Effects of Probiotic Saccharomyces boulardii Supernatant on Viability, Nano-Mechanical Properties of Cytoplasmic Membrane and Pro-Inflammatory Gene Expression in Human Gastric Cancer AGS Cells
Abstract
1. Introduction
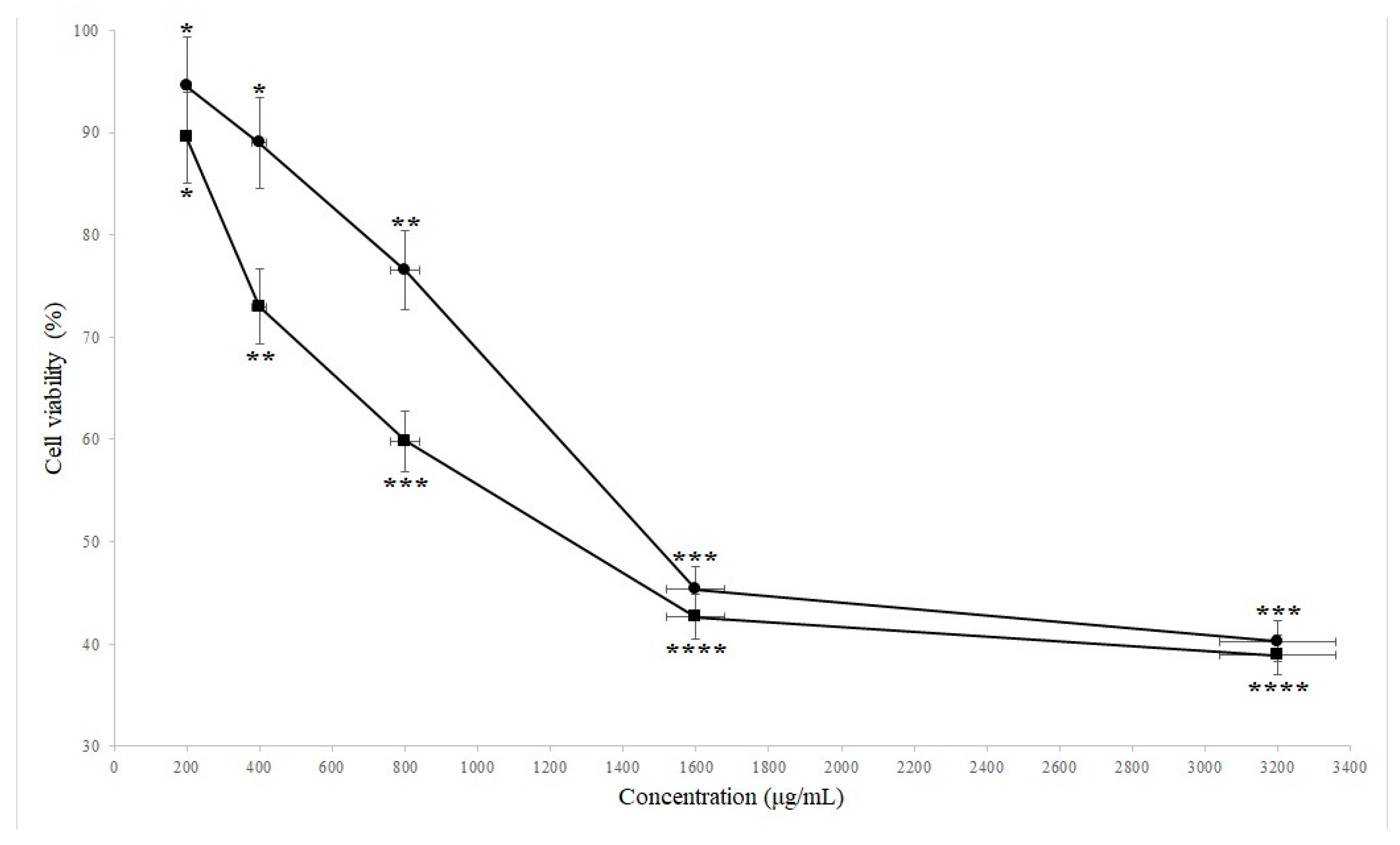
2. Results
3. Discussion
4. Materials and Methods
4.1. Saccharomyces Cerevisiae var. Boulardii Supernatant (SBS) Preparation
4.2. Cell Culture
4.3. Cell Viability
4.4. Atomic Force Microscopy Analysis
4.5. Expression of Survivin and Pro-Inflammatory Genes
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; El-Serag, H.B. Burden of gastric cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA A Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Sexton, R.E.; Al Hallak, M.N.; Diab, M.; Azmi, A.S. Gastric cancer: A comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020, 39, 1179–1203. [Google Scholar] [CrossRef]
- Pakbin, B.; Pishkhan Dibazar, S.; Allahyari, S.; Javadi, M.; Farasat, A.; Darzi, S. Probiotic Saccharomyces cerevisiae var. boulardii supernatant inhibits survivin gene expression and induces apoptosis in human gastric cancer cells. Food Sci. Nutr. 2021, 9, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Pakbin, B.; Dibazar, S.P.; Allahyari, S.; Javadi, M.; Amani, Z.; Farasat, A.; Darzi, S. Anticancer properties of probiotic Saccharomyces boulardii supernatant on human breast cancer cells. Probiotics Antimicrob. Proteins 2022, 14, 1130–1138. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Newman, A.M.; Arshad, M. The role of probiotics, prebiotics and synbiotics in combating multidrug-resistant organisms. Clin. Ther. 2020, 42, 1637–1648. [Google Scholar] [CrossRef]
- Kisan, B.S.; Kumar, R.; Ashok, S.P.; Sangita, G. Probiotic foods for human health: A review. J. Pharmacogn. Phytochem. 2019, 8, 967–971. [Google Scholar]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Vivarelli, S.; Falzone, L.; Basile, M.S.; Nicolosi, D.; Genovese, C.; Libra, M.; Salmeri, M. Benefits of using probiotics as adjuvants in anticancer therapy. World Acad. Sci. J. 2019, 1, 125–135. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, S.; Di, H.; Deng, Z.; Liu, J.; Wang, H. Gut health benefit and application of postbiotics in animal production. J. Anim. Sci. Biotechnol. 2022, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, A.; Tounian, P.; Adel-Patient, K.; Thomas, M. Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants? Nutrients 2023, 15, 1231. [Google Scholar] [CrossRef] [PubMed]
