Embryonic Stem Cells Can Generate Oral Epithelia under Matrix Instruction
Abstract
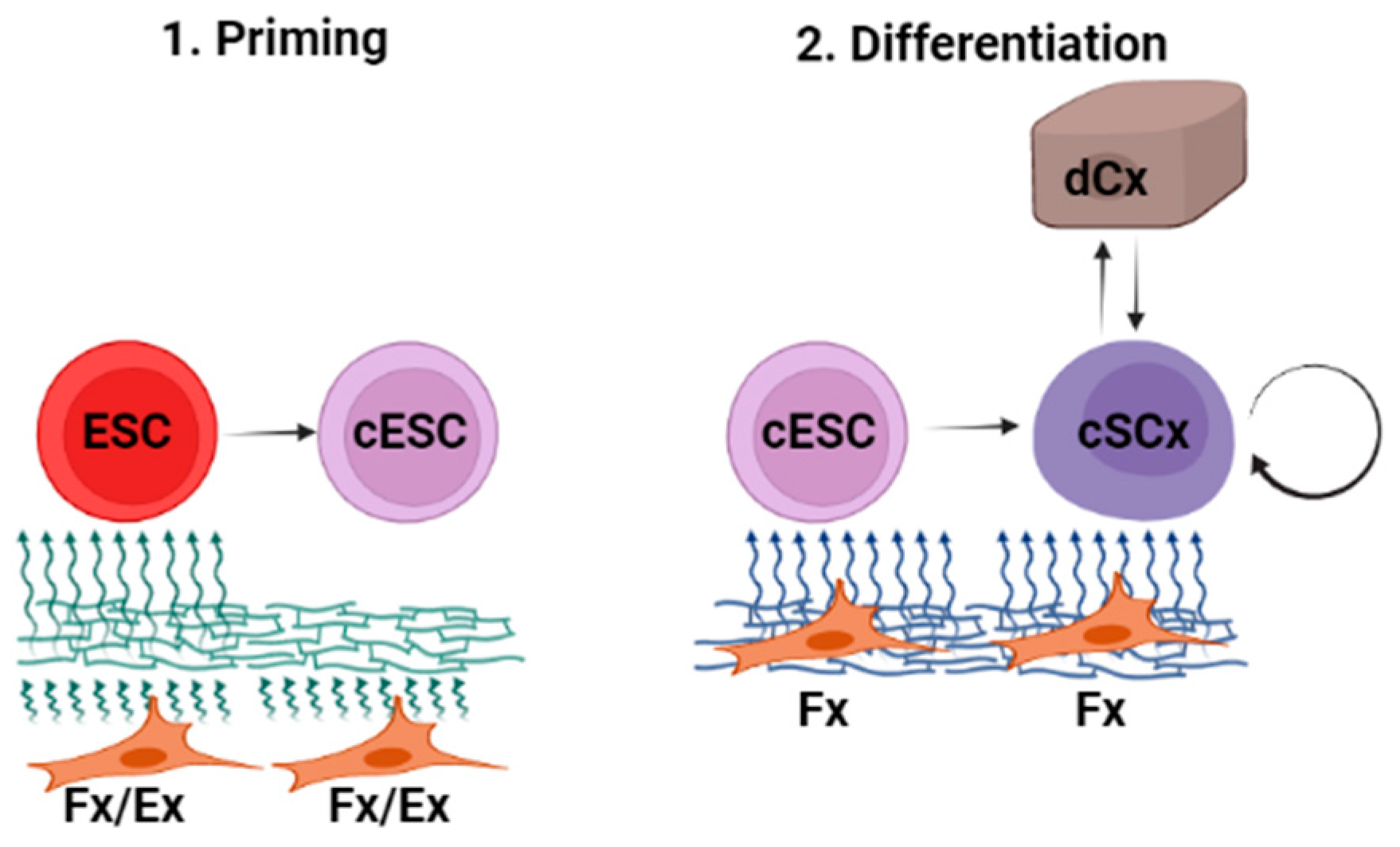
1. Introduction
2. Results
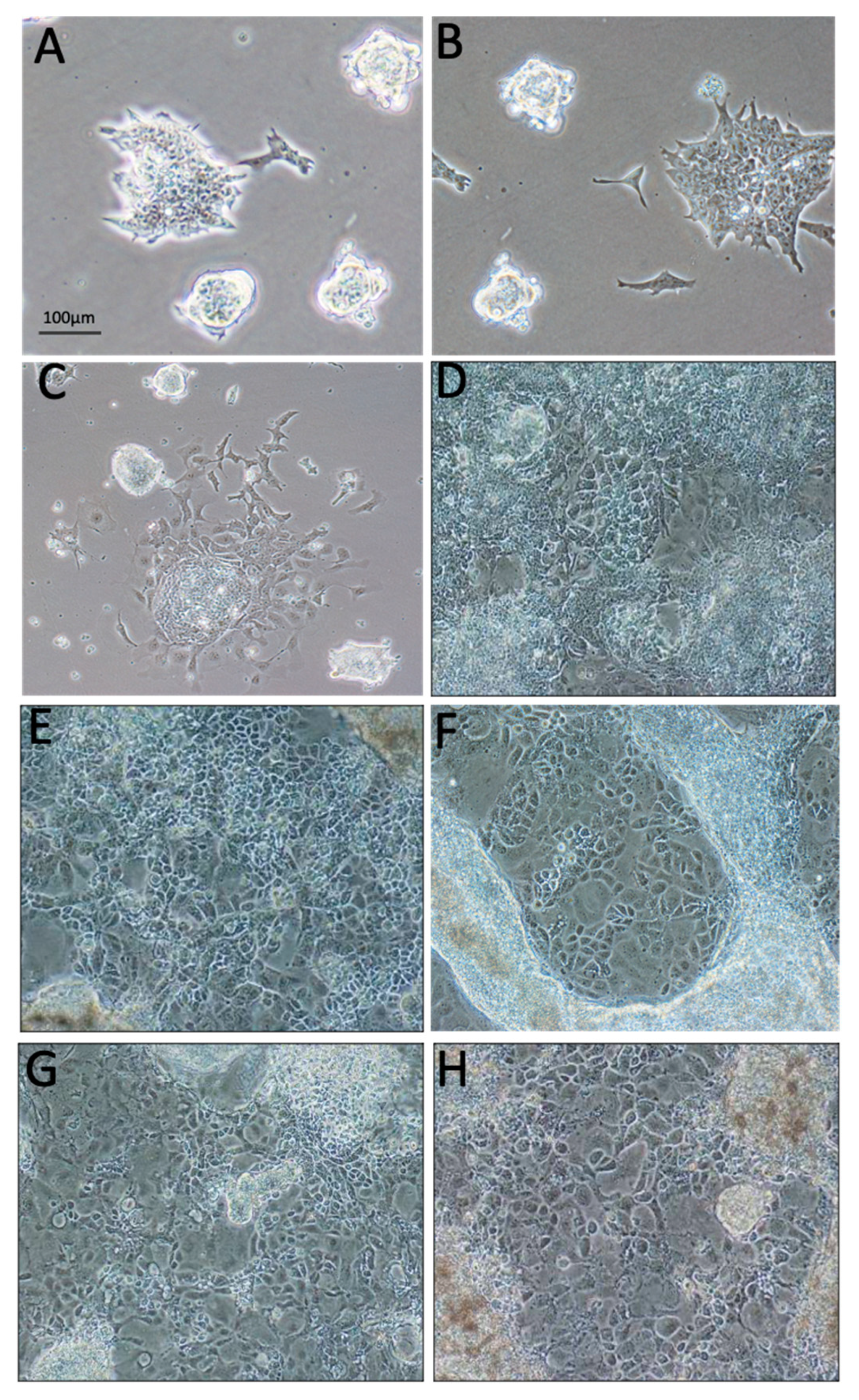
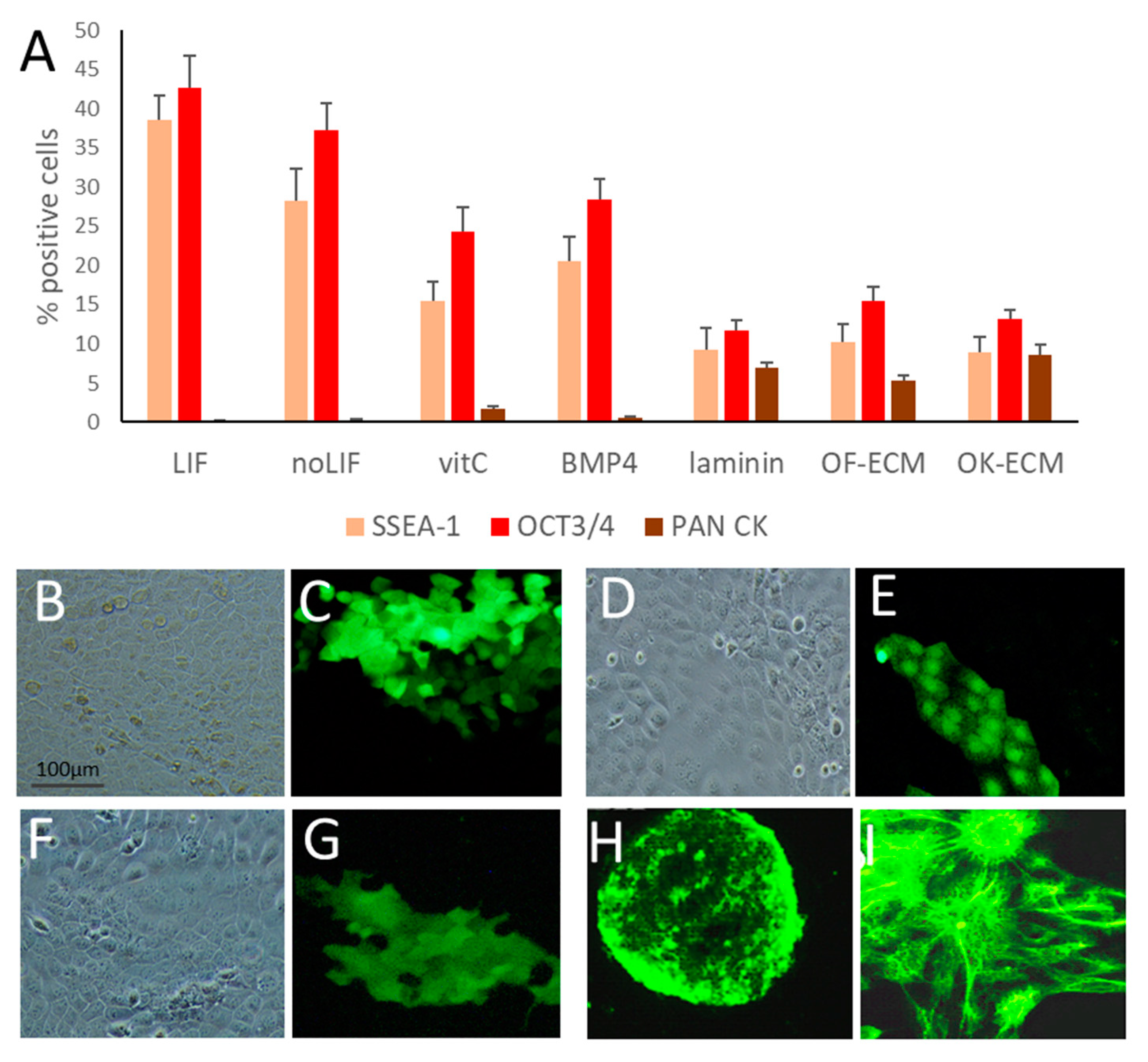
2.1. Vitamin C Alone Initiated Differentiation of ESC towards the Epithelial Lineage
2.2. ECM Generated by Both Fibroblasts and Keratinocytes Enhanced Differentiation of ESC into Epithelial Lineages
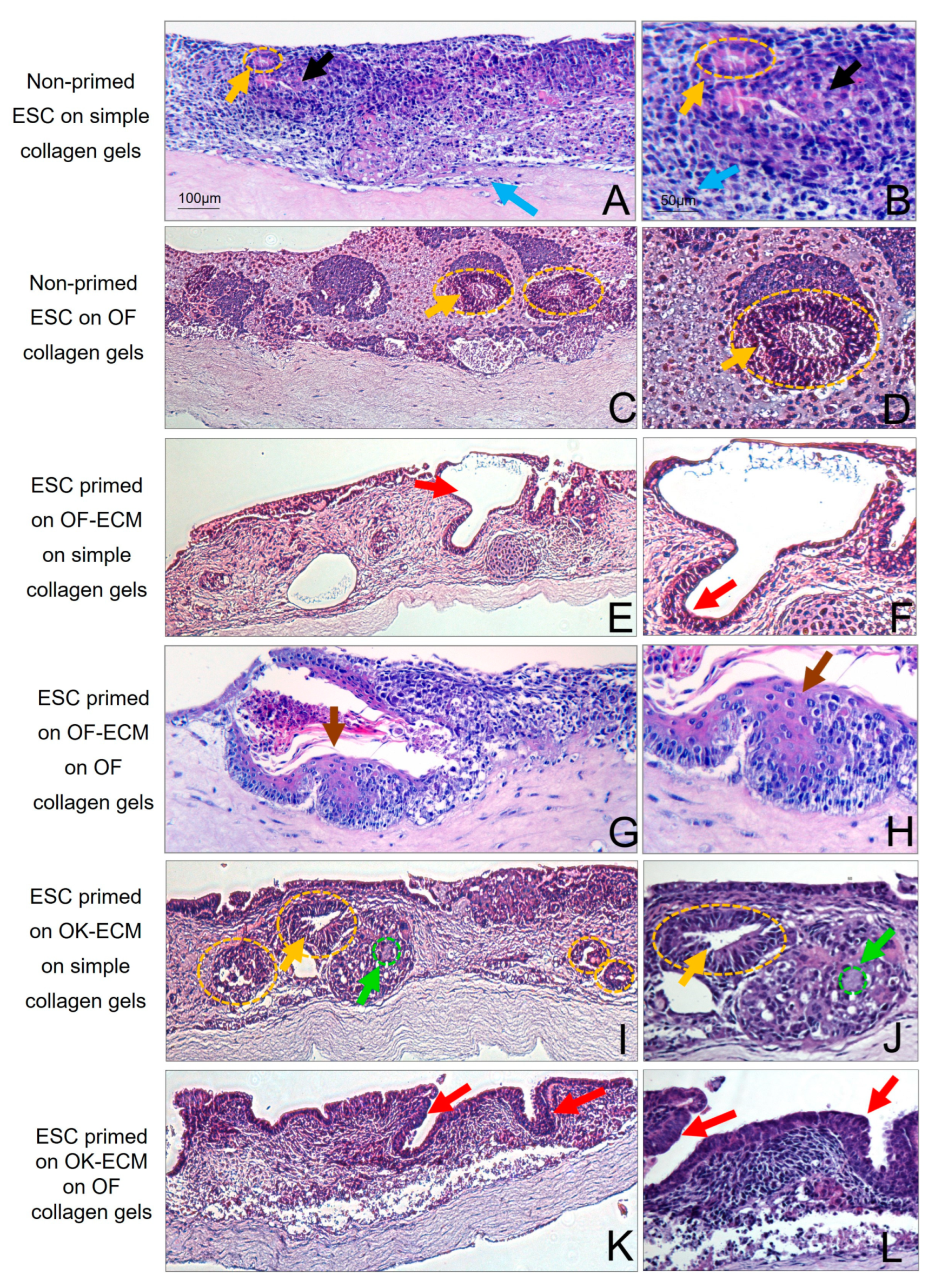
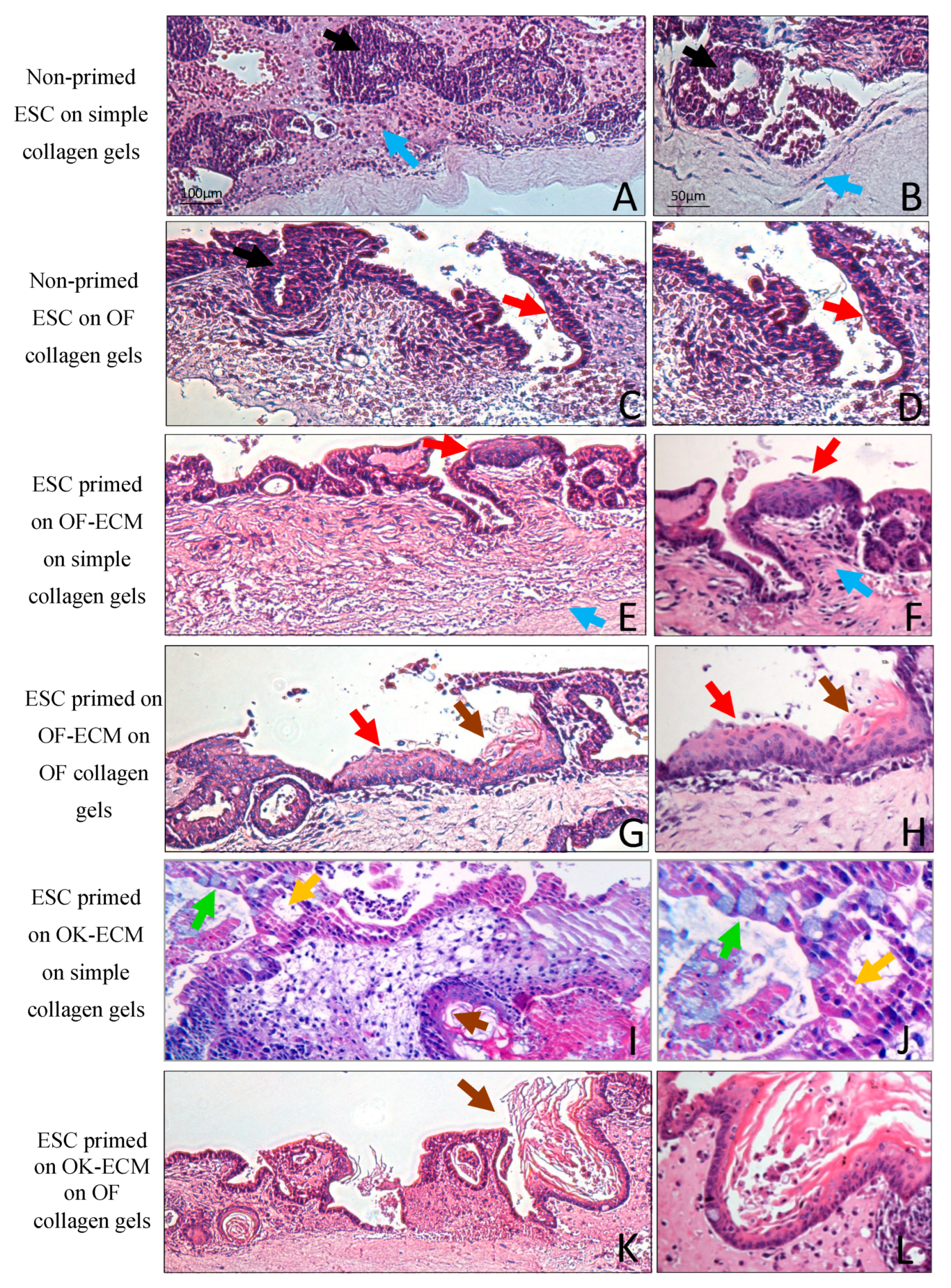
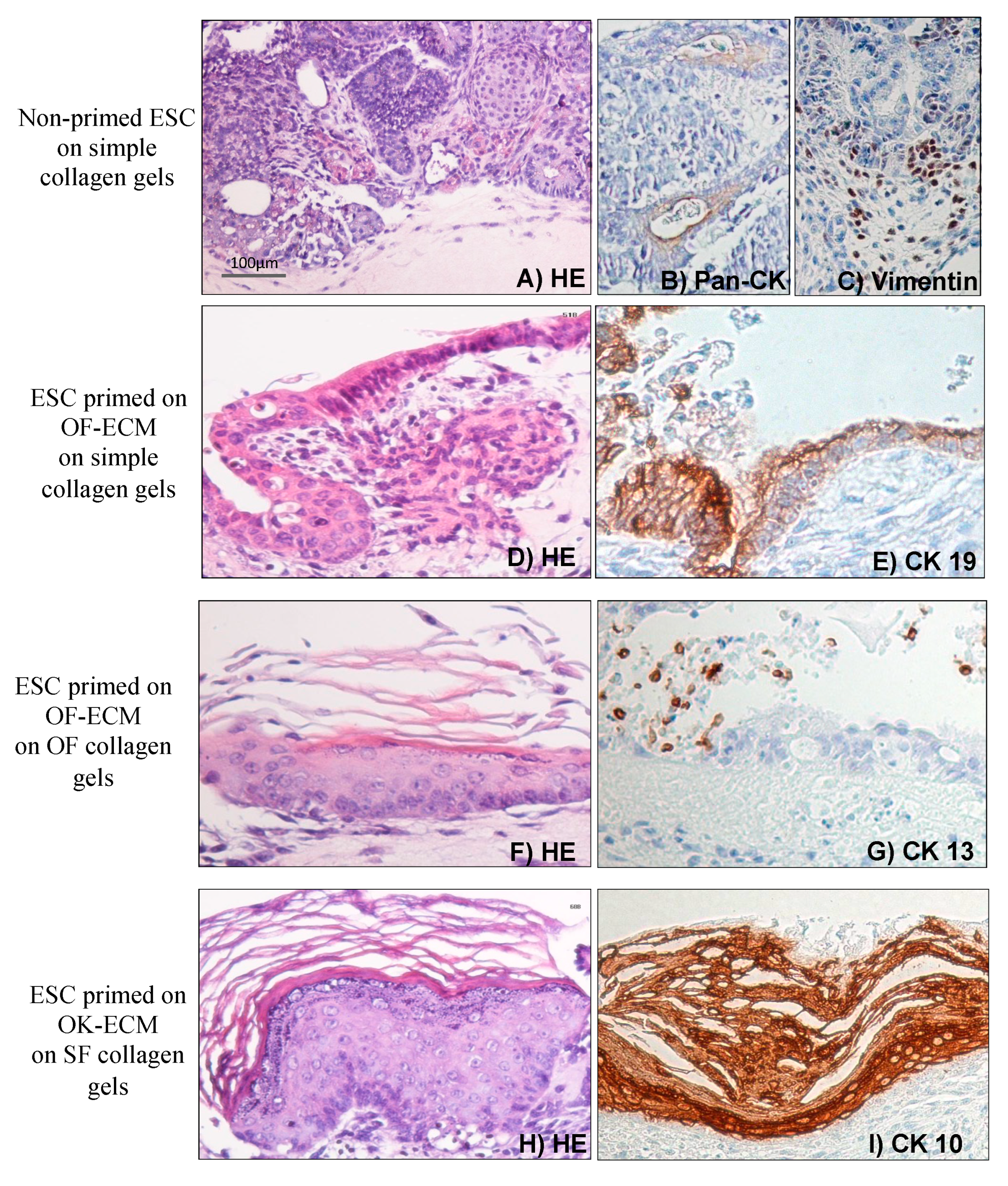
2.3. Three-Dimensional Culture on Simple Collagen Induced Further Differentiation of ESC in Epithelial and Mesenchymal Lineages but without Formation of a Lining Epithelium
2.4. Two-Dimensional Priming on Oral Fibroblast-Produced ECM and Further 3D Culture on Collagen Induced Differentiation of ESC into a Lining Epithelium
2.5. Two-Dimensional Priming on Oral Keratinocyte-Produced ECM (OK-ECM) and Further 3D Culture on Collagen Induced Differentiation of ESC into Ductal and Mucus-Producing Epithelium
2.6. Full Epithelial Phenotypic Specificity Was Achieved after 2D Priming on Matrix Products of Regionally Distinct Fibroblasts and Keratinocytes and Further 3D Culture on Fibroblast-Populated Collagen Gels
