Computational Insights and In Silico Characterization of a Novel Mini-Lipoxygenase from Nostoc Sphaeroides and Its Application in the Quality Improvement of Steamed Bread
Abstract
:1. Introduction
2. Results and Discussion
2.1. Excavation of a Hypothetical LOX from N. sphaeroides
2.2. Structural Modeling of NsLOX and Structure Analysis
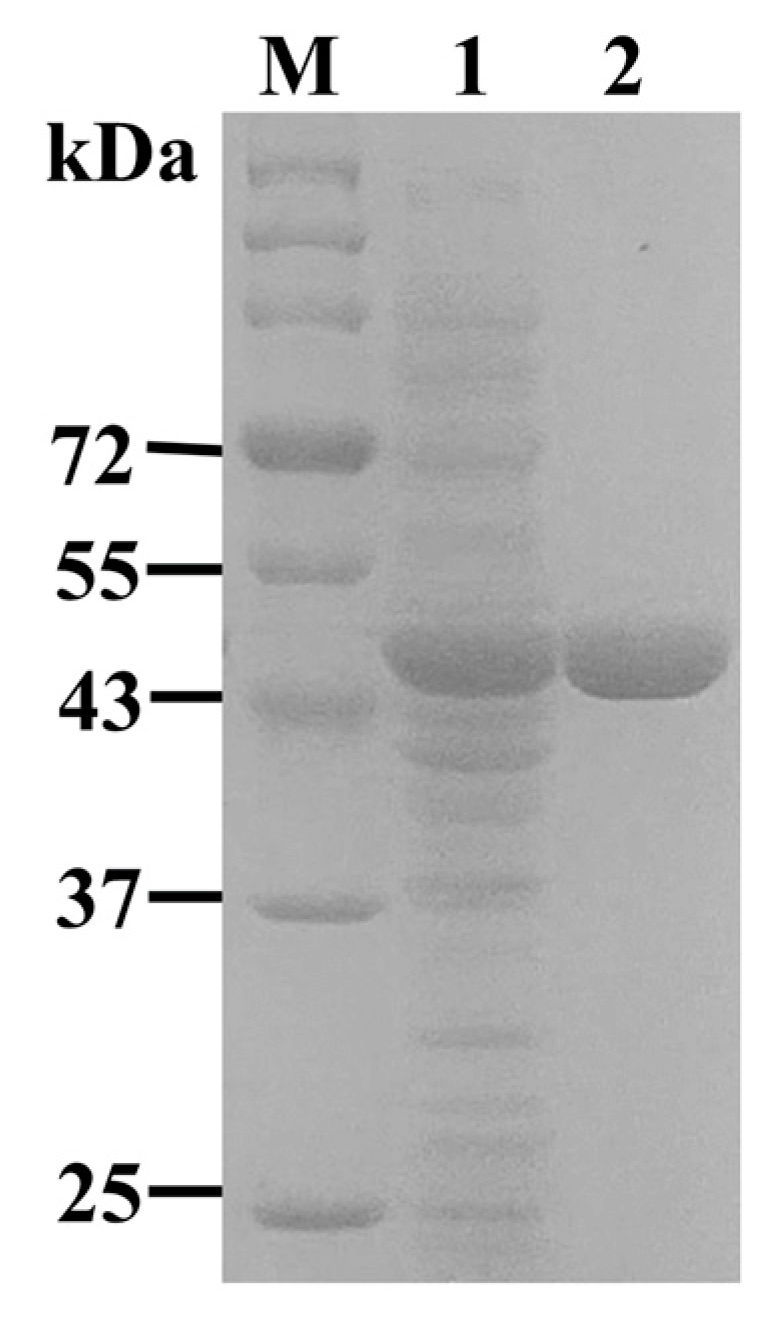
2.3. Expression and Purification of NsLOX in E. coli
2.4. Enzymatic Properties of the Purified NsLOX
2.5. Substrate Spectrum, Kinetic Parameter, and Product Analysis of Purified NsLOX
2.6. Application of Purified NsLOX in the Steamed Bread
3. Materials and Methods
3.1. Strains and Chemicals
3.2. In Silico Sequence and Phylogenetic Analysis of NsLOX Gene
3.3. Structural Modeling of NsLOX
3.4. Recombinant Expression of NsLOX Gene in E. coli
3.5. Purification of NsLOX
3.6. Spectrophotometirc Assay of the LOX Activity
3.7. Effects of pH and Temperature on the Activity and Stability of NsLOX
3.8. Effects of Metal Ions and Chemical Reagents on the Activity of NsLOX
3.9. Substrate Specificity and Kinetic Parameters of NsLOX
3.10. Product Analysis of NsLOX
3.11. Effect of NsLOX on the Quantity of Steamed Bread
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, H.; Xia, B.; He, Y.; Lu, Z.; Bie, X.; Zhao, H.; Zhang, C.; Lu, F. Expression, purification, and characterization of a novel acidic Lipoxygenase from Myxococcus xanthus. Protein Expr. Purif. 2017, 138, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Stolterfoht, H.; Rinnofner, C.; Winkler, M.; Pichler, H. Recombinant Lipoxygenases and Hydroperoxide Lyases for the Synthesis of Green Leaf Volatiles. J. Agric. Food Chem. 2019, 67, 13367–13392. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Otsuki, A.; Mori, H.; Li, P.; Kinoshita, M.; Kawakami, Y.; Tsuji, H.; Fang, D.Z.; Takahashi, Y. Two new monoterpene glycosides from Qing Shan Lu Shui tea with inhibitory effects on leukocyte-type 12-lipoxygenase activity. Molecules 2013, 18, 4257–4266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.-U.; Hong, S.-H.; Oh, D.-K. Regiospecificity of a novel bacterial lipoxygenase from Myxococcus xanthus for polyunsaturated fatty acids. BBA-Mol. Cell Biol. Lipids 2018, 1863, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Hayward, S.; Cilliers, T.; Swart, P. Lipoxygenases: From Isolation to Application. Compr. Rev. Food Sci. Food Saf. 2017, 16, 199–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Mandal, R.; Singh, A.; Pratap Singh, A. Legume lipoxygenase: Strategies for application in food industry. Legume Sci. 2020, 2, e44. [Google Scholar] [CrossRef]
- Liburdi, K.; Esti, M.; Petroselli, V.; Mendler-Drienyovszki, N.; Radicetti, E.; Mancinelli, R. Catalytic properties of lipoxygenase extracted from different varieties of Pisum sativum and Lens culinaris. J. Food Biochem. 2021, 45, e13617. [Google Scholar] [CrossRef]
- Pang, C.; Liu, S.; Zhang, G.; Zhou, J.; Du, G.; Li, J. Improving the catalytic efficiency of Pseudomonas aeruginosa lipoxygenase by semi-rational design. Enzyme Microb. Technol. 2022, 162, 110120. [Google Scholar] [CrossRef]
