A Facile Synthesis and Molecular Characterization of Certain New Anti-Proliferative Indole-Based Chemical Entities
Abstract
:1. Introduction
2. Results and Discussions
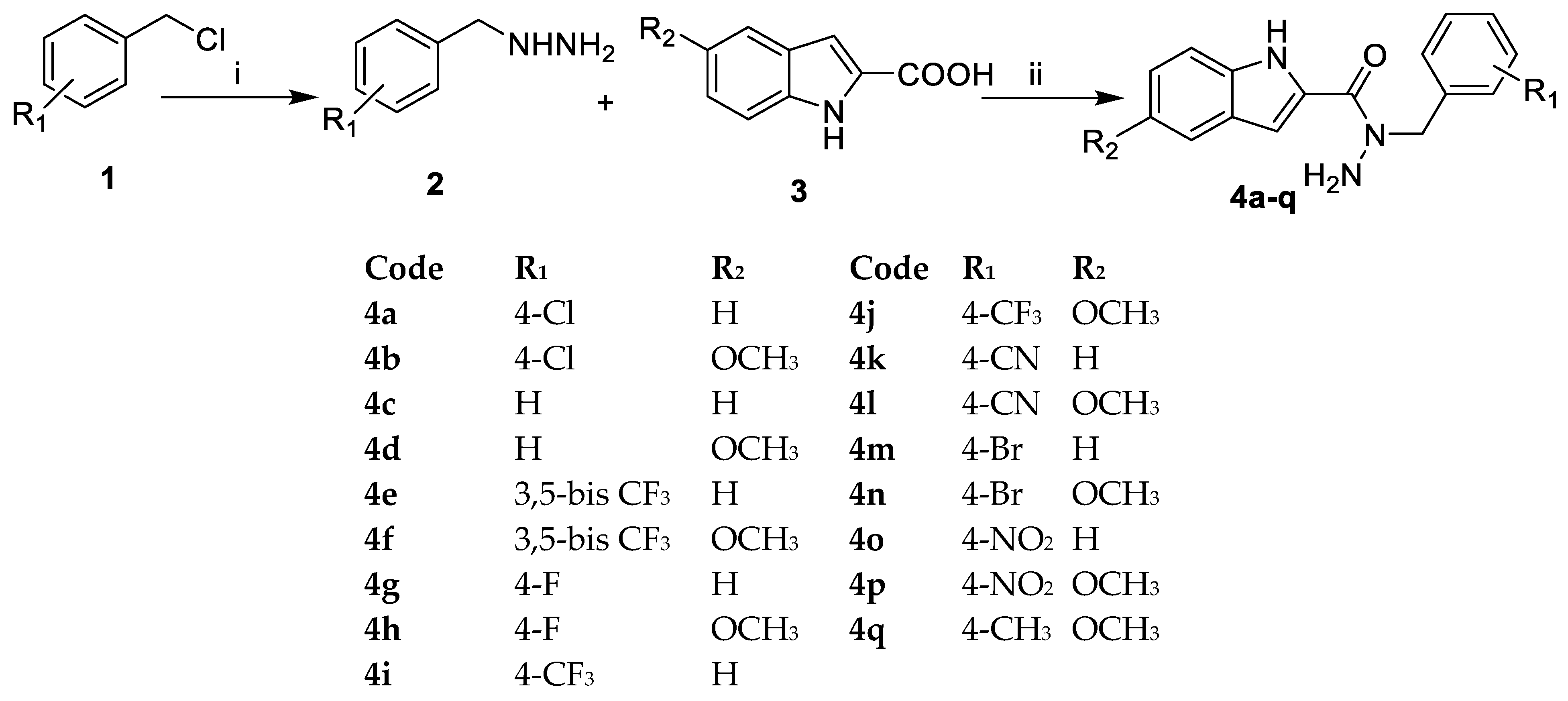
2.1. Chemistry
2.2. Antiproliferative Activity
2.3. Flowcytometry
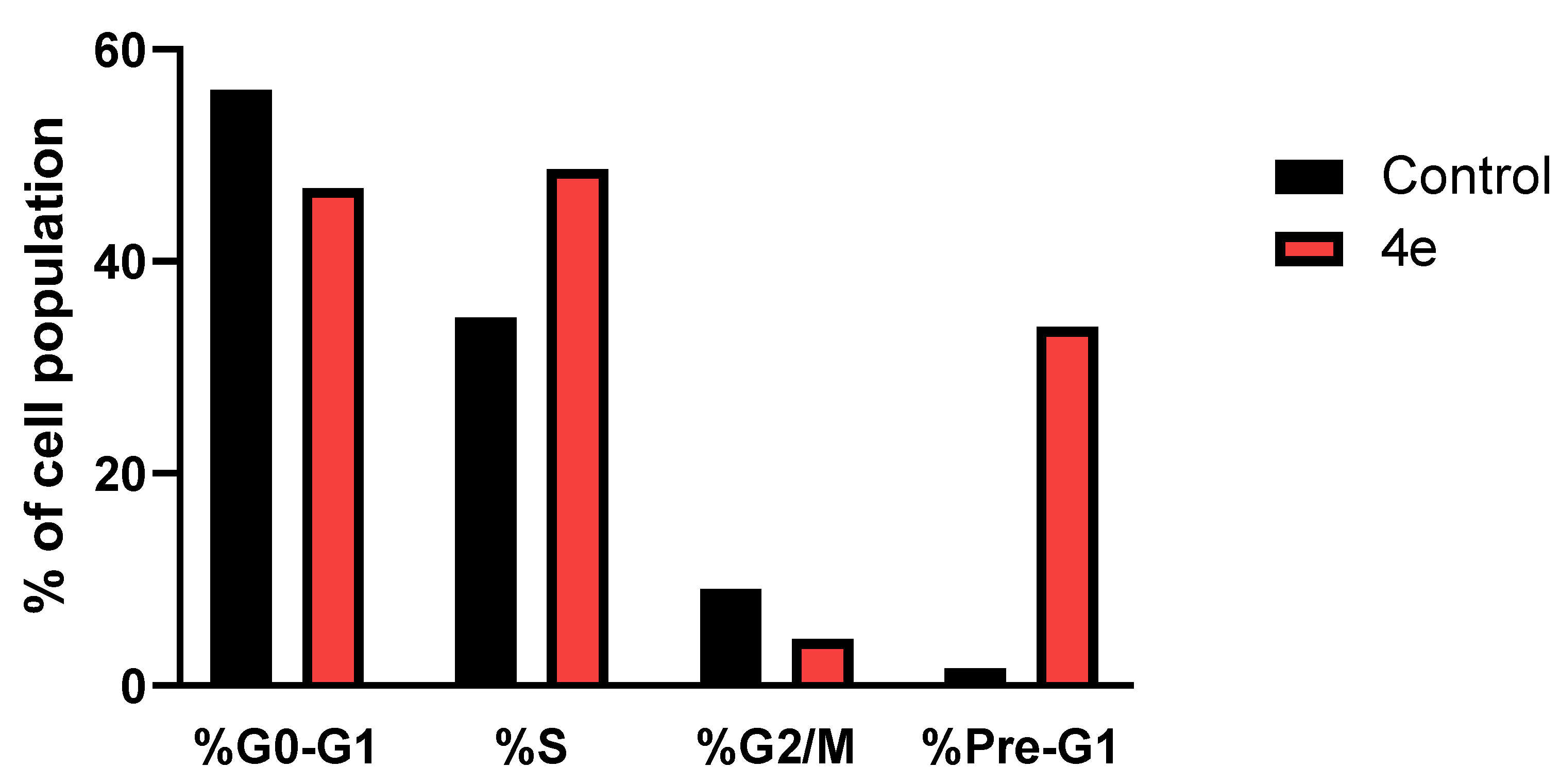
2.4. Cell Cycle Arrest
3. Materials and Methods
3.1. General
3.2. Chemistry
3.2.1. General Method for the Synthesis of Substituted Benzyl Hydrazine 3
3.2.2. General Method for the Synthesis of the Target Compounds 4a–q
3.3. MTT Assay
3.4. Flow Cytometry
3.5. Cell Cycle Arrest
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Yar, M.S. Structure-Activity Relationship (SAR) Study and Design Strategies of Nitrogen-Containing Heterocyclic Moieties for Their Anticancer Activities. Eur. J. Med. Chem. 2017, 125, 143–189. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabli, R.I.; Alsulami, M.A.; Bukhari, S.I.; Moubayed, N.M.S.; Al-Mutairi, M.S.; Attia, M.I. Design, Synthesis, and Antimicrobial Activity of Certain New Indole-1,2,4 Triazole Conjugates. Molecules 2021, 26, 2292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Chen, Q.; Yang, G.F. A Review on Recent Developments of Indole-Containing Antiviral Agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef]
- Angelova, V.T.; Rangelov, M.; Todorova, N.; Dangalov, M.; Andreeva-Gateva, P.; Kondeva-Burdina, M.; Karabeliov, V.; Shivachev, B.; Tchekalarova, J. Discovery of Novel Indole-Based Aroylhydrazones as Anticonvulsants: Pharmacophore-Based Design. Bioorg. Chem. 2019, 90, 103028. [Google Scholar] [CrossRef]