- Vera-Santander, V.E.; Hernández-Figueroa, R.H.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Health Benefits of Consuming Foods with Bacterial Probiotics, Postbiotics, and Their Metabolites: A Review. Molecules 2023, 28, 1230. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Landaburu, M.F.; Daneri, G.A.L.; Relloso, S.; Zarlenga, L.J.; Vinante, M.A.; Mujica, M.T. Fungemia following Saccharomyces cerevisiae var. boulardii probiotic treatment in an elderly patient. Rev. Argent. Microbiol. 2020, 52, 27–30. [Google Scholar] [CrossRef]
- Allahyari, S.; Dibazar, S.P.; Pakbin, B.; Mahmoudi, R.; Farasat, A.; Peymani, A.; Ghajarbeygi, P.; Gheibi, N. anticancer effect of probiotic saccharomyces boulardii supernatant on human Caco-2 cells; an in vitro study. Carpathian J. Food Sci. Technol. 2020, 12, 181–189. [Google Scholar]
- Pakbin, B.; Allahyari, S.; Dibazar, S.P.; Peymani, A.; Haghverdi, M.K.; Taherkhani, K.; Javadi, M.; Mahmoudi, R. Anticancer Properties of Saccharomyces boulardii Metabolite Against Colon Cancer Cells. Probiotics Antimicrob. Proteins 2022, 1, 1–9. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187. [Google Scholar] [CrossRef]
- Huang, J.; Lucero-Prisno III, D.E.; Zhang, L.; Xu, W.; Wong, S.H.; Ng, S.C.; Wong, M.C. Updated epidemiology of gastrointestinal cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol. 2023, 11, 1–17. [Google Scholar] [CrossRef]
- Hani, U.; Osmani, R.A.M.; Yasmin, S.; Gowda, B.J.; Ather, H.; Ansari, M.Y.; Siddiqua, A.; Ghazwani, M.; Fatease, A.A.; Alamri, A.H. Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharmaceutics 2022, 14, 1576. [Google Scholar] [CrossRef] [PubMed]
- Mohd Fuad, A.S.; Amran, N.A.; Nasruddin, N.S.; Burhanudin, N.A.; Dashper, S.; Arzmi, M.H. The mechanisms of probiotics, prebiotics, synbiotics, and postbiotics in oral cancer management. Probiotics Antimicrob. Proteins 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-H.; Lee, N.-K.; Paik, H.-D. The anti-cancer potential of heat-killed Lactobacillus brevis KU15176 upon AGS cell lines through intrinsic apoptosis pathway. Int. J. Mol. Sci. 2022, 23, 4073. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, Q.; Shen, W.; Zhu, J. Anti-proliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. Mol. Cell. Biochem. 2008, 313, 125–132. [Google Scholar] [CrossRef]
