Abstract
The wood of Michelia macclurei Dandy (MD) is an excellent material that is widely used in the furniture, handicraft, and construction industries. However, less research has been conducted on the chemical composition and biological activity of heartwood, which is the main valuable part of the wood. This study aimed to investigate the chemical composition and biological activities of the heartwood of Michelia macclurei Dandy (MDHW) and to confirm the active ingredients. Triple quadrupole gas chromatography–mass spectrometry (GC-MS) was used to characterize the volatile components of MDHW, while ultra-performance liquid chromatography–mass spectrometry was used to analyze the non-volatile components (UPLC-MS). The total reducing power, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging assays, acetylcholinesterase and α-glucosidase inhibition assays, and an antimicrobial test of 4 gram bacteria were used to describe the in vitro bioactivities. The GC-MS analysis showed that the volatile components of MDHW were mainly fatty compounds and terpenoids, with sesquiterpenes and their derivatives dominating the terpene composition. β-elemene was the main terpene component in the steam distillation (11.88%) and ultrasonic extraction (8.2%) methods. A total of 67 compounds, comprising 45 alkaloids, 9 flavonoids, 6 lignans, and others, were found by UPLC-MS analysis. The primary structural kinds of the non-volatile components were 35 isoquinoline alkaloids. Alkaloids were the predominant active constituent in all MDHW extracts, including crude extracts, alkaloid fractions, and non-alkaloid fractions. These extracts all demonstrate some biological effects in terms of antioxidant, enzyme inhibition, and bacterial inhibition. The findings of this study show that MDHW is abundant in chemical structure types, has great bioactivity assessment, and has the potential to be used to create natural antioxidants, products that postpone Alzheimer’s disease and lower blood sugar levels and antibacterial agents.
1. Introduction
Natural products are organic molecules with a variety of structural properties and biological activities that have evolved via long-term natural selection and evolution. They are safer and more affordable than synthetic products [1]. Both the medications used in conventional medicine and the chemical pharmaceuticals that are frequently prescribed in modern medicine are either analogs or derivatives of natural chemicals. The biological and chemical makeup of many of the plants used in traditional medicine and folk cures is still insufficiently understood.
Free radicals have been linked to several illnesses, including atherosclerosis, cancer, diabetes, liver disease, and organismal aging, according to a recent pathological study [2]. Antioxidants not only scavenge free radicals but also inhibit their production. Alzheimer’s disease (AD) and diabetes mellitus (DM) are considered to be increasingly prevalent global public health problems in the 21st century [3]. As a result, medications for AD and DM have drawn a lot of interest. However, many synthetic antioxidants and medications that block AD and DM are inefficient and come with adverse side effects including nausea and diarrhea [4,5,6]. There is an increasing search for non-toxic and effective natural products to treat these diseases. Additionally, drug-resistant microorganisms are emerging and persisting at an increasing rate, indicating that the available conventional antibiotics are becoming less effective. As a result, it is crucial to discover new, potent antibacterial medications [7].
Magnoliaceae is the most primitive group of angiosperms and is widespread throughout the world. It is a traditional Chinese medicine used in Asia and North America. For example, the Chinese Pharmacopoeia has officially listed the Magnolia officinalis cortex and Magnolia biondii Pamp., which are rich in magnocurarine and volatile oil [8]. Alkaloids, lignans, minor levels of flavonoids, and steroids are all present in the non-volatile composition of Magnoliaceae, which is also known for being rich in aromatic compounds [9]. MD is a Magnoliaceae (Michelia Linn.) evergreen arbor plant, which is widely used in the furniture, handicrafts, musical instrument manufacturing, plywood, and construction industries, is an important native precious timber and multifunctional high-efficiency species in southern China [10]. Regrettably, investigations into MD’s medicinal value have rarely been reported. Instead, they have mostly concentrated on biomass, breeding, and the development and utilization of essential oils [11,12]. The MD chemical composition research is reflected in the analysis of the volatile oil components of flowers, leaves, and fruits, and it has been confirmed that these volatile oils have a variety of biological activities, which are better medicinal materials [13]. In Chinese folklore, the roots and bark have traditionally been used for the treatment of inflammation and heat-clearing [14], whereas the heartwood receives less attention than the other parts. A significant amount of secondary metabolites are accumulated in the heartwood, which is a part of the xylem. The heartwood’s characteristic odor, color, and natural durability, as well as its high economic worth, are caused by a significant buildup of secondary metabolites. For example, the heartwood of Dalbergia odorifera and Santalum album is ideal for crafting artistic crafts and sculptures as well as traditional Chinese medicine and aromatherapy [15,16]. The volatile components of MDHW are reported to be rich in sesquiterpenes such as β-elemene and β-bisabolene [17]. No studies have yet reported on the non-volatile components of MD.