- Busquets, M.; Deroncele, V.; Vidal-Mas, J.; Rodriguez, E.; Guerrero, A.; Manresa, A. Isolation and characterization of a lipoxygenase from Pseudomonas 42A2 responsible for the biotransformation of oleic acid into (S)-(E)-10-hydroxy-8-octadecenoic acid. Antonie Leeuwenhoek 2004, 85, 129–139. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, C.; Lu, Z.; Xia, B.; Bie, X.; Zhao, H.; Lu, F.; Yang, G.-Y. Consensus design for improved thermostability of lipoxygenase from Anabaena sp. PCC 7120. BMC Biotechnol. 2018, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chen, M.; Xia, B.; Lu, Z.; Khoo, K.S.; Show, P.L.; Lu, F. Characterization and Preliminary Application of a Novel Lipoxygenase from Enterovibrio norvegicus. Foods 2022, 11, 2864. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-R.; An, J.-U.; Lee, S.-H.; Oh, D.-K. Selective Production of 9R-Hydroxy-10E,12Z,15Z-Octadecatrienoic Acid from alpha-Linolenic Acid in Perilla Seed Oil Hydrolyzate by a Lipoxygenase from Nostoc sp. SAG 25.82. PLoS ONE 2015, 10, e0137785. [Google Scholar] [CrossRef] [Green Version]
- Newie, J.; Neumann, P.; Werner, M.; Mata, R.A.; Ficner, R.; Feussner, I. Lipoxygenase 2 from Cyanothece sp. controls dioxygen insertion by steric shielding and substrate fixation. Sci. Rep. 2017, 7, 2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, I.; Feussner, I. Oxylipin formation in Nostoc punctiforme (PCC73102). Phytochemistry 2007, 68, 1120–1127. [Google Scholar] [CrossRef]
- Mashhadi, Z.; Boeglin, W.E.; Brash, A.R. Robust inhibitory effects of conjugated linolenic acids on a cyclooxygenase-related linoleate 10S-dioxygenase: Comparison with COX-1 and COX-2. BBA-Mol. Cell Biol. Lipids 2015, 1851, 1346–1352. [Google Scholar] [CrossRef] [Green Version]
- Joshi, N.; Hoobler, E.K.; Perry, S.; Diaz, G.; Fox, B.; Holman, T.R. Kinetic and structural investigations into the allosteric and pH effect on the substrate specificity of human epithelial 15-lipoxygenase-2. Biochemistry 2013, 52, 8026–8035. [Google Scholar] [CrossRef] [Green Version]
- Newie, J.; Andreou, A.; Neumann, P.; Einsle, O.; Feussner, I.; Ficner, R. Crystal structure of a lipoxygenase from Cyanothece sp. may reveal novel features for substrate acquisition. J. Lipid Res. 2016, 57, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Segraves, E.N.; Chruszcz, M.; Neidig, M.L.; Ruddat, V.; Zhou, J.; Wecksler, A.T.; Minor, W.; Solomon, E.I.; Holman, T.R. Kinetic, spectroscopic, and structural investigations of the soybean lipoxygenase-1 first-coordination sphere mutant, Asn694Gly. Biochemistry 2006, 45, 10233–10242. [Google Scholar] [CrossRef]
- Boutaud, O.; Brash, A. Purification and catalytic activities of the two domains of the allene oxide synthase-lipoxygenase fusion protein of the coral Plexaura homomalla. J. Biol. Chem. 1999, 274, 33764–33770. [Google Scholar] [CrossRef]