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Culture Conditions for 2D Differentiation
4.3. Three-dimensional Organotypic Cultures
4.4. Immunofluorescence
4.5. Flow Cytometry
4.6. Immunohistochemistry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Medium Additives/Matrices | LIF | No LIF | BMP4 | VitC |
|---|---|---|---|---|
| Gelatin | X | X | X | X |
| Laminin | X | |||
| Matrix formed by mouse ear fibroblasts (SF-ECM) | X | |||
| Matrix formed by oral fibroblasts (OF-ECM) | X | |||
| Matrix produced by normal human oral keratinocytes (OK-ECM) | X | |||
| Matrix produced by normal human skin keratinocytes (SK-ECM) | X |
References
- Lefebvre, J.L. Current clinical outcomes demand new treatment options for SCCHN. Ann. Oncol. 2005, 16, vi7–vi12. [Google Scholar] [CrossRef]
- Papp, A.; Sikora, S.; Evans, M.; Song, D.; Kirchhof, M.; Miliszewski, M.; Dutz, J. Treatment of toxic epidermal necrolysis by a multidisciplinary team. A review of literature and treatment results. Burns 2018, 44, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.A.; Billmire, D.A. Skin resurfacing for the burned patient. Clin. Plast. Surg. 2002, 29, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Rubegni, P.; Burroni, M.; Andreassi, A.; Fimiani, M. The Role of Dermoscopy and Digital Dermoscopy Analysis in the Diagnosis of Pigmented Skin Lesions. Arch. Dermatol. 