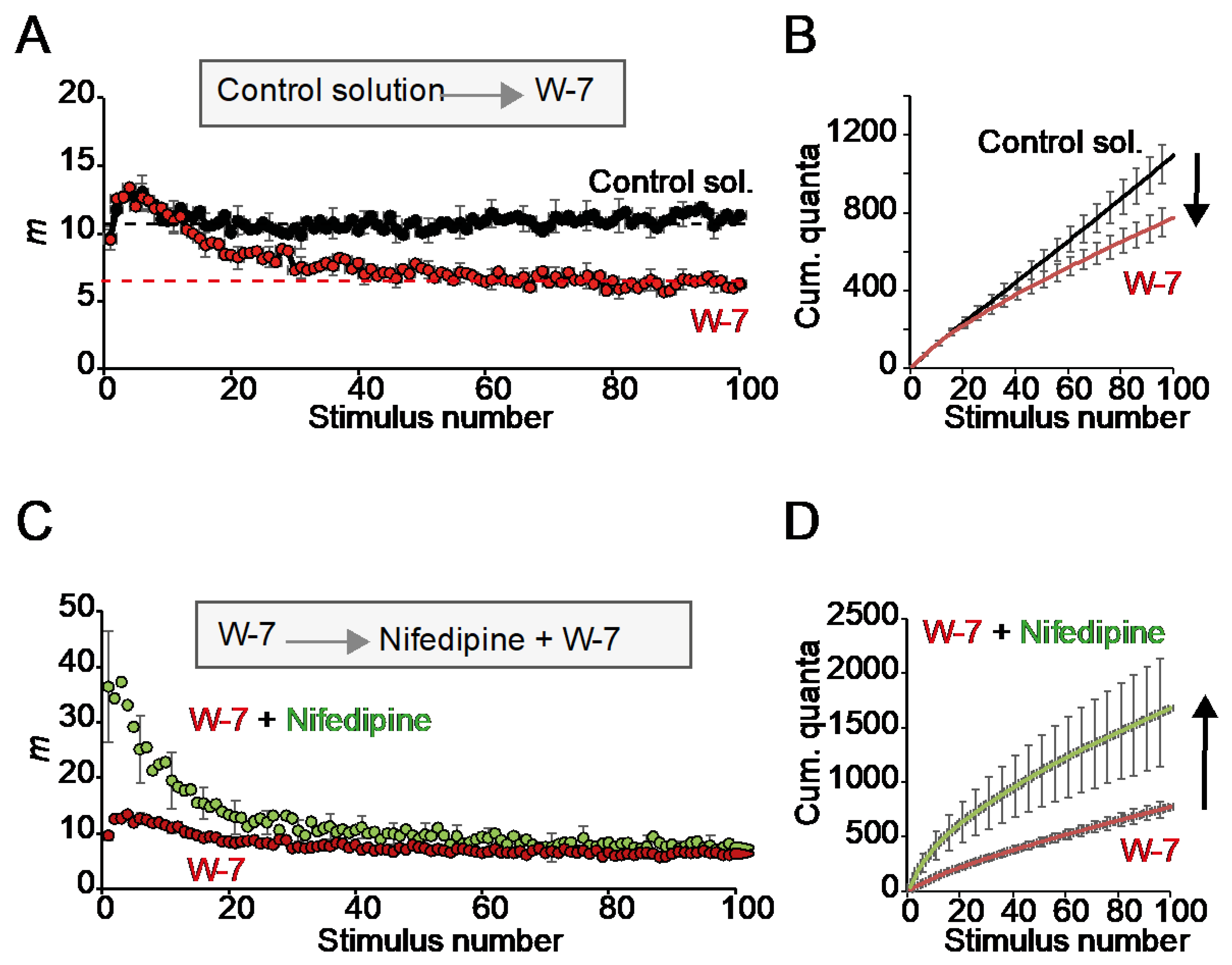
2.6. The Effect of Nifedipine on the RRP Is Calmodulin-Independent
3. Discussion
3.1. Nifedipine Beneficially Regulates the Cellular Differentiation of SMN-Deficient Motor Neurons
3.2. Age-Dependent Nifedipine Potentiation of Evoked and Spontaneous Neurotransmission at the NMJ
3.3. The Multiple Targets of L-Type Ligand Drugs and Neurotransmission
3.4. Is Nifedipine a Good Candidate for Complementary Therapy in Motor Neuron Diseases?
4. Materials and Methods
4.1. Mouse Models
4.2. Primary Embryonic Motor Neuron Cell Culture
4.3. Immunocytochemistry of Primary Cultured Embryonic Motor Neurons
4.4. Ca2+ Imaging of Primary Cultured Motor Neurons
4.5. Neuromuscular Preparation
4.6. Electrophysiological Experiments and Drugs
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and Characterization of a Spinal Muscular Atrophy-Determining Gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Burghes, A.H.M.; Beattie, C.E. Spinal Muscular Atrophy: Why Do Low Levels of Survival Motor Neuron Protein Make Motor Neurons Sick? Nat. Rev. Neurosci. 2009, 10, 597–609. [Google Scholar] [CrossRef]
- Singh, R.N.; Howell, M.D.; Ottesen, E.W.; Singh, N.N. Diverse Role of Survival Motor Neuron Protein. Biochim. Biophys. Acta-Gene Regul. Mech. 2017, 1860, 299–315. [Google Scholar] [CrossRef]
- Crawford, T.O.; Pardo, C.A. The Neurobiology of Childhood Spinal Muscular Atrophy. Neurobiol. Dis. 1996, 3, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Zerres, K.; Rudnik-Schöneborn, S.; Forrest, E.; Lusakowska, A.; Borkowska, J.; Hausmanowa-Petrusewicz, I. A Collaborative Study on the Natural History of Childhood and Juvenile Onset Proximal Spinal Muscular Atrophy (Type II and III SMA): 569 Patients. J. Neurol. Sci. 1997, 146, 67–72. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.; Mercuri, E.; Tiziano, F.D.; Bertini, E. Spinal Muscular Atrophy. Orphanet J. Rare Dis. 2011, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, J.A.; Singh, P.; Darras, B.T. Spinal Muscular Atrophy: A Clinical and Research Update. Pediatr. Neurol. 2012, 46, 1–12. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- Baranello, G.; Darras, B.T.; Day, J.W.; Deconinck, N.; Klein, A.; Masson, R.; Mercuri, E.; Rose, K.; El-Khairi, M.; Gerber, M.; et al. Risdiplam in Type 1 Spinal Muscular Atrophy. N. Engl. J. Med. 2021, 384, 915–923. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Rossoll, W.; Jablonka, S.; Andreassi, C.; Kröning, A.K.; Karle, K.; Monani, U.R.; Sendtner, M. Smn, the Spinal Muscular Atrophy-Determining Gene Product, Modulates Axon Growth and Localization of β-Actin MRNA in Growth Cones of Motoneurons. J. Cell Biol. 2003, 163, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, S.; Beck, M.; Lechner, B.D.; Mayer, C.; Sendtner, M. Defective Ca2+ Channel Clustering in Axon Terminals Disturbs Excitability in Motoneurons in Spinal Muscular Atrophy. J. Cell Biol. 2007, 179, 139–149. [Google Scholar] [CrossRef]
