HD-ZIP Transcription Factors and Brassinosteroid Signaling Play a Role in Capitulum Patterning in Chrysanthemum
Abstract
1. Introduction
2. Results
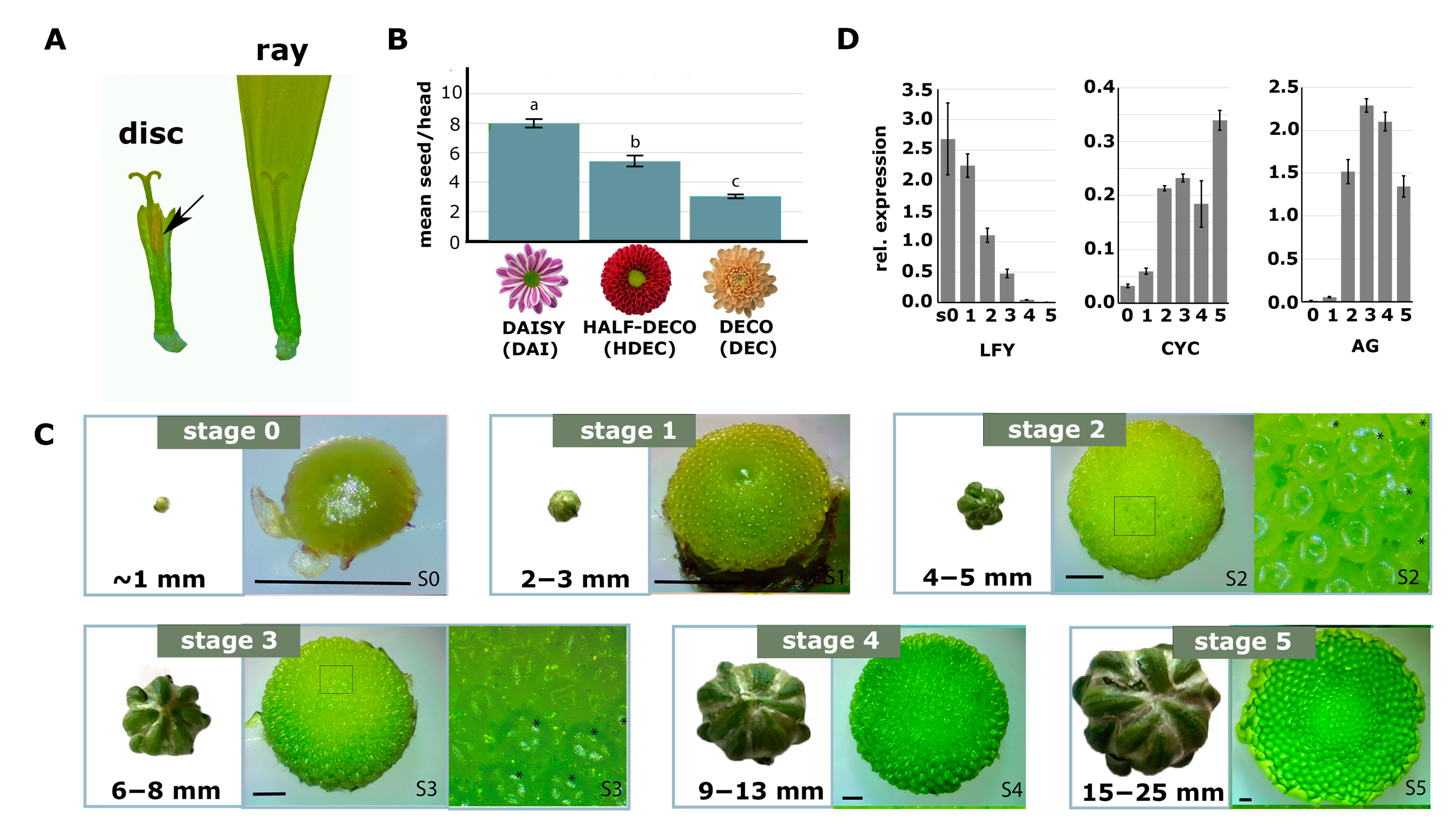
2.1. Chrysanthemum Types with Less Disc Florets Produce Fewer Seeds
2.2. Identification and Characterization of a Mutant with Altered Disc:Ray Floret Ratio
2.3. Differential Expression Analysis Reveals Genes Possibly Involved in the Regulation of the Disc:Ray Floret Ratio
2.4. Differential Gene Expression in a Second Genetic Background with Altered Disc:Ray Floret Ratio
2.5. Cycloidea Genes Are Only Differentially Expressed at Later Stages
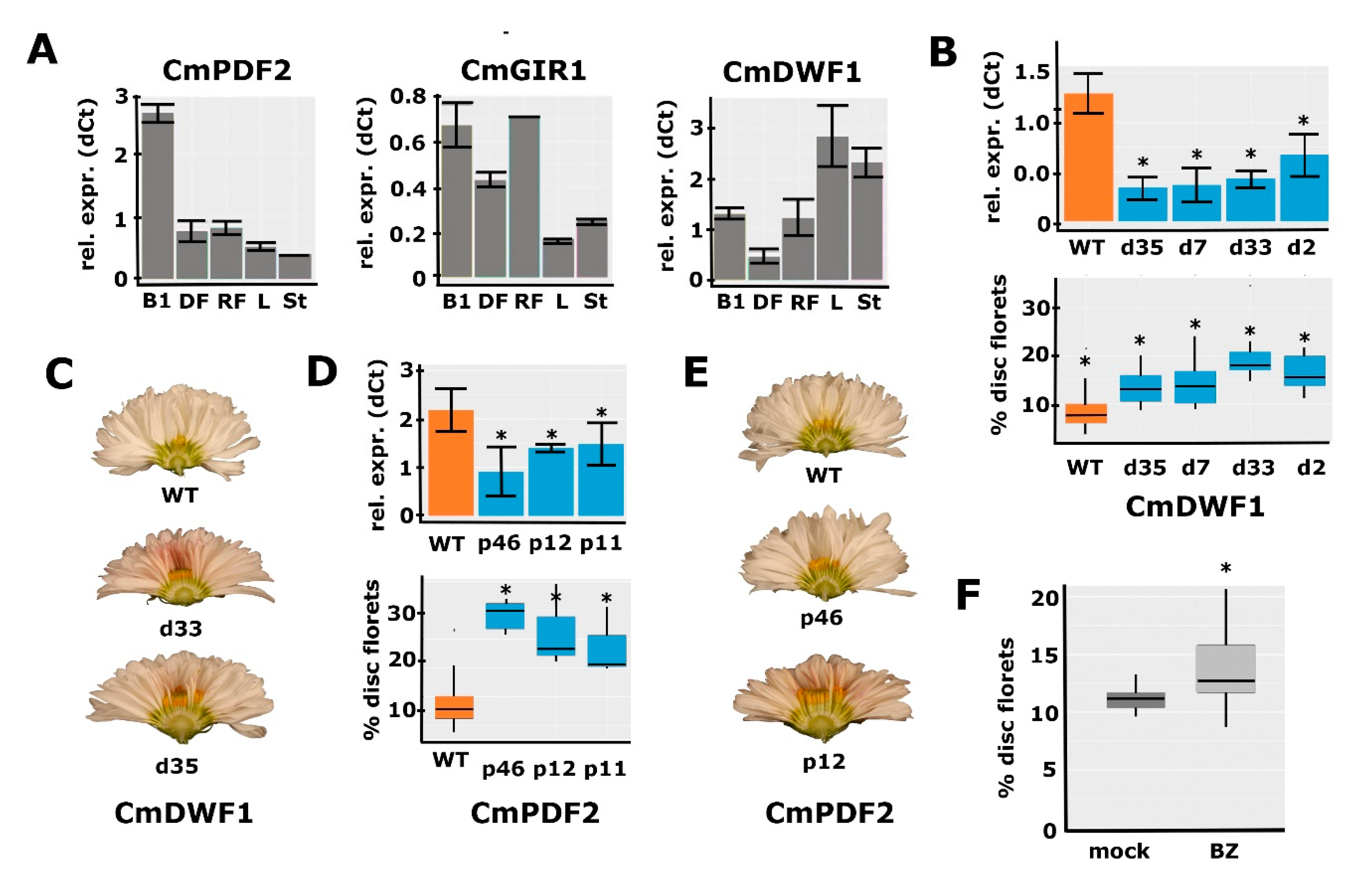
2.6. Functional Analysis for a Selection of Identified Candidate Genes
2.7. Confirmation of the Role of BR in Disc:Ray Floret Ratio by Brassinazole Treatments
3. Discussion
3.1. Pros and Cons of the Followed Transcriptomics Approach
3.2. Does BR Represent a Capitulum Patterning Hormone in Asteraceae?
3.3. A Defined Role for HD-ZIP IV Transcription Factors in the Regulation of Disc:Ray Floret Ratio
3.4. Overall Conclusions and Implementation
4. Materials and Methods
4.1. Determination of Average Seed Set Per Capitulum Type
4.2. Plant Growth Conditions and Phenotyping
4.3. Sampling and RNA-Extraction
4.4. Library Preparation for RNA-Seq
4.5. RNAseq Data Analysis
4.6. qPCR Analysis
4.7. Cloning of Constructs for Transformation
4.8. Plant Transformation
4.9. Brassinolide and Brassinazole Treatments
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gillies, A.C.M.; Cubas, P.; Coen, E.S.; Abbott, R.J. Making rays in the Asteraceae: Genetics and evolution of variation for radiate versus discoid flower heads. In Developmental Genetics and Plant Evolution; Cronk, Q.C.B., Bateman, R.M., Hawkins, J.A., Eds.; CRC Press: London, UK, 2002; pp. 233–246. [Google Scholar]
- Harris, E.M. Inflorescence and floral ontogeny in asteraceae: A synthesis of historical and current concepts. Bot. Rev. 1995, 61, 93–278. [Google Scholar] [CrossRef]
- Kim, M.; Cui, M.L.; Cubas, P.; Gillies, A.; Lee, K.; Chapman, M.A.; Abbott, R.J.; Coen, E. Regulatory genes control a key morphological and ecological trait transferred between species. Science 2008, 322, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.O. Chrysanthemum. In Flower Breeding and Genetics: Issues, Challenges and Opportunities for the 21st Century; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 389–437. [Google Scholar]
- Broholm, S.K.; Teeri, T.H.; Elomaa, P. Chapter ten—Molecular control of inflorescence development in Asteraceae. In Advances in Botanical Research; Fornara, F., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 72, pp. 297–333. [Google Scholar]