- Somappa, S.B.; Biradar, J.S.; Rajesab, P.; Rahber, S.; Sundar, M. A One-Pot Synthesis of Indole-Appended Heterocycles as Potent Anti-Inflammatory, Analgesic, and CNS Depressant Agents. Mon. Chem.-Chem. Mon. 2015, 146, 2067–2078. [Google Scholar] [CrossRef]
- Radwan, M.A.; Ragab, E.A.; Sabry, N.M.; El-Shenawy, S.M. Synthesis and Biological Evaluation of New 3-Substituted Indole Derivatives as Potential Anti-Inflammatory and Analgesic Agents. Bioorg. Med. Chem. 2007, 15, 3832–3841. [Google Scholar] [CrossRef]
- Dadashpour, S.; Emami, S. Indole in the Target-Based Design of Anticancer Agents: A Versatile Scaffold with Diverse Mechanisms. Eur. J. Med. Chem. 2018, 150, 9–29. [Google Scholar] [CrossRef]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A Privileged Scaffold for the Design of Anti-Cancer Agents. Eur. J. Med. Chem. 2019, 183, 111691. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PI3K/Akt/MTOR and Ras/Raf/MEK/ERK Signaling Pathways Inhibitors as Anticancer Agents: Structural and Pharmacological Perspectives. Eur. J. Med. Chem. 2016, 109, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Manal, M.; Chandrasekar, M.J.N.; Priya, J.G.; Nanjan, M.J. Inhibitors of Histone Deacetylase as Antitumor Agents: A Critical Review. Bioorg. Chem. 2016, 67, 18–42. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.C.; Jian, X.E.; Chen, P.; Huang, C.; Yin, J.; Huang, J.C.; Li, J.S.; Zhao, P.L. Design, Synthesis and Biological Evaluation of Novel Indole-Based Oxalamide and Aminoacetamide Derivatives as Tubulin Polymerization Inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 126816. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Shokrzadeh, M.; Modanloo, M.; Ziar, A.; Riazi, G.H.; Emami, S. New Indole-Based Chalconoids as Tubulin-Targeting Antiproliferative Agents. Bioorg. Chem. 2017, 75, 86–98. [Google Scholar] [CrossRef]