- Hennlein, L.; Ghanawi, H.; Gerstner, F.; Palominos García, E.; Yildirim, E.; Saal-Bauernschubert, L.; Moradi, M.; Deng, C.; Klein, T.; Appenzeller, S.; et al. Plastin 3 Rescues Cell Surface Translocation and Activation of TrkB in Spinal Muscular Atrophy. J. Cell Biol. 2023, 222, e202204113. [Google Scholar] [CrossRef] [PubMed]
- Dombert, B.; Balk, S.; Lüningschrör, P.; Moradi, M.; Sivadasan, R.; Saal-Bauernschubert, L. BDNF/TrkB Induction of Calcium Transients through Cav2.2 Calcium Channels in Motoneurons Corresponds to F-Actin Assembly and Growth Cone Formation on Β2-Chain Laminin (221). Front. Mol. Neurosci. 2017, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, X.; Choe, D.W.; Polley, M.; Burnett, B.G.; Bosch-Marcé, M.; Griffin, J.W.; Rich, M.M.; Sumner, C.J. Impaired Synaptic Vesicle Release and Immaturity of Neuromuscular Junctions in Spinal Muscular Atrophy Mice. J. Neurosci. 2009, 29, 842–851. [Google Scholar] [CrossRef]
- Ruiz, R.; Casañas, J.J.; Torres-Benito, L.; Cano, R.; Tabares, L. Altered Intracellular Ca2+ Homeostasis in Nerve Terminals of Severe Spinal Muscular Atrophy Mice. J. Neurosci. 2010, 30, 849–857. [Google Scholar] [CrossRef]
- Torres-Benito, L.; Neher, M.F.; Cano, R.; Ruiz, R.; Tabares, L. SMN Requirement for Synaptic Vesicle, Active Zone and Microtubule Postnatal Organization in Motor Nerve Terminals. PLoS ONE 2011, 6, e26164. [Google Scholar] [CrossRef]
- Tejero, R.; Lopez-Manzaneda, M.; Arumugam, S.; Tabares, L. Synaptotagmin-2, and -1, Linked to Neurotransmission Impairment and Vulnerability in Spinal Muscular Atrophy. Hum. Mol. Genet. 2016, 25, 4703–4716. [Google Scholar] [CrossRef]
- Tejero, R.; Balk, S.; Franco-Espin, J.; Ojeda, J.; Hennlein, L.; Drexl, H.; Dombert, B.; Clausen, J.D.; Torres-Benito, L.; Saal-Bauernschubert, L.; et al. R-Roscovitine Improves Motoneuron Function in Mouse Models for Spinal Muscular Atrophy. iScience 2020, 23, 100826. [Google Scholar] [CrossRef]