- Tähtiharju, S.; Rijpkema, A.S.; Vetterli, A.; Albert, V.A.; Teeri, T.H.; Elomaa, P. Evolution and diversification of the cyc/tb1 gene family in asteraceae—A comparative study in gerbera (mutisieae) and sunflower (heliantheae). Mol. Biol. Evol. 2012, 29, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Broholm, S.K.; Tähtiharju, S.; Laitinen, R.A.; Albert, V.A.; Teeri, T.H.; Elomaa, P. A tcp domain transcription factor controls flower type specification along the radial axis of the gerbera (asteraceae) inflorescence. Proc. Natl. Acad. Sci. USA 2008, 105, 9117–9122. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Tang, S.; Draeger, D.; Nambeesan, S.; Shaffer, H.; Barb, J.G.; Knapp, S.J.; Burke, J.M. Genetic analysis of floral symmetry in van gogh’s sunflowers reveals independent recruitment of cycloidea genes in the asteraceae. PLoS Genet. 2012, 8, e1002628. [Google Scholar] [CrossRef]
- Juntheikki-Palovaara, I.; Tähtiharju, S.; Lan, T.; Broholm, S.K.; Rijpkema, A.S.; Ruonala, R.; Kale, L.; Albert, V.A.; Teeri, T.H.; Elomaa, P. Functional diversification of duplicated cyc2 clade genes in regulation of inflorescence development in gerbera hybrida (asteraceae). Plant J. 2014, 79, 783–796. [Google Scholar] [CrossRef]
- Chen, J.; Shen, C.Z.; Guo, Y.P.; Rao, G.Y. Patterning the asteraceae capitulum: Duplications and differential expression of the flower symmetry cyc2-like genes. Front. Plant Sci. 2018, 9, 551. [Google Scholar] [CrossRef]
- Fambrini, M.; Bellanca, M.; Costa Muñoz, M.; Usai, G.; Cavallini, A.; Pugliesi, C. Ligulate inflorescence of helianthus × multiflorus, cv. Soleil d’or, correlates with a mis-regulation of a cycloidea gene characterised by insertion of a transposable element. Plant Biol. 2018, 20, 956–967. [Google Scholar] [CrossRef]
- Garcês, H.M.P.; Spencer, V.M.R.; Kim, M. Control of floret symmetry by ray3, svdiv1b, and svrad in the capitulum of senecio vulgaris. Plant Physiol. 2016, 171, 2055–2068. [Google Scholar] [CrossRef]
- Huang, D.; Li, X.; Sun, M.; Zhang, T.; Pan, H.; Cheng, T.; Wang, J.; Zhang, Q. Identification and characterization of cyc-like genes in regulation of ray floret development in chrysanthemum morifolium. Front. Plant Sci. 2016, 7, 1633. [Google Scholar] [CrossRef]
- Shen, C.Z.; Chen, J.; Zhang, C.J.; Rao, G.Y.; Guo, Y.P. Dysfunction of cyc2g is responsible for the evolutionary shift from radiate to disciform flowerheads in the chrysanthemum group (asteraceae: Anthemideae). Plant J. Cell Mol. Biol. 2021, 106, 1024–1038. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Han, M.; Yao, W.; Wang, Y. Transcriptome analysis reveals the regulation of brassinosteroids on petal growth in gerbera hybrida. PeerJ 2017, 5, e3382. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Li, L.; Peng, J.; Sun, S.; Wang, X. Transcriptome analysis of gerbera hybrida ray florets: Putative genes associated with gibberellin metabolism and signal transduction. PLoS ONE 2013, 8, e57715. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, M.; Du, D.; Pan, H.; Cheng, T.; Wang, J.; Zhang, Q. Whole-transcriptome analysis of differentially expressed genes in the vegetative buds, floral buds and buds of chrysanthemum morifolium. PLoS ONE 2015, 10, e0128009. [Google Scholar] [CrossRef]
- Liu, H.; Jia, Y.; Chai, Y.; Wang, S.; Chen, H.; Zhou, X.; Huang, C.; Guo, S.; Chen, D. Whole-transcriptome analysis of differentially expressed genes between ray and disc florets and identification of flowering regulatory genes in Chrysanthemum morifolium. Front. Plant Sci. 2022, 13, 947331. [Google Scholar] [CrossRef]
- Wen, X.; Qi, S.; Huang, H.; Wu, X.; Zhang, B.; Fan, G.; Yang, L.; Hong, Y.; Dai, S. The expression and interactions of abce-class and cyc2-like genes in the capitulum development of Chrysanthemum lavandulifolium and c. × morifolium. Plant Growth Regul. 2019, 88, 205–214. [Google Scholar] [CrossRef]
- Pu, Y.; Huang, H.; Wen, X.; Lu, C.; Zhang, B.; Gu, X.; Qi, S.; Fan, G.; Wang, W.; Dai, S. Comprehensive transcriptomic analysis provides new insights into the mechanism of ray floret morphogenesis in chrysanthemum. BMC Genom. 2020, 21, 728. [Google Scholar] [CrossRef]
- Fan, J.; Huang, J.; Pu, Y.; Niu, Y.; Zhang, M.; Dai, S.; Huang, H. Transcriptomic analysis reveals the formation mechanism of anemone-type flower in chrysanthemum. BMC Genom. 2022, 23, 846. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, J.; He, J.; Geng, Z.; Li, S.; Zhang, J.; Li, P.; Zhang, L.; Wang, Z.; Wang, L.; et al. Whole-transcriptome profiles of Chrysanthemum seticuspe improve genome annotation and shed new light on mrna–mirna–lncrna networks in ray florets and disc florets. BMC Plant Biol. 2022, 22, 515. [Google Scholar] [CrossRef]
- Zhang, T.; Elomaa, P. Don’t be fooled: False flowers in asteraceae. Curr. Opin. Plant Biol. 2021, 59, 101972. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.; Broholm, S.K.; Tähtiharju, S.; Mouhu, K.; Albert, V.A.; Teeri, T.H.; Elomaa, P. Evolutionary co-option of floral meristem identity genes for patterning of the flower-like Asteraceae inflorescence. Plant Physiol. 2016, 172, 284–296. [Google Scholar] [PubMed]
- Yang, Y.; Sun, M.; Yuan, C.; Han, Y.; Zheng, T.; Cheng, T.; Wang, J.; Zhang, Q. Interactions between wuschel- and cyc2-like transcription factors in regulating the development of reproductive organs in Chrysanthemum morifolium. Int. J. Mol. Sci. 2019, 20, 1276. [Google Scholar] [CrossRef] [PubMed]