2005, 141, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Supp, D.M.; Boyce, S.T. Engineered skin substitutes: Practices and potentials. Clin. Dermatol. 2005, 23, 403–412. [Google Scholar] [CrossRef]
- Kinikoglu, B.; Damour, O.; Hasirci, V. Tissue engineering of oral mucosa: A shared concept with skin. J. Artif. Organs 2014, 18, 8–19. [Google Scholar] [CrossRef]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2009, 7, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.W.C.; Khoo, Y.C.; Tan, B.K.; Tan, K.C.; Foo, C.L.; Chong, S.J. Skin tissue engineering advances in severe burns: Review and therapeutic applications. Burn. Trauma 2016, 4. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, F.; Hsia, H.C. Regenerative Approaches for Chronic Wounds. Annu. Rev. Biomed. Eng. 2022, 24, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Vazin, T.; Freed, W.J. Human embryonic stem cells: Derivation, culture, and differentiation: A review. Restor. Neurol. Neurosci. 2010, 28, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Bae, M.-R.; La, H.; Song, H.; Hong, K.; Do, J.-T. Efficient Generation of Neural Stem Cells from Embryonic Stem Cells Using a Three-Dimensional Differentiation System. Int. J. Mol. Sci. 2021, 22, 8322. [Google Scholar] [CrossRef]