- Meijer, L.; Borgne, A.; Mulner, O.; Chong, J.P.J.; Blow, J.J.; Inagaki, N.; Inagaki, M.; Delcros, J.G.; Moulinoux, J.P. Biochemical and Cellular Effects of Roscovitine, a Potent and Selective Inhibitor of the Cyclin-Dependent Kinases Cdc2, Cdk2 and Cdk5. Eur. J. Biochem. 1997, 243, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Chi, P.; Bibb, J.A.; Ryan, T.A.; Greengard, P. Roscovitine: A Novel Regulator of P/Q-Type Calcium Channels and Transmitter Release in Central Neurons. J. Physiol. 2002, 540, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Ojala, K.S.; Kaufhold, C.J.; Davey, M.R.; Yang, D.; Liang, M.; Wipf, P.; Badawi, Y.; Meriney, S.D. Potentiation of Neuromuscular Transmission by a Small Molecule Calcium Channel Gating Modifier Improves Motor Function in a Severe Spinal Muscular Atrophy Mouse Model. Hum. Mol. Genet. 2023, ddad019. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Ko, C.P. Novel Modulatory Effect of L-Type Calcium Channels at Newly Formed Neuromuscular Junctions. J. Neurosci. 1997, 17, 1101–1111. [Google Scholar] [CrossRef]
- Rosato Siri, M.D.; Uchitel, O.D. Calcium Channels Coupled to Neurotransmitter Release at Neonatal Rat Neuromuscular Junctions. J. Physiol. 1999, 514, 533–540. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lien, C.C.; Cheng, C.F.; Yen, T.Y.; Chen, C.J.; Tsaur, M.L. K+ Channel Kv3.4 Is Essential for Axon Growth by Limiting the Influx of Ca2+ into Growth Cones. J. Neurosci. 2017, 37, 4433–4449. [Google Scholar] [CrossRef]
- Roy, J.; Minotti, S.; Dong, L.; Figlewicz, D.A.; Durham, H.D. Glutamate Potentiates the Toxicity of Mutant Cu/Zn-Superoxide Dismutase in Motor Neurons by Postsynaptic Calcium-Dependent Mechanisms. J. Neurosci. 1998, 18, 9673–9684. [Google Scholar] [CrossRef]
- Joshi, D.C.; Singh, M.; Krishnamurthy, K.; Joshi, P.G.; Joshi, N.B. AMPA Induced Ca2+ Influx in Motor Neurons Occurs through Voltage Gated Ca2+ Channel and Ca2+ Permeable AMPA Receptor. Neurochem. Int. 2011, 59, 913–921. [Google Scholar] [CrossRef]
- Chipman, P.H.; Schachner, M.; Rafuse, V.F. Presynaptic NCAM Is Required for Motor Neurons to Functionally Expand Their Peripheral Field of Innervation in Partially Denervated Muscles. J. Neurosci. 2014, 34, 10497–10510. [Google Scholar] [CrossRef]
- Piriz, J.; Siri, M.D.R.; Pagani, R.; Uchitel, O.D. Nifedipine-Mediated Mobilization of Intracellular Calcium Stores Increases Spontaneous Neurotransmitter Release at Neonatal Rat Motor Nerve Terminals. J. Pharmacol. Exp. Ther. 2003, 306, 658–663. [Google Scholar] [CrossRef]
