Molecular and Epigenetic Aspects of Opioid Receptors in Drug Addiction and Pain Management in Sport
Abstract
:1. Introduction
2. Opioids and Opioids Receptors
3. Neuronal Networks and Mechanisms in Opioid Addiction
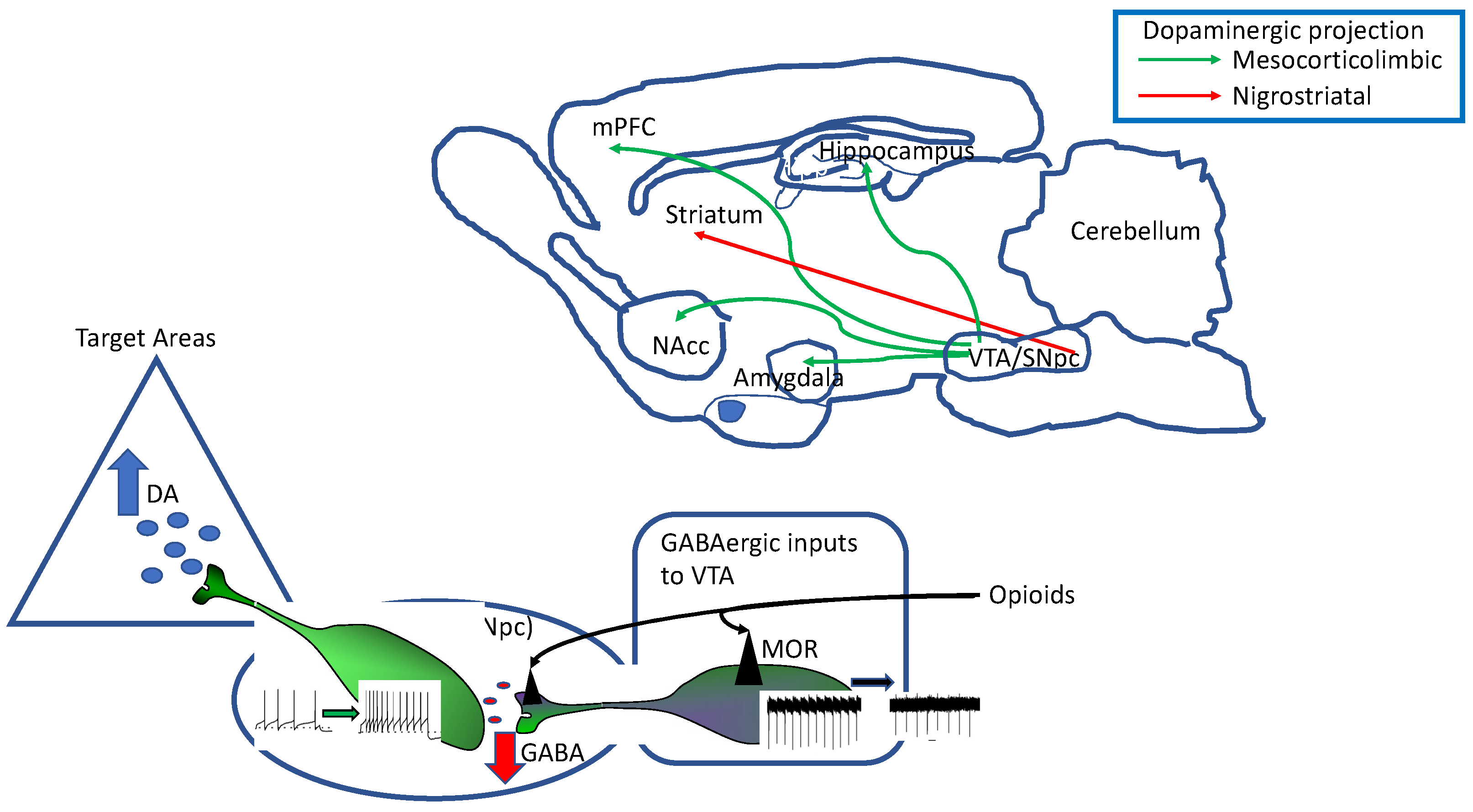
3.1. The Dopaminergic System
3.2. Opioid Receptors Expression by Dopaminergic Neurons
3.3. Mechanisms of Opioid Receptor Modulation of DA Neuron Function
3.3.1. Enkephalinergic Modulation of DA Neuron Intrinsic Excitability
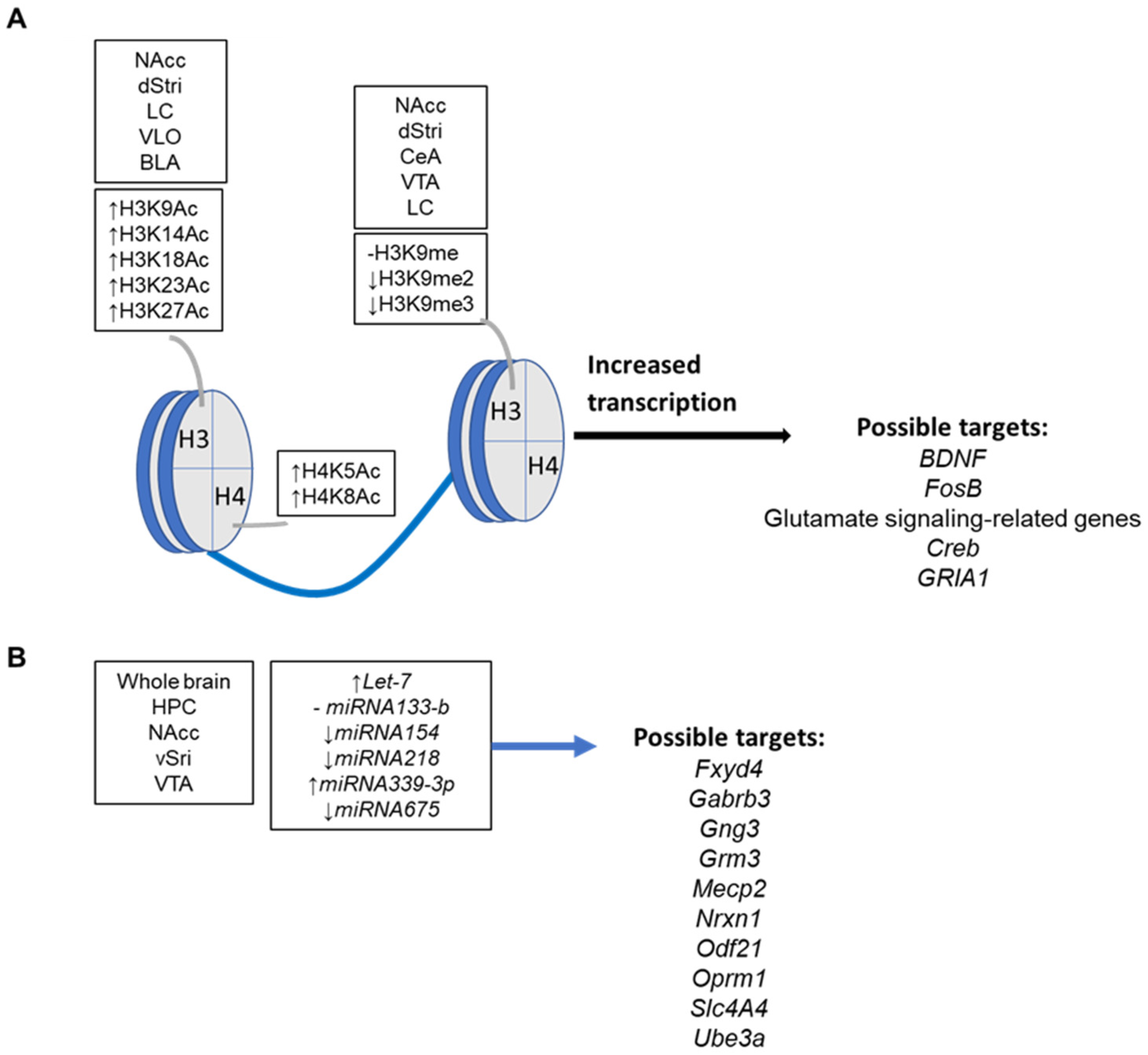
3.3.2. Epigenetic Mechanisms and Targets in Drug Addiction
4. Opioids and Pain Management in Sport
4.1. Upcoming Epigenetic Effectors in Nociception
4.2. Upcoming Epigenetic Markers in Doping
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Klenowski, P.; Morgan, M.; Bartlett, S.E. The role of δ-opioid receptors in learning and memory underlying the development of addiction. Br. J. Pharmacol. 2015, 172, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Mollereau, C.; Parmentier, M.; Mailleux, P.; Butour, J.L.; Moisand, C.; Chalon, P.; Caput, D.; Vassart, G.; Meunier, J. CORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994, 341, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Chavkin, C.; James, I.F.; Goldstein, A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 1982, 215, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Negishi, K.; Suda, M.; Sawa, A.; Fujino, M.; Wakimasu, M. Evidence that dynorphin-(1-13) acts as an agonist on opioid kappa-receptors. Eur. J. Pharmacol. 1982, 77, 137–141. [Google Scholar] [CrossRef]
- Goldstein, A.; Tachibana, S.; Lowney, L.I.; Hunkapiller, M.; Hood, L. Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc. Natl. Acad. Sci. USA 1979, 76, 6666–6670. [Google Scholar] [CrossRef]
- Kangawa, K.; Matsuo, H. alpha-Neo-endorphin: A “big” Leu-enkephalin with potent opiate activity from porcine hypothalami. Biochem. Biophys. Res. Commun. 1979, 86, 153–160. [Google Scholar] [CrossRef]
- Tejeda, H.A.; Bonci, A. Dynorphin/kappa-opioid receptor control of dopamine dynamics: Implications for negative affective states and psychiatric disorders. Brain Res. 2019, 1713, 91–101. [Google Scholar] [CrossRef]
- Bennett, M.; Paice, J.A.; Wallace, M. Pain and Opioids in Cancer Care: Benefits, Risks, and Alternatives. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 705–713. [Google Scholar] [CrossRef]
- Liu, J.F.; Li, J.X. Drug addiction: A curable mental disorder? Acta Pharmacol. Sin. 2018, 39, 1823–1829. [Google Scholar] [CrossRef]
- Koob, G.F. Antireward, compulsivity, and addiction: Seminal contributions of Dr. Athina Markou to motivational dysregulation in addiction. Psychopharmacology 2017, 234, 1315–1332. [Google Scholar] [CrossRef]
- Browne, C.J.; Godino, A.; Salery, M.; Nestler, E.J. Epigenetic Mechanisms of Opioid Addiction. Biol. Psychiatry 2020, 87, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. The epigenotype. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Hombach, S.; Kretz, M. Non-coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 2016, 937, 3–17. [Google Scholar]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef] [PubMed]
- Saw, P.E.; Xu, X.; Chen, J.; Song, E.W. Non-coding RNAs: The new central dogma of cancer biology. Sci. China Life Sci. 2021, 64, 22–50. [Google Scholar] [CrossRef]
- Kumar, P.; Kuscu, C.; Dutta, A. Biogenesis and function of transfer RNA-related fragments (tRFs). Trends Biochem. Sci. 2016, 41, 679–689. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Wang, X.; He, A. Circles reshaping the RNA world: From waste to treasure. Oncoscience 2014, 1, 674–705. [Google Scholar] [CrossRef]
- Cadet, J.L.; Jayanthi, S. Epigenetics of addiction. Neurochem. Int. 2021, 147, 105069. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, F.; Han, S.; Li, S.; Zhao, Y.; Wang, H.; Tian, J.; Cen, X. MicroRNAs in drug addiction: Current status and future perspectives. Pharmacol. Ther. 2022, 236, 108215. [Google Scholar] [CrossRef]
- Odell, D.W. Epigenetics of pain mediators. Curr. Opin. Anaesthesiol. 2018, 31, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Pan, H.L. Epigenetic Mechanisms of Neural Plasticity in Chronic Neuropathic Pain. ACS Chem. Neurosci. 2022, 13, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Ren, K.; Dubner, R. Epigenetic regulation of persistent pain. Transl. Res. 2015, 165, 177–199. [Google Scholar] [CrossRef]
- Gaudio, F.; Mortali, C.; Tini, A. Opioid epi-demic spread from Northern and Eastern Europe to Mediterranean Area. Clin. Ter. 2021, 172, 209–210. [Google Scholar] [PubMed]
- Wang, Y.; Zhuang, Y.; DiBerto, J.F.; Zhou, X.E.; Schmitz, G.P.; Yuan, Q.; Jain, M.K.; Liu, W.; Melcher, K.; Jiang, Y.; et al. Structures of the entire human opioid receptor family. Cell 2023, 186, 413–427. [Google Scholar] [CrossRef]
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid pharmacology. Pain Physician 2008, 11 (Suppl. S2), S133–S153. [Google Scholar] [CrossRef]
- Olsen, Y. What Is Addiction? History, Terminology, and Core Concepts. Med. Clin. N. Am. 2022, 106, 1–12. [Google Scholar] [CrossRef]