- Biswas, A.; Hutchins, R. Embryonic Stem Cells. Stem Cells Dev. 2007, 16, 213–222. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Cheng, Y.-Y.; Yen, C.Y.T.; Hsieh, P.C.H. Mechanisms of pluripotency maintenance in mouse embryonic stem cells. Cell. Mol. Life Sci. 2016, 74, 1805–1817. [Google Scholar] [CrossRef]
- Onishi, K.; Zandstra, P.W. LIF signaling in stem cells and development. Development 2015, 142, 2230–2236. [Google Scholar] [CrossRef]
- Smith, A.G.; Heath, J.K.; Donaldson, D.D.; Wong, G.G.; Moreau, J.; Stahl, M.; Rogers, D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 1988, 336, 688–690. [Google Scholar] [CrossRef] [PubMed]
- Paynter, J.M.; Chen, J.; Liu, X.; Nefzger, C.M. Propagation and Maintenance of Mouse Embryonic Stem Cells. Mouse Cell Cult. 2019, 33–45. [Google Scholar] [CrossRef]
- Coraux, C.; Hilmi, C.; Rouleau, M.; Spadafora, A.; Hinnrasky, J.; Ortonne, J.-P.; Dani, C.; Aberdam, D. Reconstituted Skin from Murine Embryonic Stem Cells. Curr. Biol. 2003, 13, 849–853. [Google Scholar] [CrossRef]
- Birch, H.L. Extracellular Matrix and Ageing. Biochem. Cell Biol. Ageing Part I Biomed. Sci. 2018, 169–190. [Google Scholar] [CrossRef]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.S.; Fu, J. Forcing stem cells to behave: A biophysical perspective of the cellular microenvironment. Annu. Rev. Biophys. 