- Shchennikova, A.V.; Shulga, O.A.; Immink, R.; Skryabin, K.G.; Angenent, G.C. Identification and characterization of four chrysanthemum mads-box genes, belonging to the apetala1/fruitfull and sepallata3 subfamilies. Plant Physiol. 2004, 134, 1632–1641. [Google Scholar] [CrossRef]
- Yoshioka, S.; Aida, R.; Yamamizo, C.; Shibata, M.; Ohmiya, A. The carotenoidcleavagedioxygenase4(cmccd4a) gene family encodes a key regulator of petal color mutation in chrysanthemum. Euphytica 2012, 184, 377–387. [Google Scholar] [CrossRef]
- Renault, H.; Alber, A.; Horst, N.A.; Basilio Lopes, A.; Fich, E.A.; Kriegshauser, L.; Wiedemann, G.; Ullmann, P.; Herrgott, L.; Erhardt, M.; et al. A phenol-enriched cuticle is ancestral to lignin evolution in land plants. Nat. Commun. 2017, 8, 14713. [Google Scholar] [CrossRef]
- Li, Z.; He, Y. Roles of brassinosteroids in plant reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef]
- Choe, S.; Dilkes, B.P.; Gregory, B.D.; Ross, A.S.; Yuan, H.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Tanaka, A.; Yoshida, S.; et al. The arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 1999, 119, 897–907. [Google Scholar] [CrossRef]
- Lv, M.; Li, J. Molecular mechanisms of brassinosteroid-mediated responses to changing environments in arabidopsis. Int. J. Mol. Sci. 2020, 21, 2737. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Fujioka, S.; Takatsuto, S.; Matsumoto, S.; Gou, X.; He, K.; Russell, S.D.; Li, J. Ben1, a gene encoding a dihydroflavonol 4-reductase (dfr)-like protein, regulates the levels of brassinosteroids in Arabidopsis thaliana. Plant J. 2007, 51, 220–233. [Google Scholar] [CrossRef]
- Guo, H.; Li, L.; Ye, H.; Yu, X.; Algreen, A.; Yin, Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2009, 106, 7648–7653. [Google Scholar] [CrossRef]
- Nakamura, M.; Katsumata, H.; Abe, M.; Yabe, N.; Komeda, Y.; Yamamoto, K.T.; Takahashi, T. Characterization of the class iv homeodomain-leucine zipper gene family in arabidopsis. Plant Physiol. 2006, 141, 1363–1375. [Google Scholar] [CrossRef]
- Wu, R.; Citovsky, V. Adaptor proteins gir1 and gir2. I. Interaction with the repressor glabra2 and regulation of root hair development. Biochem. Biophys. Res. Commun. 2017, 488, 547–553. [Google Scholar] [CrossRef]
- Wu, R.; Citovsky, V. Adaptor proteins gir1 and gir2. Ii. Interaction with the co-repressor topless and promotion of histone deacetylation of target chromatin. Biochem. Biophys. Res. Commun. 2017, 488, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Melsen, K.; van de Wouw, M.; Contreras, R. Mutation breeding in ornamentals. HortScience Horts 2021, 56, 1154–1165. [Google Scholar] [CrossRef]