- D’Agostino, B.; Orlotti, D.; Calò, G.; Sullo, N.; Russo, M.; Guerrini, R.; De Nardo, M.; Mazzeo, F.; Candeletti, S.; Rossi, F. Nociceptin modulates bronchoconstriction induced by sensory nerve activation in mouse lung. Am. J. Respir. Cell Mol. Biol. 2010, 42, 250–254. [Google Scholar] [CrossRef]
- Stevens, C.W. The evolution of vertebrate opioid receptors. Front. Biosci. 2009, 14, 1247–1269. [Google Scholar] [CrossRef]
- Dreborg, S.; Sundström, G.; Larsson, T.A.; Larhammar, D. Evolution of vertebrate opioid receptors. Proc. Natl. Acad. Sci. USA 2008, 105, 15487–15492. [Google Scholar] [CrossRef]
- Dannals, R.F. Positron emission tomography radioligands for the opioid system. J. Label. Comp. Radiopharm. 2013, 56, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Stella, L.; D’Ambra, C.; Mazzeo, F.; Capuano, A.; Del Franco, F.; Avolio, A.; Ambrosino, F. Naltrexone plus benzodiazepine aids abstinence in opioid-dependent patients. Life Sci. 2005, 77, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.M. Recent developments in the study of opioid receptors. Mol. Pharmacol. 2013, 83, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Norn, S.; Kruse, P.R.; Kruse, E. Opiumsvalmuen og morfin gennem tiderne [History of opium poppy and morphine]. Dan. Med. Arbog. 2005, 33, 171–184. [Google Scholar]
- Gendron, L.; Cahill, C.M.; von Zastrow, M.; Schiller, P.W.; Pineyro, G. Molecular Pharmacology of δ-Opioid Receptors. Pharmacol. Rev. 2016, 68, 631–700. [Google Scholar] [CrossRef]
- Ballantyne, J.C.; Shin, N.S. Efficacy of opioids for chronic pain: A review of the evidence. Clin. J. Pain 2008, 24, 469–478. [Google Scholar] [CrossRef]
- Franklin, G.M. Opioids for chronic noncancer pain: A position paper of the American Academy of Neurology. Neurology 2014, 83, 1277–1284. [Google Scholar] [CrossRef]
- Yao, Y.; Jiang, J.; Lin, W.; Yu, Y.; Guo, Y.; Zheng, X. Efficacy of systemic lidocaine on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: A randomized controlled trial. J. Clin. Anesth. 2021, 71, 110223. [Google Scholar] [CrossRef]
- Bujedo, B.M.; Santos, S.G.; Azpiazu, A.U. A review of epidural and intrathecal opioids used in the management of postoperative pain. J. Opioid Manag. 2012, 8, 177–192. [Google Scholar] [CrossRef]
- Kristek, G.; Radoš, I.; Kristek, D.; Kapural, L.; Nešković, N.; Škiljić, S.; Horvat, V.; Mandić, S.; Haršanji-Drenjančević, I. Influence of postoperative analgesia on systemic inflammatory response and postoperative cognitive dysfunction after femoral fractures surgery: A randomized controlled trial. Reg. Anesth. Pain Med. 2019, 44, 59–68. [Google Scholar] [CrossRef]
- Sawynok, J. The therapeutic use of heroin: A review of the pharmacological literature. Can. J. Physiol. Pharmacol. 1986, 64, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Chatterjee, O.; Ravishankar, N.; Suresh, S.; Raju, R.; Mahadevan, A.; Prasad, T.S.K. Opioid receptors signaling network. J. Cell Commun. Signal. 2022, 16, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Nordt, S.P.; Clark, R.F. Midazolam: A review of therapeutic uses and toxicity. J. Emerg. Med. 1997, 15, 357–365. [Google Scholar] [CrossRef]
- Bell, H.J.; Azubike, E.; Haouzi, P. The “other” respiratory effect of opioids: Suppression of spontaneous augmented (“sigh”) breaths. J. Appl. Physiol. 2011, 111, 1296–1303. [Google Scholar] [CrossRef]
- Phillips, S.N.; Fernando, R.; Girard, T. Parenteral opioid analgesia: Does it still have a role? Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Foldes, F.F. Pain control with intrathecally and peridurally administered opioids and other drugs. Anaesthesiol. Reanim. 1991, 16, 287–298. [Google Scholar] [PubMed]
- Terashvili, M.; Talluri, B.; Palangmonthip, W.; Iczkowski, K.A.; Sanvanson, P.; Medda, B.K.; Banerjee, B.; Cunningham, C.W.; Sengupta, J.N. Peripheral antinociceptive effects of a bifunctional μ and δ opioid receptor ligand in rat model of inflammatory bladder pain. Neuropharmacology 2021, 196, 108701. [Google Scholar] [CrossRef]
- Truong, W.; Cheng, C.; Xu, Q.G.; Li, X.Q.; Zochodne, D.W. Mu opioid receptors and analgesia at the site of a peripheral nerve injury. Ann. Neurol. 2003, 53, 366–375. [Google Scholar] [CrossRef]
- Loffreda, A.; Falcone, G.; Motola, G.; Mazzeo, F.; Iacobelli, M.; Ferrari, P.; Rossi, F. Use of naltrexone for the treatment of opiate addiction in campania, italy: The role of family. J. Subst. Use 2003, 8, 182–185. [Google Scholar] [CrossRef]
- Stella, L.; Cassese, F.; Barone, S.; Barchetta, A.; Iacobelli, M.; Motola, G.; Mazzeo, M.; Rossi, F. Naltrexone to keep a drug-free condition. Res. Comm. Alcohol Sub. Abuse 1999, 20, 91–98. [Google Scholar]
- Brackley, A.D.; Sarrami, S.; Gomez, R.; Guerrero, K.A.; Jeske, N.A. Identification of a signaling cascade that maintains constitutive δ-opioid receptor incompetence in peripheral sensory neurons. J. Biol. Chem. 2017, 26, 8762–8772. [Google Scholar] [CrossRef] [PubMed]
- Brackley, A.D.; Gomez, R.; Akopian, A.N.; Henry, M.A.; Jeske, N.A. GRK2 Constitutively Governs Peripheral Delta Opioid Receptor Activity. Cell Rep. 2016, 16, 2686–2698. [Google Scholar] [CrossRef]