2012, 41, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Kshitiz; Park, J.; Kim, P.; Helen, W.; Engler, A.J.; Levchenko, A.; Kim, D.-H. Control of stem cell fate and function by engineering physical microenvironments. Integr. Biol. 2012, 4, 1008–1018. [Google Scholar] [CrossRef]
- Catani, M.V.; Savini, I.; Rossi, A.; Melino, G.; Avigliano, L. Biological Role of Vitamin C in Keratinocytes. Nutr. Rev. 2005, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Rossi, A.; Duranti, G.; Avigliano, L.; Catani, M.V.; Melino, G. Characterization of Keratinocyte Differentiation Induced by Ascorbic Acid: Protein Kinase C Involvement and Vitamin C Homeostasis11The authors declared not to have a conflict of interest. J. Investig. Dermatol. 2002, 118, 372–379. [Google Scholar] [CrossRef]
- Guenou, H.; Nissan, X.; Larcher, F.; Feteira, J.; Lemaitre, G.; Saidani, M.; Del Rio, M.; Barrault, C.C.; Bernard, F.-X.; Peschanski, M.; et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: A preclinical study. Lancet 2009, 374, 1745–1753. [Google Scholar] [CrossRef]
- Mathieu, M.-E.; Saucourt, C.; Mournetas, V.; Gauthereau, X.; Thézé, N.; Praloran, V.; Thiébaud, P.; Bœuf, H. LIF-Dependent Signaling: New Pieces in the Lego. Stem Cell Rev. Rep. 2011, 8, 1–15. [Google Scholar] [CrossRef]
- Hirai, H.; Karian, P.; Kikyo, N. Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochem. J. 2011, 438, 11–23. [Google Scholar] [CrossRef]
- Shen, C.-N.; Burke, Z.D.; Tosh, D. Transdifferentiation, Metaplasia and Tissue Regeneration. Organogenesis 2014, 1, 36–44. [Google Scholar] [CrossRef]
- Sakulpaptong, W.; Clairmonte, I.A.; Blackstone, B.N.; Leblebicioglu, B.; Powell, H.M. 3D engineered human gingiva fabricated with electrospun collagen scaffolds provides a platform for in vitro analysis of gingival seal to abutment materials. PLoS ONE 2022, 17, e0263083. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ma, W.; Shen, Q.; Zhang, M.; Du, Z.; Wu, C.; Niu, B.; Liu, W.; Hua, J. Reconstitution of male germline cell specification from mouse embryonic stem cells using defined factors in vitro. Cell Death Differ. 2019, 26, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Holton, K.L.; Lanza, R. Efficient Differentiation of Functional Hepatocytes from Human Embryonic Stem Cells. Stem Cells 2008, 26, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Rohwedel, J.; Wobus, A.M. Embryonic stem cell differentiation models: Cardiogenesis, myogenesis, neurogenesis, epithelial and vascular smooth muscle cell differentiation in vitro. Cytotechnology 1999, 30, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, W.