- Costa, M.M.; Fox, S.; Hanna, A.I.; Baxter, C.; Coen, E. Evolution of regulatory interactions controlling floral asymmetry. Development 2005, 132, 5093–5101. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Xiao, W.; Guo, W.; Yao, X.; Xiao, J.; Ye, Z.; Wang, N.; Jiao, K.; Lei, M.; Peng, Q.; et al. The cycloidea–radialis module regulates petal shape and pigmentation, leading to bilateral corolla symmetry in Torenia fournieri (linderniaceae). New Phytol. 2017, 215, 1582–1593. [Google Scholar] [CrossRef]
- Valoroso, M.C.; Sobral, R.; Saccone, G.; Salvemini, M.; Costa, M.M.R.; Aceto, S. Evolutionary conservation of the orchid myb transcription factors div, rad, and drif. Front. Plant Sci. 2019, 10, 1359. [Google Scholar] [CrossRef] [PubMed]
- Fukai, S.; Jong, J.d.; Rademaker, W. Efficient genetic transformation of chrysanthemum (Dendranthema grandiflorum (ramat.) kitamura) using stem segments. Jpn. J. Breed. 1995, 45, 179–184. [Google Scholar] [CrossRef]
- Visser, P.B.; de Maagd, R.A.; Jongsma, M.A. Chrysanthemum. In Transgenic Crops VI; Pua, E.-C., Davey, M.R., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2007; pp. 253–272. [Google Scholar]
- Neumann, M.; Xu, X.; Smaczniak, C.; Schumacher, J.; Yan, W.; Blüthgen, N.; Greb, T.; Jönsson, H.; Traas, J.; Kaufmann, K.; et al. A 3d gene expression atlas of the floral meristem based on spatial reconstruction of single nucleus rna sequencing data. Nat. Commun. 2022, 13, 2838. [Google Scholar] [CrossRef] [PubMed]
- Zoulias, N.; Duttke, S.H.C.; Garcês, H.; Spencer, V.; Kim, M. The role of auxin in the pattern formation of the asteraceae flower head (capitulum). Plant Physiol. 2018, 179, 391–401. [Google Scholar] [CrossRef]
- Zhang, T.; Cieslak, M.; Owens, A.; Wang, F.; Broholm, S.K.; Teeri, T.H.; Elomaa, P.; Prusinkiewicz, P. Phyllotactic patterning of gerbera flower heads. Proc. Natl. Acad. Sci. USA 2021, 118, e2016304118. [Google Scholar] [CrossRef]
- Ceserani, T.; Trofka, A.; Gandotra, N.; Nelson, T. Vh1/brl2 receptor-like kinase interacts with vascular-specific adaptor proteins vit and vik to influence leaf venation. Plant J. 2009, 57, 1000–1014. [Google Scholar] [CrossRef]
- Xie, Z.; Nolan, T.; Jiang, H.; Tang, B.; Zhang, M.; Li, Z.; Yin, Y. The ap2/erf transcription factor tiny modulates brassinosteroid-regulated plant growth and drought responses in arabidopsis. Plant Cell 2019, 31, 1788–1806. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Liu, Y.; Yang, Y.; Chen, H.; Cheng, H.; Hu, Q.; Zhang, Z.; Gao, J.; Zhang, J.; Ding, L.; et al. Cmbes1 is a regulator of boundary formation in chrysanthemum ray florets. Hortic. Res. 2020, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Li, J.; Wang, L.; Lu, C.; Gao, Q.; Xu, P.; Pu, Y.; Zhang, Q.; Hong, Y.; Hong, L.; et al. The Chrysanthemum lavandulifolium genome and the molecular mechanism underlying diverse capitulum types. Hortic. Res. 2022, 9, uhab022. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Thames, S.; Best, N.B.; Jiang, H.; Huang, P.; Dilkes, B.P.; Eveland, A.L. Brassinosteroids modulate meristem fate and differentiation of unique inflorescence morphology in Setaria viridis. Plant Cell 2017, 30, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Kamata, N.; Okada, H.; Komeda, Y.; Takahashi, T. Mutations in epidermis-specific hd-zip iv genes affect floral organ identity in Arabidopsis thaliana. Plant J. 2013, 75, 430–440. [Google Scholar] [CrossRef]
- Kamata, N.; Sugihara, A.; Komeda, Y.; Takahashi, T. Allele-specific effects of pdf2 on floral morphology in Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e27417. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Katsumata, H.; Komeda, Y.; Takahashi, T. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 2003, 130, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Takahashi, T.; Komeda, Y. Identification of a cis-regulatory element for l1 layer-specific gene expression, which is targeted by an l1-specific homeodomain protein. Plant J. 2001, 26, 487–494. [Google Scholar] [CrossRef]
- Takada, S.; Takada, N.; Yoshida, A. Atml1 promotes epidermal cell differentiation in Arabidopsis shoots. Development 2013, 140, 1919–1923. [Google Scholar] [CrossRef]
- Nagata, K.; Abe, M. A conserved mechanism determines the activity of two pivotal transcription factors that control epidermal cell differentiation in Arabidopsis thaliana. J. Plant Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Savaldi-Goldstein, S.; Peto, C.; Chory, J. The epidermis both drives and restricts plant shoot growth. Nature 2007, 446, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yan, A.; Li, L.; Zhu, Y.; Feng, B.; Liu, X.; Zhou, Y. A signal cascade originated from epidermis defines apical-basal patterning of arabidopsis shoot apical meristems. Nat. Commun. 2020, 11, 1214. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Yamada, Y.; Sezaki, N.; Kosaka, S.; Kondo, H.; Kamata, N.; Abe, M.; Komeda, Y.; Takahashi, T. Atml1 and pdf2 play a redundant and essential role in arabidopsis embryo development. Plant Cell Physiol. 2015, 56, 1183–1192. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Li, S.; Zhang, W.; Yin, C.; Lin, Y. Regulation of phytohormones on the growth and development of plant root hair. Front. Plant Sci. 2022, 13, 865302. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.-Y.; Bai, M.-Y.; Arenhart, R.A.; Sun, Y.; Wang, Z.-Y. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife 2014, 3, e03031. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Yang, L.; Wen, X.; Hong, Y.; Song, X.; Zhang, M.; Dai, S. Reference gene selection for rt-qpcr analysis of flower development in Chrysanthemum morifolium and Chrysanthemum lavandulifolium. Front. Plant Sci. 2016, 7, 287. [Google Scholar] [CrossRef]