- Guo, J.; Coker, A.R.; Wood, S.P.; Cooper, J.B.; Chohan, S.M.; Rashid, N.; Akhtar, M. Structure and function of the thermostable L-asparaginase from Thermococcus kodakarensis. Acta Crystallogr. D. 2017, 73, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Razvi, A.; Scholtz, J.M. Lessons in stability from thermophilic proteins. Protein Sci. 2006, 15, 1569–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.-U.; Kim, S.-E.; Oh, D.-K. Molecular insights into lipoxygenases for biocatalytic synthesis of diverse lipid mediators. Prog. Lipid Res. 2021, 83, 101110. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The lid domain in lipases: Structural and functional determinant of enzymatic properties. Front. Bioeng. Biotech. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dainese, E.; Angelucci, C.B.; Sabatucci, A.; De Filippis, V.; Mei, G.; Maccarrone, M. A novel role for iron in modulating the activity and membrane-binding ability of a trimmed soybean lipoxygenase-1. FASEB J. 2010, 24, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Walther, M.; Anton, M.; Wiedmann, M.; Fletterick, R.; Kuhn, H. The N-terminal domain of the reticulocyte-type 15-lipoxygenase is not essential for enzymatic activity but contains determinants for membrane binding. J. Biol. Chem. 2002, 277, 27360–27366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, M.; Hofheinz, K.; Vogel, R.; Roffeis, J.; Kuehn, H. The N-terminal beta-barrel domain of mammalian lipoxygenases including mouse 5-lipoxygenase is not essential for catalytic activity and membrane binding but exhibits regulatory functions. Arch. Biochem. Biophys. 2011, 516, 1–9. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Feng, Y.; Liu, S.; Zhou, X.; Du, G.; Chen, J. The N-terminal α-helix domain of Pseudomonas aeruginosa lipoxygenase is required for its soluble expression in Escherichia coli but not for catalysis. J. Microbiol. Biotechn. 2016, 26, 1701–1707. [Google Scholar] [CrossRef] [Green Version]
- Corbin, J.A.; Evans, J.H.; Landgraf, K.E.; Falke, J.J. Mechanism of specific membrane targeting by C2 domains: Localized pools of target lipids enhance Ca2+ affinity. Biochemistry 2007, 46, 4322–4336. [Google Scholar] [CrossRef] [Green Version]
- Di Venere, A.; Horn, T.; Stehling, S.; Mei, G.; Masgrau, L.; Gonzalez-Lafont, A.; Kuehn, H.; Ivanov, I. Role of Arg403 for thermostability and catalytic activity of rabbit 12/15-lipoxygenase. BBA-Mol. Cell Biol. Lipids 2013, 1831, 1079–1088. [Google Scholar] [CrossRef]
- Wang, X.; Lu, F.; Zhang, C.; Lu, Y.; Bie, X.; Xie, Y.; Lu, Z. Effects of recombinated Anabaena sp. lipoxygenase on the protein component and dough property of wheat flour. J. Agric. Food Chem. 2014, 62, 9885–9892. [Google Scholar] [CrossRef]
- An, J.-U.; Kim, B.-J.; Hong, S.-H.; Oh, D.-K. Characterization of an omega-6 linoleate lipoxygenase from Burkholderia thailandensis and its application in the production of 13-hydroxyoctadecadienoic acid. Appl. Microbiol. Biotechnol. 2015, 99, 5487–5497. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Oliw, E. Manganese lipoxygenase: Purification and characterization. J. Biol. Chem. 1998, 273, 13072–13079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karrer, D.; Ruehl, M. A new lipoxygenase from the agaric fungus Agrocybe aegerita: Biochemical characterization and kinetic properties. PLoS ONE 2019, 14, e0218625. [Google Scholar] [CrossRef]