- Bueno, L.; Fioramonti, J. Action of opiates on gastrointestinal function. Baillieres Clin. Gastroenterol. 1988, 2, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Przewłocki, R.; Przewłocka, B. Opioids in chronic pain. Eur. J. Pharmacol. 2001, 429, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J.; Akbarali, H.I. Molecular physiology of enteric opioid receptors. Am. J. Gastroenterol. 2014, 10, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Cahill, C.M.; Taylor, A.M.; Cook, C.; Ong, E.; Morón, J.A.; Evans, C.J. Does the kappa opioid receptor system contribute to pain aversion? Front. Pharmacol. 2014, 17, 5–253. [Google Scholar] [CrossRef]
- Moon, S.W.; Park, E.H.; Suh, H.R.; Ko, D.H.; Kim, Y.I.; Han, H.C. The contribution of activated peripheral kappa opioid receptors (kORs) in the inflamed knee joint to anti-nociception. Brain Res. 2016, 1648 Pt A, 11–18. [Google Scholar] [CrossRef]
- Nagasaka, H.; Awad, H.; Yaksh, T.L. Peripheral and spinal actions of opioids in the blockade of the autonomic response evoked by compression of the inflamed knee joint. Anesthesiology 1996, 85, 808–816. [Google Scholar] [CrossRef]
- Shook, J.E.; Pelton, J.T.; Hruby, V.J.; Burks, T.F. Peptide opioid antagonist separates peripheral and central opioid antitransit effects. J. Pharmacol. Exp. Ther. 1987, 243, 492–500. [Google Scholar]
- Culpepper-Morgan, J.; Kreek, M.J.; Holt, P.R.; LaRoche, D.; Zhang, J.; O’Bryan, L. Orally administered kappa as well as mu opiate agonists delay gastrointestinal transit time in the guinea pig. Life Sci. 1988, 42, 2073–2077. [Google Scholar] [CrossRef]
- Blandini, F.; Nappi, G.; Tassorelli, C.; Martignoni, E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog. Neurobiol. 2000, 62, 63–88. [Google Scholar] [CrossRef] [PubMed]
- French, I.T.; Muthusamy, K.A. A Review of the Pedunculopontine Nucleus in Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.P.; Mark, G.P.; Williams, J.T. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J. Neurosci. 2006, 26, 2788–2797. [Google Scholar] [CrossRef]
- Wise, R.A. Neurobiology of addiction. Curr. Opin. Neurobiol. 1996, 6, 243–251. [Google Scholar] [CrossRef]
- Koob, G.F. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 1992, 13, 177–184. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Wang, M.; Paspalas, C.D. Dopamine’s Actions in Primate Prefrontal Cortex: Challenges for Treating Cognitive Disorders. Pharmacol. Rev. 2015, 67, 681–696. [Google Scholar] [CrossRef]
- Berke, J.D. What does dopamine mean? Nat. Neurosci. 2018, 21, 787–793. [Google Scholar] [CrossRef]
- Lammel, S.; Lim, B.K.; Malenka, R.C. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 2014, 76 Pt B, 351–359. [Google Scholar] [CrossRef]
- Pignatelli, M.; Bonci, A. Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron 2015, 86, 1145–1157. [Google Scholar] [CrossRef]
- Wise, R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004, 5, 483–494. [Google Scholar] [CrossRef]
- Williams, J.T.; Christie, M.J.; Manzoni, O. Cellular and synaptic adaptations mediating opioid dependence. Physiol. Rev. 2001, 81, 299–343. [Google Scholar] [CrossRef]
- Borst, J.G.; Sakmann, B. Effect of changes in action potential shape on calcium currents and transmitter release in a calyx-type synapse of the rat auditory brainstem. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Pachenari, N.; Azizi, H.; Semnaniann, S. Adolescent Morphine Exposure in Male Rats Alters the Electrophysiological Properties of Locus Coeruleus Neurons of the Male Offspring. Neuroscience 2019, 410, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Gantz, S.C.; Ford, C.P.; Morikawa, H.; Williams, J.T. The Evolving Understanding of Dopamine Neurons in the Substantia Nigra and Ventral Tegmental Area. Annu. Rev. Physiol. 2018, 80, 219–241. [Google Scholar] [CrossRef]
- Ledonne, A.; Massaro Cenere, M.; Paldino, E.; D’Angelo, V.; D’Addario, S.L.; Casadei, N.; Nobili, A.; Berretta, N.; Fusco, F.R.; Ventura, R.; et al. Morpho-Functional Changes of Nigral Dopamine Neurons in an α-Synuclein Model of Parkinson’s Disease. Mov. Disord. 2023, 38, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, E.; Cucchiaroni, M.L.; Mercuri, N.B. Substantia nigra control of basal ganglia nuclei. J. Neural Transm. Suppl. 2009, 73, 91–101. [Google Scholar]
- Grillner, P.; Mercuri, N.B. Intrinsic membrane properties and synaptic inputs regulating the firing activity of the dopamine neurons. Behav. Brain Res. 2002, 130, 149–169. [Google Scholar] [CrossRef]
- Rice, M.E.; Cragg, S.J.; Greenfield, S.A. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J. Neurophysiol. 1997, 77, 853–862. [Google Scholar] [CrossRef]
- Tepper, J.M.; Creese, I.; Schwartz, D.H. Stimulus-evoked changes in neostriatal dopamine levels in awake and anesthetized rats as measured by microdialysis. Brain Res. 1991, 559, 283–292. [Google Scholar] [CrossRef]
- Björklund, A.; Lindvall, O. Dopamine in dendrites of substantia nigra neurons: Suggestions for a role in dendritic terminals. Brain Res. 1975, 83, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Geffen, L.B.; Jessell, T.M.; Cuello, A.C.; Iversen, L.L. Release of dopamine from dendrites in rat substantia nigra. Nature 1976, 260, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Beckstead, M.J.; Grandy, D.K.; Wickman, K.; Williams, J.T. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 2004, 42, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Nakajima, Y.; Nakajima, S. G protein-coupled inward rectifier modulated by dopamine agonists in cultured substantia nigra neurons. Neuroscience 1995, 69, 1145–1158. [Google Scholar] [CrossRef]