- Hwang, D.-J.; Wang, J.; Li, W.; Miller, D.D. Structural Optimization of Indole Derivatives Acting at Colchicine Binding Site as Potential Anticancer Agents. ACS Med. Chem. Lett. 2015, 6, 993–997. [Google Scholar] [CrossRef] [Green Version]
- Cong, H.; Zhao, X.; Castle, B.T.; Pomeroy, E.J.; Zhou, B.; Lee, J.; Wang, Y.; Bian, T.; Miao, Z.; Zhang, W.; et al. An Indole-Chalcone Inhibits Multidrug-Resistant Cancer Cell Growth by Targeting Microtubules. Mol. Pharm. 2018, 15, 3892–3900. [Google Scholar] [CrossRef]
- Dhiman, A.; Sharma, R.; Singh, R.K. Target-Based Anticancer Indole Derivatives and Insight into Structure-activity Relationship: A Mechanistic Review Update (2018–2021). Acta Pharm. Sin. B 2022, 12, 3006–3027. [Google Scholar] [CrossRef]
- Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. The Importance of Indole and Azaindole Scaffold in the Development of Antitumor Agents. Eur. J. Med. Chem. 2020, 203, 112506. [Google Scholar] [CrossRef]
- Sherer, C.; Snape, T.J. Heterocyclic Scaffolds as Promising Anticancer Agents against Tumours of the Central Nervous System: Exploring the Scope of Indole and Carbazole Derivatives. Eur. J. Med. Chem. 2015, 97, 552–560. [Google Scholar] [CrossRef]
- Devi, N.; Kaur, K.; Biharee, A.; Jaitak, V. Recent Development in Indole Derivatives as Anticancer Agent: A Mechanistic Approach. Anticancer Agents Med. Chem. 2021, 21, 1802–1824. [Google Scholar] [CrossRef]