- Liu, S.; Han, B. Differential expression pattern of an acidic 9/13-lipoxygenase in flower opening and senescence and in leaf response to phloem feeders in the tea plant. BMC Plant Biol. 2010, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, H.; Takemura, M.; Ohyama, K. Cloning and expression of three lipoxygenase genes from liverwort, Marchantia polymorpha L., in Escherichia coli. Phytochemistry 2012, 77, 70–78. [Google Scholar] [CrossRef]
- Oldham, M.; Brash, A.; Newcomer, M. Insights from the X-ray crystal structure of coral 8R-lipoxygenase: Calcium activation via a C2-like domain and a structural basis of product chirality. J. Biol. Chem. 2005, 280, 39545–39552. [Google Scholar] [CrossRef] [Green Version]
- Ritter, A.; Goulitquer, S.; Salaun, J.P.; Tonon, T.; Correa, J.A.; Potin, P. Copper stress induces biosynthesis of octadecanoid and eicosanoid oxygenated derivatives in the brown algal kelp Laminaria digitata. New Phytol. 2008, 180, 809–821. [Google Scholar] [CrossRef]
- Kim, M.-J.; Seo, M.-J.; Shin, K.-C.; Oh, D.-K. Production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid by whole recombinant Escherichia coli cells expressing 10S-dioxygenase from Nostoc punctiforme PCC 73102 with the aid of a chaperone. Biotechnol. Lett. 2017, 39, 133–139. [Google Scholar] [CrossRef]
- Cao, S.; Chen, H.; Zhang, C.; Tang, Y.; Liu, J.; Qi, H. Heterologous Expression and Biochemical Characterization of Two Lipoxygenases in Oriental Melon, Cucumis melo var. makuwa Makino. PLoS ONE 2016, 11, e0153801. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.; Heydeck, D.; Hofheinz, K.; Roffeis, J.; O’Donnell, V.B.; Kuhn, H.; Walther, M. Molecular enzymology of lipoxygenases. Arch. Biochem. Biophys. 2010, 503, 161–174. [Google Scholar] [CrossRef]
- Tong, Q.; Zhang, X.; Wu, F.; Tong, J.; Zhang, P.; Zhang, J. Effect of honey powder on dough rheology and bread quality. Food Res. Int. 2010, 43, 2284–2288. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Lu, Z.; Bie, X.; Zhao, H.; Wang, X.; Lu, F. Effects of recombinant lipoxygenase on wheat flour, dough and bread properties. Food Res. Int. 2013, 54, 26–32. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiong, W.; Wang, L.; Ju, X. Insight into the effect of gluten-starch ratio on the properties of Chinese steamed bread (Mantou). Int. J. Biol. Macromol. 2020, 163, 1821–1827. [Google Scholar] [CrossRef]
- Junqueira, R.M.; Castro, I.A.; Areas, J.A.G.; Silva, A.C.C.; Scholz, M.B.S.; Mendes, S.; Oliveira, K.C. Application of response surface methodology for the optimization of oxidants in wheat flour. Food Chem. 2007, 101, 131–139. [Google Scholar] [CrossRef]
- Chi, H.; Xia, B.; Shen, J.; Zhu, X.; Lu, Z.; Lu, F.; Zhu, P. Characterization of a novel and glutaminase-free type II L-asparaginase from Corynebacterium glutamicum and its acrylamide alleviation efficiency in potato chips. Int. J. Biol. Macromol. 2022, 221, 1384–1393. [Google Scholar] [CrossRef] [PubMed]




| Purification Steps | Total Activity (U) | Total Protein (mg) | Specific Activity (U/mg Protein) | Purification Fold | Yield (%) |