-D.; Van der Merwe, L.; Dai, W.-T.; Lin, C. Efficacy of stem cell therapy for burn wounds: A systematic review and meta-analysis of preclinical studies. Stem Cell Res. Ther. 2020, 11, 322. [Google Scholar] [CrossRef]
- Bishop, A.E.; Buttery, L.D.; Polak, J.M. Embryonic stem cells. J. Pathol. 2002, 197, 424–429. [Google Scholar] [CrossRef]
- Milstone, D.S.; Tamm, C.; Pijuan Galitó, S.; Annerén, C. A Comparative Study of Protocols for Mouse Embryonic Stem Cell Culturing. PLoS ONE 2013, 8, e081156. [Google Scholar] [CrossRef]
- Smith, A.G. Culture and differentiation of embryonic stem cells. J. Tissue Cult. Methods 1991, 13, 89–94. [Google Scholar] [CrossRef]
- Costea, D.E.; Loro, L.L.; Dimba, E.A.; Vintermyr, O.K.; Johannessen, A.C. Crucial effects of fibroblasts and keratinocyte growth factor on morphogenesis of reconstituted human oral epithelium. J. Investig. Dermatol. 2003, 121, 1479–1486. [Google Scholar] [CrossRef]
- Dabija-Wolter, G.; Bakken, V.; Cimpan, M.R.; Johannessen, A.C.; Costea, D.E. In vitro reconstruction of human junctional and sulcular epithelium. J. Oral Pathol. Med. 2013, 42, 396–404. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, R.; Harper, L.; Kitajima, K.; Osman, T.A.-H.; Cimpan, M.R.; Johannssen, A.C.; Suliman, S.; Mackenzie, I.C.; Costea, D.-E. Embryonic Stem Cells Can Generate Oral Epithelia under Matrix Instruction. Int. J. Mol. Sci. 2023, 24, 7694. https://doi.org/10.3390/ijms24097694
Das R, Harper L, Kitajima K, Osman TA-H, Cimpan MR, Johannssen AC, Suliman S, Mackenzie IC, Costea D-E. Embryonic Stem Cells Can Generate Oral Epithelia under Matrix Instruction. International Journal of Molecular Sciences. 2023; 24(9):7694. https://doi.org/10.3390/ijms24097694
Chicago/Turabian StyleDas, Ridhima, Lisa Harper, Kayoko Kitajima, Tarig Al-Hadi Osman, Mihaela Roxana Cimpan, Anne Chr. Johannssen, Salwa Suliman, Ian C. Mackenzie, and Daniela-Elena Costea. 2023. "Embryonic Stem Cells Can Generate Oral Epithelia under Matrix Instruction" International Journal of Molecular Sciences 24, no. 9: 7694. https://doi.org/10.3390/ijms24097694
APA StyleDas, R., Harper, L., Kitajima, K., Osman, T. A.-H., Cimpan, M. R., Johannssen, A. C., Suliman, S., Mackenzie, I. C., & Costea, D.-E. (2023). Embryonic Stem Cells Can Generate Oral Epithelia under Matrix Instruction. International Journal of Molecular Sciences, 24(9), 7694. https://doi.org/10.3390/ijms24097694