- Torres-Benito, L.; Ruiz, R.; Tabares, L. Synaptic Defects in Spinal Muscular Atrophy Animal Models. Dev. Neurobiol. 2012, 72, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Bogatcheva, P.O.; Ezhova, E.V.; Balezina, O.P. Mechanism of Inhibition of Acetylcholine Secretion in Newly Formed Mouse Synapses Involving Ca2+-Dependent Kinases and Voltage-Gated K+-Channels. Bull. Exp. Biol. Med. 2010, 149, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Bogatcheva, P.O.; Balezina, O.P. Multidirectional Effects of Calmodulin Kinase II on Transmitter Release in Mature and Newly Formed Mouse Motor Synapses. Bull. Exp. Biol. Med. 2013, 154, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Elmqvist, D.; Quastel, D.M. A Quantitative Study of End—Plate Potentials in Isolated Human Muscle. J. Physiol. 1965, 178, 505–529. [Google Scholar] [CrossRef] [PubMed]
- Zucker, R.S.; Regehr, W.G. Short-Term Synaptic Plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Cano, R.; Casañas, J.J.; Gaffield, M.A.; Betz, W.J.; Tabares, L. Active Zones and the Readily Releasable Pool of Synaptic Vesicles at the Neuromuscular Junction of the Mouse. J. Neurosci. 2011, 31, 2000–2008. [Google Scholar] [CrossRef]
- Lopez-Manzaneda, M.; Franco-Espin, J.; Tejero, R.; Cano, R.; Tabares, L. Calcium Is Reduced in Presynaptic Mitochondria of Motor Nerve Terminals during Neurotransmission in SMA Mice. Hum. Mol. Genet. 2021, 30, 629–643. [Google Scholar] [CrossRef]
- Cano, R.; Torres-Benito, L.; Tejero, R.; Biea, A.I.; Ruiz, R.; Betz, W.J.; Tabares, L. Structural and Functional Maturation of Active Zones in Large Synapses. Mol. Neurobiol. 2013, 47, 209–219. [Google Scholar] [CrossRef]
- Kim, J.K.; Jha, N.N.; Awano, T.; Caine, C.; Gollapalli, K.; Welby, E.; Kim, S.S.; Fuentes-Moliz, A.; Wang, X.; Feng, Z.; et al. A Spinal Muscular Atrophy Modifier Implicates the SMN Protein in SNARE Complex Assembly at Neuromuscular Synapses. Neuron 2023, 111, 1–17. [Google Scholar] [CrossRef]
- Lopez-Manzaneda, M.; Fuentes-Moliz, A.; Tabares, L. Presynaptic Mitochondria Communicate With Release Sites for Spatio-Temporal Regulation of Exocytosis at the Motor Nerve Terminal. Front. Synaptic Neurosci. 2022, 14, 858340. [Google Scholar] [CrossRef]
- Sakaba, T.; Neher, E. Calmodulin Mediates Rapid Recruitment of Fast-Releasing Synaptic Vesicles at a Calyx-Type Synapse. Neuron 2001, 32, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Junge, H.J.; Rhee, J.S.; Jahn, O.; Varoqueaux, F.; Spiess, J.; Waxham, M.N.; Rosenmund, C.; Brose, N. Calmodulin and Munc13 Form a Ca2+ Sensor/Effector Complex That Controls Short-Term Synaptic Plasticity. Cell 2004, 118, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Lipstein, N.; Sakaba, T.; Cooper, B.H.; Lin, K.H.; Strenzke, N.; Ashery, U.; Rhee, J.S.; Taschenberger, H.; Neher, E.; Brose, N. Dynamic Control of Synaptic Vesicle Replenishment and Short-Term Plasticity by Ca2+-Calmodulin-Munc13-1 Signaling. Neuron 2013, 79, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Ojala, K.S.; Reedich, E.J.; Didonato, C.J.; Meriney, S.D. In Search of a Cure: The Development of Therapeutics to Alter the Progression of Spinal Muscular Atrophy. Brain Sci. 2021, 11, 194. [Google Scholar] [CrossRef]