- Pan, W.-R.; Chen, P.-W.; Chen, Y.-L.; Hsu, H.-C.; Lin, C.-C.; Chen, W.-J. Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J. Dairy Sci. 2013, 96, 7511–7520. [Google Scholar] [CrossRef]
- Saber, A.; Alipour, B.; Faghfoori, Z.; Khosroushahi, A.Y. Secretion metabolites of dairy Kluyveromyces marxianus AS41 isolated as probiotic, induces apoptosis in different human cancer cell lines and exhibit anti-pathogenic effects. J. Funct. Foods 2017, 34, 408–421. [Google Scholar] [CrossRef]
- Gharamaleki, A.S.; Alipour, B.; Faghfoori, Z.; YariKhosroushahi, A. Prophylactic effects of dairy Kluyveromyces marxianus YAS through overexpression of BAX, CASP 3, CASP 8 and CASP 9 on human colon cancer cell lines. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2016, 10, 142–152. [Google Scholar]
- Fortin, O.; Aguilar-Uscanga, B.; Vu, K.D.; Salmieri, S.; Lacroix, M. Cancer chemopreventive, antiproliferative, and superoxide anion scavenging properties of Kluyveromyces marxianus and Saccharomyces cerevisiae var. boulardii cell wall components. Nutr. Cancer 2018, 70, 83–96. [Google Scholar] [CrossRef]
- Abbasi, A.; Rad, A.H.; Maleki, L.A.; Kafil, H.S.; Baghbanzadeh, A. Antigenotoxicity and Cytotoxic Potentials of Cell-Free Supernatants Derived from Saccharomyces cerevisiae var. boulardii on HT-29 Human Colon Cancer Cell Lines. Probiotics Antimicrob. Proteins 2023, 1, 1–13. [Google Scholar] [CrossRef]
- Martínez-García, D.; Manero-Rupérez, N.; Quesada, R.; Korrodi-Gregório, L.; Soto-Cerrato, V. Therapeutic strategies involving survivin inhibition in cancer. Med. Res. Rev. 2019, 39, 887–909. [Google Scholar] [CrossRef]
- Altieri, D.C. Targeting survivin in cancer. Cancer Lett. 2013, 332, 225–228. [Google Scholar] [CrossRef]
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Purcell, R.V.; Permain, J.; Keenan, J.I. Enterotoxigenic Bacteroides fragilis activates IL-8 expression through Stat3 in colorectal cancer cells. Gut Pathog. 2022, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Kirkiles-Smith, N.C.; McNiff, J.M.; Pober, J.S. TRAIL induces apoptosis and inflammatory gene expression in human endothelial cells. J. Immunol. 2003, 171, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Cang, H.; Guo, B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019, 8, 4709–4721. [Google Scholar] [CrossRef]
- Vivek, R.; Kannan, S.; Achiraman, S.; Thirumurugan, R.; Ganesh, D.S.; Krishnan, M. Survivin deficiency leads to imparalization of cytokinesis in cancer cells. Asian Pac. J. Cancer Prev. 2011, 12, 1675–1679. [Google Scholar]