- Al-Wabli, R.I.; Almomen, A.A.; Almutairi, M.S.; Keeton, A.B.; Piazza, G.A.; Attia, M.I. New Isatin–Indole Conjugates: Synthesis, Characterization, and a Plausible Mechanism of Their in vitro Antiproliferative Activity. Drug Des. Dev. Ther. 2020, 14, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eldehna, W.M.; Al-Wabli, R.I.; Almutairi, M.S.; Keeton, A.B.; Piazza, G.A.; Abdel-Aziz, H.A.; Attia, M.I. Synthesis and Biological Evaluation of Certain Hydrazonoindolin-2-One Derivatives as New Potent Anti-Proliferative Agents. J. Enzym. Inhib. Med. Chem. 2018, 33, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Valeur, E.; Bradley, M. Amide Bond Formation: Beyond the Myth of Coupling Reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
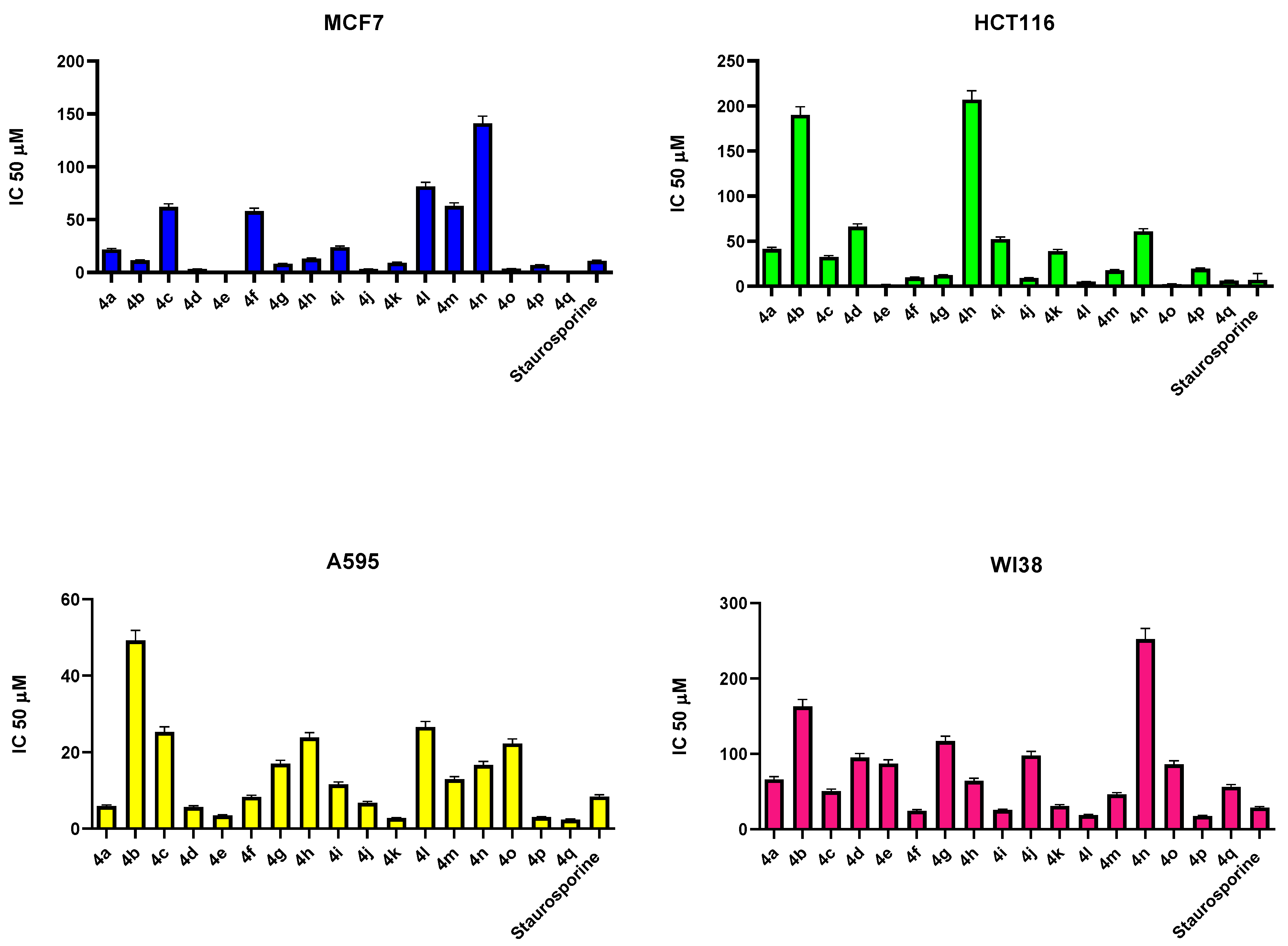


| Sample | Cytotoxicity IC50 (uM) | ||||
|---|---|---|---|---|---|
| Code | M.W (g/mol) | MCF7 | HCT116 | A549 | WI38 |
| 4a | 299 | 21.7 ± 1.04 | 41.3 ± 1.98 | 5.9 ± 0.32 | 66.2 ± 3.75 |
| 4b | 329 | 11.5 ± 0.55 | 190 ± 9.12 | 49.2 ± 2.68 | 163 ± 9.25 |
| 4c | 265 | 62 ± 2.97 | 32.5 ± 1.56 | 25.3 ± 1.38 | 50.4 ± 2.86 |
| 4d | 295 | 3.23 ± 0.16 | 66.2 ± 3.17 | 5.69 ± 0.31 | 95.2 ± 5.39 |
| 4e | 401 | 0.57 ± 0.03 | 1.95 ± 0.09 | 3.49 ± 0.19 | 87.2 ± 4.94 |
| 4f | 432 | 58.1 ± 2.78 | 9.79 ± 0.47 | 8.33 ± 0.45 | 24.4 ± 1.38 |
| 4g | 283 | 8.31 ± 0.4 | 12.3 ± 0.59 | 17 ± 0.93 | 117 ± 6.65 |
| 4h | 313 | 13.1 ± 0.63 | 207 ± 9.94 | 23.9 ± 1.3 | 64.1 ± 3.63 |
| 4i | 383 | 23.9 ± 1.15 | 52.2 ± 2.5 | 11.6 ± 0.63 | 25.3 ± 1.43 |
| 4j | 364 | 3.27 ± 0.16 | 9.16 ± 0.44 | 6.78 ± 0.37 | 97.9 ± 5.54 |
| 4k | 290 | 9.21 ± 0.44 | 39.1 ± 1.88 | 2.65 ± 0.14 | 30.8 ± 1.74 |
| 4l | 321 | 81.4 ± 3.9 | 5.02 ± 0.24 | 26.6 ± 1.45 | 18.5 ± 1.05 |