- Aida, R.; Komano, M.; Saito, M.; Nakase, K.; Murai, K. Chrysanthemum flower shape modification by suppression of chrysanthemum-agamous gene. Plant Biotechnol. 2008, 25, 55–59. [Google Scholar] [CrossRef]
- Ma, Y.-P.; Zhao, L.; Zhang, W.-J.; Zhang, Y.-H.; Xing, X.; Duan, X.-X.; Hu, J.; Harris, A.; Liu, P.-L.; Dai, S.-L.; et al. Origins of cultivars of chrysanthemum—Evidence from the chloroplast genome and nuclear lfy gene. J. Syst. Evol. 2020, 58, 925–944. [Google Scholar] [CrossRef]
- Weber, E.; Engler, C.; Gruetzner, R.; Werner, S.; Marillonnet, S. A modular cloning system for standardized assembly of multigene constructs. PLoS ONE 2011, 6, e16765. [Google Scholar] [CrossRef] [PubMed]
- Engler, C.; Youles, M.; Gruetzner, R.; Ehnert, T.M.; Werner, S.; Jones, J.D.; Patron, N.J.; Marillonnet, S. A golden gate modular cloning toolbox for plants. ACS Synth. Biol. 2014, 3, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Inzé, D.; Depicker, A. Gateway vectors for agrobacterium-mediated plant transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castricum, A.; Bakker, E.H.; de Vetten, N.C.M.H.; Weemen, M.; Angenent, G.C.; Immink, R.G.H.; Bemer, M. HD-ZIP Transcription Factors and Brassinosteroid Signaling Play a Role in Capitulum Patterning in Chrysanthemum. Int. J. Mol. Sci. 2023, 24, 7655. https://doi.org/10.3390/ijms24087655
Castricum A, Bakker EH, de Vetten NCMH, Weemen M, Angenent GC, Immink RGH, Bemer M. HD-ZIP Transcription Factors and Brassinosteroid Signaling Play a Role in Capitulum Patterning in Chrysanthemum. International Journal of Molecular Sciences. 2023; 24(8):7655. https://doi.org/10.3390/ijms24087655
Chicago/Turabian StyleCastricum, Annemarie, Erin H. Bakker, Nick C. M. H. de Vetten, Mieke Weemen, Gerco C. Angenent, Richard G. H. Immink, and Marian Bemer. 2023. "HD-ZIP Transcription Factors and Brassinosteroid Signaling Play a Role in Capitulum Patterning in Chrysanthemum" International Journal of Molecular Sciences 24, no. 8: 7655. https://doi.org/10.3390/ijms24087655
APA StyleCastricum, A., Bakker, E. H., de Vetten, N. C. M. H., Weemen, M., Angenent, G. C., Immink, R. G. H., & Bemer, M. (2023). HD-ZIP Transcription Factors and Brassinosteroid Signaling Play a Role in Capitulum Patterning in Chrysanthemum. International Journal of Molecular Sciences, 24(8), 7655. https://doi.org/10.3390/ijms24087655