- Lacey, M.G.; Mercuri, N.B.; North, R.A. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J. Physiol. 1987, 392, 397–416. [Google Scholar] [CrossRef]
- Guatteo, E.; Rizzo, F.R.; Federici, M.; Cordella, A.; Ledonne, A.; Latini, L.; Nobili, A.; Viscomi, M.T.; Biamonte, F.; Landrock, K.K.; et al. Functional alterations of the dopaminergic and glutamatergic systems in spontaneous α-synuclein overexpressing rats. Exp. Neurol. 2017, 287 Pt 1, 21–33. [Google Scholar] [CrossRef]
- Guatteo, E.; Berretta, N.; Monda, V.; Ledonne, A.; Mercuri, N.B. Pathophysiological Features of Nigral Dopaminergic Neurons in Animal Models of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 4508. [Google Scholar] [CrossRef]
- Krashia, P.; Martini, A.; Nobili, A.; Aversa, D.; D’Amelio, M.; Berretta, N.; Guatteo, E.; Mercuri, N.B. On the properties of identified dopaminergic neurons in the mouse substantia nigra and ventral tegmental area. Eur. J. Neurosci. 2017, 45, 92–105. [Google Scholar] [CrossRef]
- Neal, C.R., Jr.; Mansour, A.; Reinscheid, R.; Nothacker, H.P.; Civelli, O.; Watson, S.J., Jr. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J. Comp. Neurol. 1999, 406, 503–547. [Google Scholar] [CrossRef]
- Norton, C.S.; Neal, C.R.; Kumar, S.; Akil, H.; Watson, S.J. Nociceptin/orphanin FQ and opioid receptor-like receptor mRNA expression in dopamine systems. J. Comp. Neurol. 2002, 444, 358–368. [Google Scholar] [CrossRef]
- Mercatelli, D.; Pisanò, C.A.; Novello, S.; Morari, M. NOP Receptor Ligands and Parkinson’s Disease. Handb. Exp. Pharmacol. 2019, 254, 213–232. [Google Scholar] [PubMed]
- Gramsch, C.; Höllt, V.; Pasi, A.; Mehraein, P.; Herz, A. Immunoreactive dynorphin in human brain and pituitary. Brain Res. 1982, 233, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Vincent, S.; Hökfelt, T.; Christensson, I.; Terenius, L. Immunohistochemical evidence for a dynorphin immunoreactive striato-nigral pathway. Eur. J. Pharmacol. 1982, 85, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Zamir, N.; Palkovits, M.; Brownstein, M.J. Distribution of immunoreactive dynorphin in the central nervous system of the rat. Brain Res. 1983, 280, 81–93. [Google Scholar] [CrossRef]
- Fallon, J.H.; Leslie, F.M.; Cone, R.I. Dynorphin-containing pathways in the substantia nigra and ventral tegmentum: A double labeling study using combined immunofluorescence and retrograde tracing. Neuropeptides 1985, 5, 457–460. [Google Scholar] [CrossRef]
- DePaoli, A.M.; Hurley, K.M.; Yasada, K.; Reisine, T.; Bell, G. Distribution of kappa opioid receptor mRNA in adult mouse brain: An in situ hybridization histochemistry study. Mol. Cell. Neurosci. 1994, 5, 327–335. [Google Scholar] [CrossRef]
- Meng, F.; Xie, G.X.; Thompson, R.C.; Mansour, A.; Goldstein, A.; Watson, S.J.; Akil, H. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 9954–9958. [Google Scholar] [CrossRef]
- Simonin, F.; Befort, K.; Gavériaux-Ruff, C.; Matthes, H.; Nappey, V.; Lannes, B.; Micheletti, G.; Kieffer, B. The human delta-opioid receptor: Genomic organization, cDNA cloning, functional expression, and distribution in human brain. Mol. Pharmacol. 1994, 46, 1015–1021. [Google Scholar]
- Margolis, E.B.; Fujita, W.; Devi, L.A.; Fields, H.L. Two delta opioid receptor subtypes are functional in single ventral tegmental area neurons, and can interact with the mu opioid receptor. Neuropharmacology 2017, 123, 420–432. [Google Scholar] [CrossRef]
- Corkrum, M.; Rothwell, P.E.; Thomas, M.J.; Kofuji, P.; Araque, A. Opioid-Mediated Astrocyte-Neuron Signaling in the Nucleus Accumbens. Cells 2019, 8, 586. [Google Scholar] [CrossRef]
- Lecca, S.; Melis, M.; Luchicchi, A.; Muntoni, A.L.; Pistis, M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology 2012, 37, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.E.; Long, H.; Pei, J.; Kukutla, P.; Phero, A.; Hadaegh, F.; Abdelnabi, A.; Solt, K.; Brenner, G.J. The rostromedial tegmental nucleus: A key modulator of pain and opioid analgesia. Pain 2019, 160, 2524–2534. [Google Scholar] [CrossRef] [PubMed]
- Margolis, E.B.; Hjelmstad, G.O.; Fujita, W.; Fields, H.L. Direct bidirectional μ-opioid control of midbrain dopamine neurons. J. Neurosci. 2014, 34, 14707–14716. [Google Scholar] [CrossRef]
- Johnson, S.W.; North, R.A. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J. Physiol. 1992, 450, 455–468. [Google Scholar] [CrossRef]
- Matsui, A.; Jarvie, B.C.; Robinson, B.G.; Hentges, S.T.; Williams, J.T. Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron 2014, 82, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Bonci, A.; Malenka, R.C. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J. Neurosci. 1999, 19, 3723–3730. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Y.; Yang, H.; Luan, W.; Song, J.; Cui, D.; Dong, Y.; Lai, B.; Ma, L.; Zheng, P. Morphine disinhibits glutamatergic input to VTA dopamine neurons and promotes dopamine neuron excitation. Elife 2015, 4, e09275. [Google Scholar] [CrossRef] [PubMed]