- Nishimune, H.; Sanes, J.R.; Carlson, S.S. A Synaptic Laminin-Calcium Channel Interaction Organizes Active Zones in Motor Nerve Terminals. Nature 2004, 432, 580–587. [Google Scholar] [CrossRef]
- Jun, K.; Piedras-Rentería, E.S.; Smith, S.M.; Wheeler, D.B.; Lee, S.B.; Lee, T.G.; Chin, H.; Adams, M.E.; Scheller, R.H.; Tsien, R.W.; et al. Ablation of P/Q-Type Ca2+ Channel Currents, Altered Synaptic Transmission, and Progressive Ataxia in Mice Lacking the α(1A)-Subunit. Proc. Natl. Acad. Sci. USA 1999, 96, 15245–15250. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J. Multiple Calcium Channels and Neuronal Function. Science 1987, 235, 46–52. [Google Scholar] [CrossRef]
- Cho, S.; Meriney, S.D. The Effects of Presynaptic Calcium Channel Modulation by Roscovitine on Transmitter Release at the Adult Frog Neuromuscular Junction. Eur. J. Neurosci. 2006, 23, 3200–3208. [Google Scholar] [CrossRef]
- Bos, R.; Harris-Warrick, R.M.; Brocard, C.; Demianenko, L.E.; Manuel, M.; Zytnicki, D.; Korogod, S.M.; Brocard, F. Kv1.2 Channels Promote Nonlinear Spiking Motoneurons for Powering Up Locomotion. Cell Rep. 2018, 22, 3315–3327. [Google Scholar] [CrossRef]
- Buraei, Z.; Schofield, G.; Elmslie, K.S. Roscovitine Differentially Affects CaV2 and Kv Channels by Binding to the Open State. Neuropharmacology 2007, 52, 883–894. [Google Scholar] [CrossRef]
- Cernuda, B.; Fernandes, C.T.; Allam, S.M.; Orzillo, M.; Suppa, G.; Chang, Z.C.; Athanasopoulos, D.; Buraei, Z. The Molecular Determinants of R-Roscovitine Block of HERG Channels. PLoS ONE 2019, 14, e0217733. [Google Scholar] [CrossRef] [PubMed]
- Tse, F.W.; Tse, A.; Hille, B.; Horstmann, H.; Almers, W. Local Ca2+ Release from Internal Stores Controls Exocytosis in Pituitary Gonadotrophs. Neuron 1997, 18, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, M.; Pittman, Q.J. Nifedipine Facilitates Neurotransmitter Release Independently of Calcium Channels. Proc. Natl. Acad. Sci. USA 2003, 100, 6139–6144. [Google Scholar] [CrossRef]