- Zolghadr, L.; Behbehani, G.R.; Pakbin, B.; Hosseini, S.A.; Gheibi, N. A New Insight Into the Anti Proliferative and Apoptotic Effects of Fulvic and Humic Acids as Bio Product of Humus on Breast Cancer Cells, Optimized by Response Surface Methodology. Waste Biomass Valorization 2022, 14, 859–872. [Google Scholar] [CrossRef]
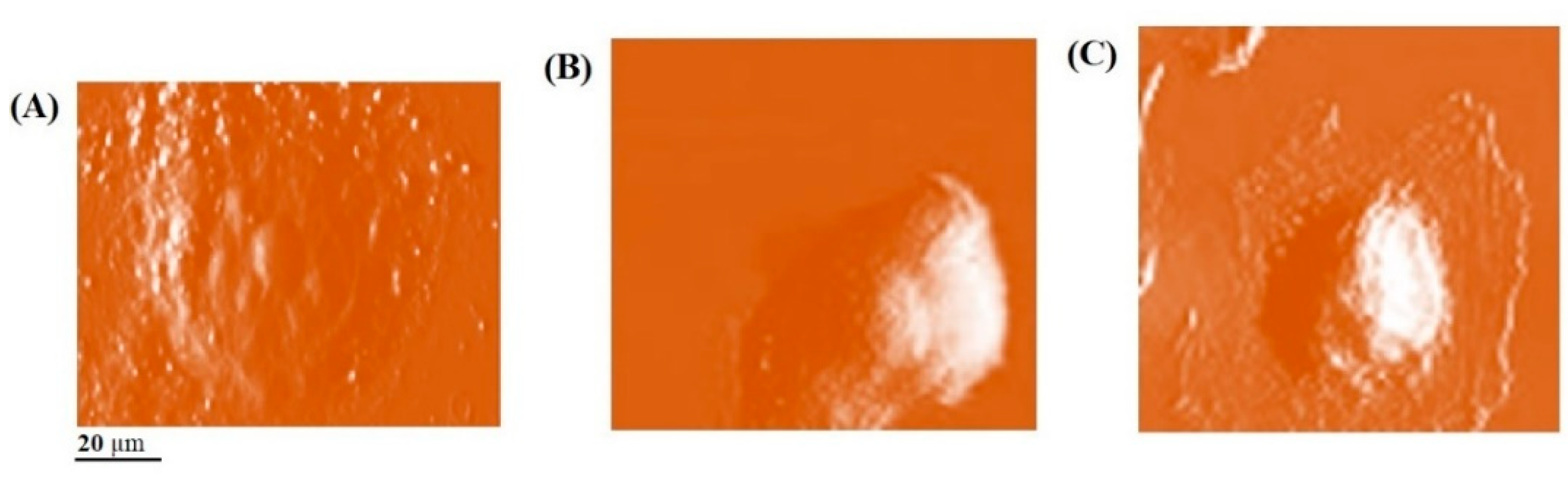

| Treatment | Mean Young’s Modulus Value (kpa) ± SD * | Mean Adhesion Force (pN) ± SD | Mean Z Pulling (µm) ± SD |
|---|---|---|---|
| Control | 0.95 ± 0.025 a | 148 ± 4.02 a | 0.61 ± 0.012 a |
| After 24 h | 1.57 ± 0.180 b | 128 ± 5.20 b | 1.23 ± 0.120 b |
| After 48 h | 1.62 ± 0.220 b | 112 ± 4.20 b | 1.58 ± 0.025 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakbin, B.; Allahyari, S.; Dibazar, S.P.; Zolghadr, L.; Chermahini, N.K.; Brück, W.M.; Brück, T.B.; Mahmoudi, R. Effects of Probiotic Saccharomyces boulardii Supernatant on Viability, Nano-Mechanical Properties of Cytoplasmic Membrane and Pro-Inflammatory Gene Expression in Human Gastric Cancer AGS Cells. Int. J. Mol. Sci. 2023, 24, 7945. https://doi.org/10.3390/ijms24097945
Pakbin B, Allahyari S, Dibazar SP, Zolghadr L, Chermahini NK, Brück WM, Brück TB, Mahmoudi R. Effects of Probiotic Saccharomyces boulardii Supernatant on Viability, Nano-Mechanical Properties of Cytoplasmic Membrane and Pro-Inflammatory Gene Expression in Human Gastric Cancer AGS Cells. International Journal of Molecular Sciences. 2023; 24(9):7945. https://doi.org/10.3390/ijms24097945
Chicago/Turabian StylePakbin, Babak, Samaneh Allahyari, Shaghayegh Pishkhan Dibazar, Leila Zolghadr, Neda Karami Chermahini, Wolfram Manuel Brück, Thomas B. Brück, and Razzagh Mahmoudi. 2023. "Effects of Probiotic Saccharomyces boulardii Supernatant on Viability, Nano-Mechanical Properties of Cytoplasmic Membrane and Pro-Inflammatory Gene Expression in Human Gastric Cancer AGS Cells" International Journal of Molecular Sciences 24, no. 9: 7945. https://doi.org/10.3390/ijms24097945
APA StylePakbin, B., Allahyari, S., Dibazar, S. P., Zolghadr, L., Chermahini, N. K., Brück, W. M., Brück, T. B., & Mahmoudi, R. (2023). Effects of Probiotic Saccharomyces boulardii Supernatant on Viability, Nano-Mechanical Properties of Cytoplasmic Membrane and Pro-Inflammatory Gene Expression in Human Gastric Cancer AGS Cells. International Journal of Molecular Sciences, 24(9), 7945. https://doi.org/10.3390/ijms24097945