- Baimel, C.; Lau, B.K.; Qiao, M.; Borgland, S.L. Projection-Target-Defined Effects of Orexin and Dynorphin on VTA Dopamine Neurons. Cell Rep. 2017, 18, 1346–1355. [Google Scholar] [CrossRef]
- Margolis, E.B.; Hjelmstad, G.O.; Bonci, A.; Fields, H.L. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J. Neurosci. 2003, 23, 9981–9986. [Google Scholar] [CrossRef]
- Margolis, E.B.; Lock, H.; Chefer, V.I.; Shippenberg, T.S.; Hjelmstad, G.O.; Fields, H.L. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 2938–2942. [Google Scholar] [CrossRef]
- Ford, C.P.; Beckstead, M.J.; Williams, J.T. Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J. Neurophysiol. 2007, 97, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.L.; Wessendorf, M.W.; Williams, J.T. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience 1997, 77, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A.; Williams, J.T. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J. Neurosci. 2011, 31, 17729–17735. [Google Scholar] [CrossRef] [PubMed]
- Popova, D.; Desai, N.; Blendy, J.A.; Pang, Z.P. Synaptic Regulation by OPRM1 Variants in Reward Neurocircuitry. J. Neurosci. 2019, 39, 5685–5696. [Google Scholar] [CrossRef] [PubMed]
- Margolis, E.B.; Fields, H.L.; Hjelmstad, G.O.; Mitchell, J.M. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J. Neurosci. 2008, 28, 12672–12681. [Google Scholar] [CrossRef]
- Zheng, F.; Grandy, D.K.; Johnson, S.W. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br. J. Pharmacol. 2002, 136, 1065–1071. [Google Scholar] [CrossRef]
- Driscoll, J.R.; Wallace, T.L.; Mansourian, K.A.; Martin, W.J.; Margolis, E.B. Differential Modulation of Ventral Tegmental Area Circuits by the Nociceptin/Orphanin FQ System. eNeuro 2020, 7, ENEURO.0376-19.2020. [Google Scholar] [CrossRef]
- Margolis, E.B.; Wallace, T.L.; Van Orden, L.J.; Martin, W.J. Differential effects of novel kappa opioid receptor antagonists on dopamine neurons using acute brain slice electrophysiology. PLoS ONE 2020, 15, e0232864. [Google Scholar] [CrossRef]
- Isaacs, D.P.; Leman, R.P.; Everett, T.J.; Lopez-Beltran, H.; Hamilton, L.R.; Oleson, E.B. Buprenorphine is a weak dopamine releaser relative to heroin, but its pretreatment attenuates heroin-evoked dopamine release in rats. Neuropsychopharmacol. Rep. 2020, 40, 355–364. [Google Scholar] [CrossRef]
- Charbogne, P.; Kieffer, B.L.; Befort, K. 15 years of genetic approaches in vivo for addiction research: Opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 2014, 76 Pt B, 204–217. [Google Scholar] [CrossRef]
- Moran, T.D.; Abdulla, F.A.; Smith, P.A. Cellular neurophysiological actions of nociceptin/orphanin FQ. Peptides 2000, 21, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; He, Y.; Ha, S.G.; Loh, H.H.; Wei, L.N. Epigenetic regulation of kappa opioid receptor gene in neuronal differentiation. Neuroscience 2008, 151, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Flaisher-Grinberg, S.; Persaud, S.D.; Loh, H.H.; Wei, L.N. Stress-induced epigenetic regulation of κ-opioid receptor gene involves transcription factor c-Myc. Proc. Natl. Acad. Sci. USA 2012, 109, 9167–9172. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, M.; Seddigh, A.; Ebrahimi Barough, S.; Fazeli, S.A.S.; Ai, J. Potential of Extracellular Vesicles in Neurodegenerative Diseases: Diagnostic and Therapeutic Indications. J. Mol. Neurosci. 2018, 66, 172–179. [Google Scholar] [CrossRef]
- Rao, P.S.S.; O’Connel, K.; Finnerty, T.K. Potential Role of Extracellular Vesicles in the Pathophysiology of Drug Addiction. Mol. Neurobiol. 2018, 55, 6906–6913. [Google Scholar] [CrossRef]
- Vishnoi, A.; Rani, S. MiRNA Biogenesis and Regulation of Diseases: An Overview. Methods Mol. Biol. 2017, 1509, 1–10. [Google Scholar]
- Salim, U.; Kumar, A.; Kulshreshtha, R.; Vivekanandan, P. Biogenesis, characterization, and functions of mirtrons. Wiley Interdiscip. Rev. RNA 2022, 13, e1680. [Google Scholar] [CrossRef]
- Braga, E.A.; Fridman, M.V.; Moscovtsev, A.A.; Filippova, E.A.; Dmitriev, A.A.; Kushlinskii, N.E. LncRNAs in Ovarian Cancer Progression, Metastasis, and Main Pathways: ceRNA and Alternative Mechanisms. Int. J. Mol. Sci. 2020, 21, 8855. [Google Scholar] [CrossRef]
- Dykes, I.M.; Emanueli, C. Transcriptional and Post-transcriptional Gene Regulation by Long Non-coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef]
- Chen, L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef]
- Liu, C.X.; Chen, L.L. Circular RNAs: Characterization, cellular roles, and applications. Cell 2022, 185, 2016–2034. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lai, X.; Wang, X.; Ying, J.; Zhang, L.; Zhou, B.; Liu, X.; Zhang, J.; Wei, G.; Hua, F. Long Non-coding RNAs and Circular RNAs: Insights Into Microglia and Astrocyte Mediated Neurological Diseases. Front. Mol. Neurosci. 2021, 14, 745066. [Google Scholar] [CrossRef] [PubMed]
- Du, G.-H. Morphine. In Natural Small Molecule Drugs from Plants; Kong, L.-L., Wang, J.-H., Du, G.-H., Eds.; Springer: Singapore, 2018; Volume 1, pp. 295–302. [Google Scholar]