- Fedchyshyn, M.J.; Wang, L.Y. Developmental Transformation of the Release Modality at the Calyx of Held Synapse. J. Neurosci. 2005, 25, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Leão, R.M.; von Gersdorff, H. Synaptic Vesicle Pool Size, Release Probability and Synaptic Depression Are Sensitive to Ca2+ Buffering Capacity in the Developing Rat Calyx of Held. Brazilian J. Med. Biol. Res. 2009, 42, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Santafé, M.M.; Salon, I.; Garcia, N.; Lanuza, M.A.; Uchitel, O.D.; Tomàs, J. Muscarinic Autoreceptors Related with Calcium Channels in the Strong and Weak Inputs at Polyinnervated Developing Rat Neuromuscular Junctions. Neuroscience 2004, 123, 61–73. [Google Scholar] [CrossRef]
- Zhilyakov, N.; Arkhipov, A.; Malomouzh, A.; Samigullin, D. Activation of Neuronal Nicotinic Receptors Inhibits Acetylcholine Release in the Neuromuscular Junction by Increasing Ca2+ Flux through Cav1 Channels. Int. J. Mol. Sci. 2021, 22, 9031. [Google Scholar] [CrossRef]
- Li, X.T.; Li, X.Q.; Hu, X.M.; Qiu, X.Y. The Inhibitory Effects of Ca2+ Channel Blocker Nifedipine on Rat Kv2.1 Potassium Channels. PLoS ONE 2015, 10, e0124602. [Google Scholar] [CrossRef]
- Monani, U.R.; Coovert, D.D.; Burghes, A.H.M. Animal Models of Spinal Muscular Atrophy. Hum. Mol. Genet. 2000, 9, 2451–2457. [Google Scholar] [CrossRef]
- McLachlan, E.M.; Martin, A.R. Non-Linear Summation of End-Plate Potentials in the Frog and Mouse. J. Physiol. 1981, 311, 307–324. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejero, R.; Alsakkal, M.; Hennlein, L.; Lopez-Cabello, A.M.; Jablonka, S.; Tabares, L. Nifedipine Ameliorates Cellular Differentiation Defects of Smn-Deficient Motor Neurons and Enhances Neuromuscular Transmission in SMA Mice. Int. J. Mol. Sci. 2023, 24, 7648. https://doi.org/10.3390/ijms24087648
Tejero R, Alsakkal M, Hennlein L, Lopez-Cabello AM, Jablonka S, Tabares L. Nifedipine Ameliorates Cellular Differentiation Defects of Smn-Deficient Motor Neurons and Enhances Neuromuscular Transmission in SMA Mice. International Journal of Molecular Sciences. 2023; 24(8):7648. https://doi.org/10.3390/ijms24087648
Chicago/Turabian StyleTejero, Rocio, Mohammad Alsakkal, Luisa Hennlein, Ana M. Lopez-Cabello, Sibylle Jablonka, and Lucia Tabares. 2023. "Nifedipine Ameliorates Cellular Differentiation Defects of Smn-Deficient Motor Neurons and Enhances Neuromuscular Transmission in SMA Mice" International Journal of Molecular Sciences 24, no. 8: 7648. https://doi.org/10.3390/ijms24087648
APA StyleTejero, R., Alsakkal, M., Hennlein, L., Lopez-Cabello, A. M., Jablonka, S., & Tabares, L. (2023). Nifedipine Ameliorates Cellular Differentiation Defects of Smn-Deficient Motor Neurons and Enhances Neuromuscular Transmission in SMA Mice. International Journal of Molecular Sciences, 24(8), 7648. https://doi.org/10.3390/ijms24087648