|---|---|---|---|---|---|
| Cell free homogenate | 672,800 ± 18,013.33 | 408.30 ± 24.58 | 1647.81 ± 44.12 | 1 | 100 |
| Nickel affinity purified NsLOX | 492,216.32 ± 4814.21 | 6.304 ± 0.22 | 78,080 ± 763.68 | 47.38 | 73.16 |
| Metal Ions/ Chemical Reagents | Relative Activity (%) | |
|---|---|---|
| 1 mmol/L | 5 mmol/L | |
| CK | 100.00 ± 1.16 | 100.00 ± 1.26 |
| Zn2+ | 1.39 ± 0.34 | ND |
| Fe2+ | 76.01 ± 18.59 | ND |
| Ca2+ | 267.91 ± 4.05 | 405.25 ± 5.79 |
| Mg2+ | 139.81 ± 1.17 | 149.57 ± 1.50 |
| Cu2+ | ND | ND |
| Li2+ | 84.89 ± 0.66 | 59.92 ± 0.50 |
| Mn2+ | 48.40 ± 1.76 | ND |
| Fe3+ | 16.20 ± 3.43 | ND |
| EDTA | ND | ND |
| SDS | ND | ND |
| β-Mercaptoethanol | 94.96 ± 4.14 | 87.55 ± 1.46 |
| Urea | 91.88 ± 6.03 | 87.52 ± 0.88 |
| Substrate | Structure | Relative Activity (%) |
|---|---|---|
| Oleic acid (OA) |  | 29.55 |
| Linoleic acid (LA) |  | 100 |
| α-Linolenic acid (ALA) |  | 83.12 |
| γ-Linolenic acid (GLA) |  | 10.93 |
| Arachidonic acid (AA) |  | 65.57 |
| Docosahexaenoic acid (DHA) |  | 12.96 |
| Substrate | Km (μM) | Vmax (μM s−1) | kcat (s−1) | kcat/Km (μM−1 s−1) |
|---|---|---|---|---|
| LA | 19.46 ± 1.24 | 0.64 ± 0.01 | 9199.75 ± 105.63 | 473.85 |
| ALA | 150.94 ± 6.39 | 0.66 ± 0.07 | 9394.34 ± 992.93 | 62.13 |
| AA | 571.22 ± 26.88 | 0.51 ± 0.01 | 7303.52 ± 144.68 | 12.79 |
| Gloup | Hardness (N) | Springiness (mm) | Gumminess (N) | Chewiness (mj) |
|---|---|---|---|---|
| Blank | 9.41 ± 1.24 a | 9.96 ± 0.17 a | 6.5 ± 0.89 a | 64.68 ± 8.95 a |
| Commercial LOX | 8.34 ± 0.61 ab | 10.14 ± 0.56 a | 5.74 ± 0.43 ab | 58.09 ± 1.46 ab |
| NsLOX | 7.16 ± 0.68 b | 10.21 ± 0.44 a | 4.97 ± 0.48 b | 50.75 ± 5.46 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, B.; Chi, H.; Zhang, B.; Lu, Z.; Liu, H.; Lu, F.; Zhu, P. Computational Insights and In Silico Characterization of a Novel Mini-Lipoxygenase from Nostoc Sphaeroides and Its Application in the Quality Improvement of Steamed Bread. Int. J. Mol. Sci. 2023, 24, 7941. https://doi.org/10.3390/ijms24097941
Xia B, Chi H, Zhang B, Lu Z, Liu H, Lu F, Zhu P. Computational Insights and In Silico Characterization of a Novel Mini-Lipoxygenase from Nostoc Sphaeroides and Its Application in the Quality Improvement of Steamed Bread. International Journal of Molecular Sciences. 2023; 24(9):7941. https://doi.org/10.3390/ijms24097941
Chicago/Turabian StyleXia, Bingjie, Huibing Chi, Bingjie Zhang, Zhaoxin Lu, Huawei Liu, Fengxia Lu, and Ping Zhu. 2023. "Computational Insights and In Silico Characterization of a Novel Mini-Lipoxygenase from Nostoc Sphaeroides and Its Application in the Quality Improvement of Steamed Bread" International Journal of Molecular Sciences 24, no. 9: 7941. https://doi.org/10.3390/ijms24097941
APA StyleXia, B., Chi, H., Zhang, B., Lu, Z., Liu, H., Lu, F., & Zhu, P. (2023). Computational Insights and In Silico Characterization of a Novel Mini-Lipoxygenase from Nostoc Sphaeroides and Its Application in the Quality Improvement of Steamed Bread. International Journal of Molecular Sciences, 24(9), 7941. https://doi.org/10.3390/ijms24097941