- Kim, J.; Ham, S.; Hong, H.; Moon, C.; Im, H.I. Brain reward circuits in morphine addiction. Mol. Cells 2016, 39, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Peng, C.; Wu, L.; Gao, S.; Wang, Z.; Dai, L.; Wu, H. Long non-coding RNA MEG3 attends to morphine-mediated autophagy of HT22 cells through modulating ERK pathway. Pharm. Biol. 2019, 57, 536–542. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Chen, Y.M.; He, X.Z.; Wang, S.M.; Xia, Y. δ-Opioid Receptors, microRNAs, and Neuroinflammation in Cerebral Ischemia/Hypoxia. Front. Immunol. 2020, 11, 421. [Google Scholar] [CrossRef]
- Marino, M.; Mele, E.; Pastorino, G.M.G.; Meccariello, R.; Operto, F.F.; Santoro, A.; Viggiano, A. Neuroinflammation: Molecular Mechanisms And Therapeutic Perspectives. Cent. Nerv. Syst. Agents Med. Chem. 2022, 22, 160–174. [Google Scholar]
- Xu, C.; Loh, H.H.; Law, P.Y. Effects of addictive drugs on adult neural stem/progenitor cells. Cell. Mol. Life Sci. 2016, 73, 327–348. [Google Scholar] [CrossRef]
- Xu, C.; Fan, W.; Zhang, Y.; Loh, H.H.; Law, P.Y. Kappa opioid receptor controls neural stem cell differentiation via a miR-7a/Pax6 dependent pathway. Stem Cells 2021, 39, 600–616. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Zheng, H.; Loh, H.H.; Law, P.Y. Morphine modulates mouse hippocampal progenitor cell lineages by upregulating miR-181a level. Stem Cells 2014, 32, 2961–2972. [Google Scholar] [CrossRef]
- Jia, M.; Wang, X.; Zhang, H.; Wang, X.; Ma, H.; Yang, M.; Li, Y.; Cui, C. MicroRNA-132 is involved in morphine dependence via modifying the structural plasticity of the dentate gyrus neurons in rats. Addict. Biol. 2022, 27, e13086. [Google Scholar] [CrossRef]
- Wang, Y.L.; An, X.H.; Zhang, X.Q.; Liu, J.H.; Wang, J.W.; Yang, Z.Y. Morphine induces the apoptosis of mouse hippocampal neurons HT-22 through upregulating miR-181-5p. Eur. Rev Med. Pharmacol. Sci. 2020, 24, 7114–7121. [Google Scholar]
- Gillespie, A.; Mayberry, H.L.; Wimmer, M.E.; Sillivan, S.E. microRNA expression levels in the nucleus accumbens correlate with morphine-taking but not morphine-seeking behaviour in male rats. Eur. J. Neurosci. 2022, 55, 1742–1755. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Hu, Z.; Yao, W.; Le, Q.; Xu, B.; Liu, X.; Ma, L. MiR-218 targets MeCP2 and inhibits heroin seeking behavior. Sci. Rep. 2017, 7, 40413. [Google Scholar] [CrossRef] [PubMed]
- Hoffbuhr, K.C.; Moses, L.M.; Jerdonek, M.A.; Naidu, S.; Hoffman, E.P. Associations between MeCP2 mutations, X-chromosome inactivation, and phenotype. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hong, Q.; Lin, Z.; Ma, H.; Chen, W.; Zhuang, D.; Zhu, H.; Lai, M.; Fu, D.; Zhou, W.; et al. Role of nucleus accumbens microRNA-181a and MeCP2 in incubation of heroin craving in male rats. Psychopharmacology 2021, 238, 2313–2324. [Google Scholar] [CrossRef]
- Mavrikaki, M.; Anastasiadou, E.; Ozdemir, R.A.; Potter, D.; Helmholz, C.; Slack, F.J.; Chartoff, E.H. Overexpression of miR-9 in the Nucleus Accumbens Increases Oxycodone Self-Administration. Int. J. Neuropsychopharmacol. 2019, 22, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Shahjin, F.; Guda, R.S.; Schaal, V.L.; Odegaard, K.; Clark, A.; Gowen, A.; Xiao, P.; Lisco, S.J.; Pendyala, G.; Yelamanchili, S.V. Brain-Derived Extracellular Vesicle microRNA Signatures Associated with In Utero and Postnatal Oxycodone Exposure. Cells 2019, 9, 21. [Google Scholar] [CrossRef]
- Chen, F.; Xu, Y.; Shi, K.; Zhang, Z.; Xie, Z.; Wu, H.; Ma, Y.; Zhou, Y.; Chen, C.; Yang, J.; et al. Multi-omics study reveals associations among neurotransmitter, extracellular vesicle-derived microRNA and psychiatric comorbidities during heroin and methamphetamine withdrawal. Biomed. Pharmacother. 2022, 155, 113685. [Google Scholar] [CrossRef]
- Floris, G.; Gillespie, A.; Zanda, M.T.; Dabrowski, K.R.; Sillivan, S.E. Heroin Regulates Orbitofrontal Circular RNAs. Int. J. Mol. Sci. 2022, 23, 1453. [Google Scholar] [CrossRef]
- Yu, H.; Xie, B.; Zhang, J.; Luo, Y.; Galaj, E.; Zhang, X.; Shen, Q.; Liu, Y.; Cong, B.; Wen, D.; et al. The role of circTmeff-1 in incubation of context-induced morphine craving. Pharmacol. Res. 2021, 170, 105722. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhang, J.; Ma, C.; Yu, H.; Ni, Z.; Cong, B.; Wen, D. Roles of miR-592-3p and Its Target Gene, TMEFF1, in the Nucleus Accumbens During Incubation of Morphine Craving. Int. J. Neuropsychopharmacol. 2022, 25, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Sorg, B.A. Reconsolidation of drug memories. Neurosci. Biobehav. Rev. 2012, 36, 1400–1417. [Google Scholar] [CrossRef]
- Shen, Q.; Xie, B.; Galaj, E.; Yu, H.; Li, X.; Lu, Y.; Zhang, M.; Wen, D.; Ma, C. CircTmeff-1 in the nucleus accumbens regulates the reconsolidation of cocaine-associated memory. Brain Res. Bull. 2022, 185, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E. Treatment of neuropathic pain. J. Korean Med. Assoc. 2021, 64, 484–490. [Google Scholar] [CrossRef]
- NIDA. Prescription Opioids DrugFacts. 1 June 2021. Available online: http://nida.nih.gov/publications/drugfacts/prescription-opioids (accessed on 25 March 2023).