| 4m | 343 | 62.9 ± 3.01 | 17.5 ± 0.84 | 12.9 ± 0.7 | 46.1 ± 2.61 |
| 4n | 375 | 141 ± 6.74 | 61 ± 2.92 | 16.7 ± 0.91 | 252 ± 14.3 |
| 4o | 310 | 3.66 ± 0.18 | 2.41 ± 0.12 | 22.3 ± 1.21 | 86 ± 4.87 |
| 4p | 341 | 7.01 ± 0.34 | 19.4 ± 0.93 | 3.02 ± 0.16 | 17.4 ± 0.99 |
| 4q | 310 | 1.01 ± 0.05 | 6.45 ± 0.31 | 2.4 ± 0.13 | 56.2 ± 3.18 |
| Staurosporine | 466.5 | 11.1 ± 0.53 | 7.02 ± 0.34 | 8.42 ± 0.46 | 28.5 ± 1.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wabli, R.I.; Issa, I.S.; Al-mutairi, M.S.; Almomen, A.A.; Attia, M.I. A Facile Synthesis and Molecular Characterization of Certain New Anti-Proliferative Indole-Based Chemical Entities. Int. J. Mol. Sci. 2023, 24, 7862. https://doi.org/10.3390/ijms24097862
Al-Wabli RI, Issa IS, Al-mutairi MS, Almomen AA, Attia MI. A Facile Synthesis and Molecular Characterization of Certain New Anti-Proliferative Indole-Based Chemical Entities. International Journal of Molecular Sciences. 2023; 24(9):7862. https://doi.org/10.3390/ijms24097862
Chicago/Turabian StyleAl-Wabli, Reem I., Iman S. Issa, Maha S. Al-mutairi, Aliyah A. Almomen, and Mohamed I. Attia. 2023. "A Facile Synthesis and Molecular Characterization of Certain New Anti-Proliferative Indole-Based Chemical Entities" International Journal of Molecular Sciences 24, no. 9: 7862. https://doi.org/10.3390/ijms24097862
APA StyleAl-Wabli, R. I., Issa, I. S., Al-mutairi, M. S., Almomen, A. A., & Attia, M. I. (2023). A Facile Synthesis and Molecular Characterization of Certain New Anti-Proliferative Indole-Based Chemical Entities. International Journal of Molecular Sciences, 24(9), 7862. https://doi.org/10.3390/ijms24097862