- Tai, B.; Dobbins, R.; Blackeney, Q.; Liu, D.; Moran, L. The NIDA clinical trials network: Evolving, expanding, and addressing the opioid epidemic. Addict Sci. Clin. Pract. 2021, 16, 28. [Google Scholar] [CrossRef]
- Mazzeo, F.; Santamaria, S.; Monda, V.; Tafuri, D.; Dalia, C.; Varriale, L.; de Blasio, S.; Esposito, V.; Messina, G.; Monda, M. Dietary supplements use in competitive and non-competitive boxer: An exploratory study. Biol. Med. 2016, 8, 2–8. [Google Scholar]
- Mazzeo, F. Attitude and practice of substance misuse and dietary supplements to improve performance in sport. J. Subst. Use 2019, 24, 581–586. [Google Scholar] [CrossRef]
- Vernec, A.; Pipe, A.; Slack, A. A painful dilemma? Analgesic use in sport and the role of anti-doping. Br. J. Sports Med. 2017, 51, 1243–1244. [Google Scholar] [CrossRef]
- Volkow, N.; Benveniste, H.; McLellan, A.T. Use and Misuse of Opioids in Chronic Pain. Annu. Rev. Med. 2018, 69, 451–465. [Google Scholar] [CrossRef]
- Nafziger, A.N.; Barkin, R.L. Opioid Therapy in Acute and Chronic Pain. J. Clin. Pharmacol. 2018, 58, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Toce, M.S.; Chai, P.R.; Burns, M.M.; Boyer, E.W. Pharmacologic Treatment of Opioid Use Disorder: A Review of Pharmacotherapy, Adjuncts, and Toxicity. J. Med. Toxicol. 2018, 14, 306–322. [Google Scholar] [CrossRef]
- Antunes, F.J.R. Musculoskeletal traumatic pain. DOLOR 2022, 37, 86–90. [Google Scholar]
- Montesano, P.; Palmieri, S.; Massa, B.; Mazzeo, F. From “sliding” to “winding” filaments theory: A narrative review of mechanisms behind skeletal muscle contraction. J. Hum. Sport Exerc. 2020, 15, S806–S814. [Google Scholar]
- Pietro, M.; Rocco, S.; Felice, S.; Madonna, G.; Filomena, M. Soccer fields in synthetic and natural grass: A comparative study on muscular injuries of the lower limb. Sport Sci. 2020, 14, 7–12. [Google Scholar]
- Holgado, D.; Hopker, J.; Sanabria, D.; Zabala, M. Analgesics and sport performance: Beyond the pain-modulating effects. PM R 2018, 10, 72–82. [Google Scholar] [CrossRef]
- Mazzeo, F.; Santamaria, S.; Donisi, A.; Montesano, P. Use and attitudes toward dietary supplements and drugs amongst Italian elite athletes and its correlation with banned doping substances. J. Hum. Sport Exerc. 2019, 14, S970–S980. [Google Scholar]
- Motola, G.; Russo, F.; Mazzeo, F.; Rinaldi, B.; Capuano, A.; Rossi, F.; Filippelli, A. Over-the-counter oral nonsteroidal anti-inflammatory drugs: A pharmacoepidemiologic study in southern Italy. Adv. Ther. 2001, 18, 216–222. [Google Scholar] [CrossRef]
- Schenone, S.; Bruno, O.; Ranise, A.; Brullo, C.; Bondavalli, F.; Filippelli, W.; Mazzeo, F.; Capuano, A.; Falcone, G. 2-aryl-3-phenylamino-4,5-dihydro-2h-benz[g]indazoles with analgesic activity. Farmaco 2003, 58, 845–849. [Google Scholar] [CrossRef]
- Mazzeo, F.; Santamaria, S.; Onofrio, V.D. Data investigation on the performance-enhancing drugs spread in Italy among young athletes: Prevention trough education and the fight against doping in sport. J. Hum. Sport Exerc. 2021, 16, 705–715. [Google Scholar]
- Mazzeo, F.; Santamaria, S.; Montesano, P.; Rinaldi, M.; Madonna, G. Updated evidence report for the anti-doping research: Analysis from 2008 to 2018 for performance-enhancing drugs and gene doping test development. J. Phys. Ed. Sport 2020, 20, 2378–2385. [Google Scholar]
- Ekhtiari, S.; Yusuf, I.; AlMakadma, Y.; MacDonald, A.; Leroux, T.; Khan, M. Opioid Use in Athletes: A Systematic Review. Sports Health 2020, 12, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, F. Anabolic steroid use in sports and in physical activity: Overview and analysis. Sport Mont. 2018, 16, 113–118. [Google Scholar] [CrossRef]
- Mazzeo, F.; Monda, M.; Messina, G.; Santamaria, S.; Messina, A.; Montesano, M.; Tafuri, D. Doping in Italy: An analysis of its spread in ten years. Biol. Med. 2016, 8, 1. [Google Scholar] [CrossRef]
- Del Tredici, A. Optimizing Opioid Therapy with Pharmacogenetics. Pract. Pain Manag. 2021, 21. Available online: https://www.practicalpainmanagement.com/treatments/pharmacological/opioids/optimizing-opioid-therapy-pharmacogenetics (accessed on 30 March 2023).
- Smith, H.S. Variations in opioid responsiveness. Pain Physician 2008, 11, 237–248. [Google Scholar] [CrossRef]
- Owusu Obeng, A.; Hamadeh, I.; Smith, M. Review of Opioid Pharmacogenetics and Considerations for Pain Management. Pharmacotherapy 2017, 37, 1105–1121. [Google Scholar] [CrossRef]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Callaghan, J.T.; Samer, C.F.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin. Pharmacol. Ther. 2021, 110, 888–896. [Google Scholar] [CrossRef]
- Mazzeo, F.; Volpe, R.A. From gene doping to athlete biological passport. Sport Sci. 2016, 9, 97–103. [Google Scholar]
- Singh, A.; Zai, C.; Mohiuddin, A.G.; Kennedy, J.L. The pharmacogenetics of opioid treatment for pain management. J. Psychopharmacol. 2020, 34, 1200–1209. [Google Scholar] [CrossRef]
- Mazzeo, F.; Ascione, A. New technology and no drugs in sport: Gene doping regulation, education and research. Sport Sci. 2020, 14, 18–23. [Google Scholar]
- Ahmadi, S.; Zobeiri, M.; Mohammadi Talvar, S.; Masoudi, K.; Khanizad, A.; Fotouhi, S.; Bradburn, S. Differential expression of H19, BC1, MIAT1, and MALAT1 long non-coding RNAs within key brain reward regions after repeated morphine treatment. Behav. Brain Res. 2021, 414, 113478. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Y.; Zhang, K.; Huang, W.; Mu, Y.; Li, Y.; Ouyang, H. CircNf1-mediated CXCL12 expression in the spinal cord contributes to morphine analgesic tolerance. Brain Behav. Immun. 2023, 107, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Hilla, A.M.; Baehr, A.; Leibinger, M.; Andreadaki, A.; Fischer, D. CXCR4/CXCL12-mediated entrapment of axons at the injury site compromises optic nerve regeneration. Proc. Natl. Acad. Sci. USA 2021, 118, e2016409118. [Google Scholar] [CrossRef]
- Li, M.; Ransohoff, R.-M. Multiple roles of chemokine CXCL12 in the central nervous system: A migration from immunology to neurobiology. Prog. Neurobiol. 2008, 84, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H. Impact of physical activity and doping on epigenetic gene regulation. Drug Test. Anal. 2011, 3, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Postnikov, P.V.; Efimova, Y.A.; Pronina, I.V. Circulating MicroRNAs as a New Class of Biomarkers of Physiological Reactions of the Organism to the Intake of Dietary Supplements and Drugs. Microrna 2022, 11, 25–35. [Google Scholar]
- Marie-Claire, C.; Jourdaine, C.; Lépine, J.P.; Bellivier, F.; Bloch, V.; Vorspan, F. Pharmacoepigenomics of opiates and methadone maintenance treatment: Current data and perspectives. Pharmacogenomics 2017, 18, 1359–1372. [Google Scholar] [CrossRef]
- Zandonai, T.; Escorial, M.; Peiró, A.M. Codeine and Tramadol Use in Athletes: A Potential for Abuse. Front. Pharmacol. 2021, 12, 661781. [Google Scholar] [CrossRef]
| Ligands | Opioid Receptors | ||
|---|---|---|---|
| (Opioid Agonists) | MOR | DOR | KOR |
| Etorphine | +++ | +++ | +++ |
| Fentanyl | +++ | ||
| Hydromorphone | +++ | ||
| Levorphanol | +++ | ||
| Methadone | +++ | ||
| Morphine | +++ | ||
| Sufentanil | +++ | ||
| DAMGO | +++ | ||
| Bremazocine | + | +++ | |
| Buprenorphine | P | ||
| Butorphanol | P | +++ | |
| Nalbuphine. | - - | ++ | |
| [D-Pen2,D-Pen5]-Enkephalin (DPDPE) | +++ | ||
| U50,488 | ++ | ||
| Ligands | Opioid Receptors | ||
|---|---|---|---|
| (Opioid Antagonists) | MOR | DOR | KOR |
| Naloxone | - - - | - | - - |
| Naltrexone | - - - | - | |
| CTOP D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 | - - - | ||
| Diprenorphine | - - - | - - | - - - |
| beta-funaltrexamine | - - - | - | ++ |
| Naloxonazine | - - - | - | - |
| nor-binaltorfimina | - | - | - - - |
| Naltrindole | - | - - - | - |
| naloxone benzoylhydrazone | - - - | - | - |
| Receptor | Site of Action | Effects | References |
|---|---|---|---|
| MOR | Systemic | Analgesia, euphoria, constipation, respiratory depression, Nausea/vomiting | [32,38,39,40,41,42,43,44,45,46] |
| Peripheral | Analgesia, constipation, reduced inflammation | [40,42,47,48,49,50] | |
| DOR | Systemic | Analgesia, convulsions, anxiolysis | [51,52,53] |
| Peripheral | Analgesia, Decreases colonic transit time (constipation), | [48,54,55] | |
| KOR | Systemic | Analgesia, diuresis, dysphoria | [56,57] |
| Peripheral | Analgesia, reduced inflammation, Visceral nociception antagonist | [25,39,58,59,60] |
| Type of Receptor | Type of DA Neurons | Functional Response | Ref |
|---|---|---|---|
| MOR | VTA (tertiary cells) | Membrane hyperpolarization | [109,112] |
| VTA (Ih+) | Increase firing (indirect effect) | [109] | |
| VTA (Ih+) | No effect | [109] | |
| VTA | Increase firing (indirect effect) | [104] | |
| VTA/SNpc | Inhibition of GABA-A IPSC | [113] | |
| VTA | Excited via CdCl2-sensitive cond. | [103] | |
| VTA--> NAcc med shell | Increased firing (indirect) | [114] | |
| KOR | VTA--> BLA | Firing inhibition GIRK activation | [63,108,115] |
| VTA principal cells | Firing inhibition GIRK activation | [109] | |
| VTA--> mPFC | Firing inhibition/GIRK activation | [108,110,111] | |
| VTA--> NAcc | No effect/Firing inhibition | ||
| SNpc | Mild Firing inhibition/GIRK activation | [111] | |
| VTA | Integration of excitatory inputs | [8] | |
| DOR 1/DOR 2 | VTA (DA and non-DA neurons) | K+-mediated hyperpolarization | [99] |
| VTA | Ca2+-dependent excitation | [99] | |
| VTA | Inhibit GABA release | [115] | |
| NOP | VTA | Big outward current | [116] |
| VTA--> mPFC/NAcc | Small outward current | [117] | |
| VTA--> pACC | Small inward current | [117] |
| ncRNA | Targets | References | |
|---|---|---|---|
| Addictive-like behavior | miRNA592-3p | DRD2 REST | [148] |
| Heroin craving | miRNA181a | MeCP2 | [147] |
| Heroin seeking | miRNA218 | MeCP2 | [145] |
| Morphine craving | circTmeff-1 | miRNA541-5p miRNA6934-3p | [153] |
| miRNA592-3p | TMFF1 | [153] | |
| Morphine-taking behaviour | miRNA1298-5p | - | [144] |
| miRNA32-5p | Dusp5 Btg2 Cldn11 Dcaf6 | [144] | |
| Morphine withdrawal symptoms | miRNA132-3p | - | [142] |
| Reconsolidation of cocaine-associated memory | circTmeff-1 | miRNA206 BDNF | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzeo, F.; Meccariello, R.; Guatteo, E. Molecular and Epigenetic Aspects of Opioid Receptors in Drug Addiction and Pain Management in Sport. Int. J. Mol. Sci. 2023, 24, 7831. https://doi.org/10.3390/ijms24097831
Mazzeo F, Meccariello R, Guatteo E. Molecular and Epigenetic Aspects of Opioid Receptors in Drug Addiction and Pain Management in Sport. International Journal of Molecular Sciences. 2023; 24(9):7831. https://doi.org/10.3390/ijms24097831
Chicago/Turabian StyleMazzeo, Filomena, Rosaria Meccariello, and Ezia Guatteo. 2023. "Molecular and Epigenetic Aspects of Opioid Receptors in Drug Addiction and Pain Management in Sport" International Journal of Molecular Sciences 24, no. 9: 7831. https://doi.org/10.3390/ijms24097831
APA StyleMazzeo, F., Meccariello, R., & Guatteo, E. (2023). Molecular and Epigenetic Aspects of Opioid Receptors in Drug Addiction and Pain Management in Sport. International Journal of Molecular Sciences, 24(9), 7831. https://doi.org/10.3390/ijms24097831