Therefore, the purpose of this study was to carry out a comprehensive analysis of the chemical components of MDHW in terms of both volatile and non-volatile components using GC-MS and UPLC-MS to explore the biological potential of MDHW extracts, and to identify the main effective bioactive components. To determine the MDHW’s primary bioactive components, the antioxidant, enzyme inhibition, and antibacterial properties of the crude extract, alkaloid, and non-alkaloid fractions were assessed. This research aimed to provide guidelines for assessing the economic value and sensible application of MD. The study’s findings will provide new ideas for the development and utilization of MDHW in pharmaceutical, cosmetic, and nutraceutical products.
2. Results and Discussion
2.1. Volatile Components of MDHW Determined by GC-MS
In this study, two different extraction methods were used to analyze the volatile oil of MDHW using GC-MS. Table 1 displays the results of the top ten and the same essential oil components produced by steam distillation (SD) and ultrasonic extraction (UE), and Supplementary Table S1 displays all essential oil components. Respectively, 81 and 115 peaks were detected by SD and UE, among which 59 and 38 compounds were identified, and 97.54% and 76.15% accounted for the peak area. According to the species, oxygenated sesquiterpenes (57.66%), sesquiterpene hydrocarbons (38.74%), monoterpene hydrocarbons (0.78%), and aromatic compounds (0.37%) made up the majority of the essential oil composition discovered by SD. Fatty compounds (59.93%) and sesquiterpene hydrocarbons (13.23%), as well as tiny quantities of aromatic compounds (2.38%), oxygenated sesquiterpenes (0.54%), and monoterpene hydrocarbons (0.07%) constituted the majority of the essential oils extracted from UE. These data indicate that the composition and content of the essential oils produced by these two extraction methods are significant differences.

Table 1.
Part of the volatile components of MDHW obtained by the two extraction methods.
UE extracts a wider variety of essential oils, but the terpenoid level was not very high. The most common procedure for extracting essential oils is SD, and the compounds detected are mainly terpenoids and their derivatives with high purity [18], as also confirmed by our results. For a preliminary investigation into plant essential oil constituents, UE is preferable since it is quick, simple to use, and can detect a wider variety of chemicals. SD is more suited for realistic production applications due to its high terpene extraction rate, low cost, and ease of use.
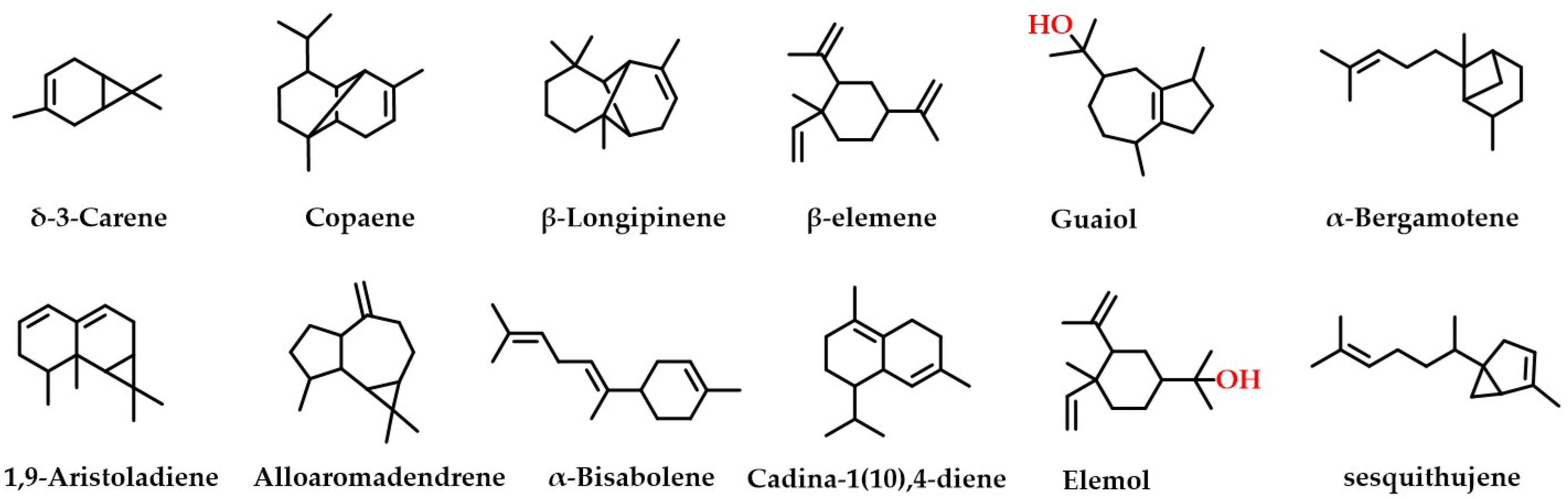
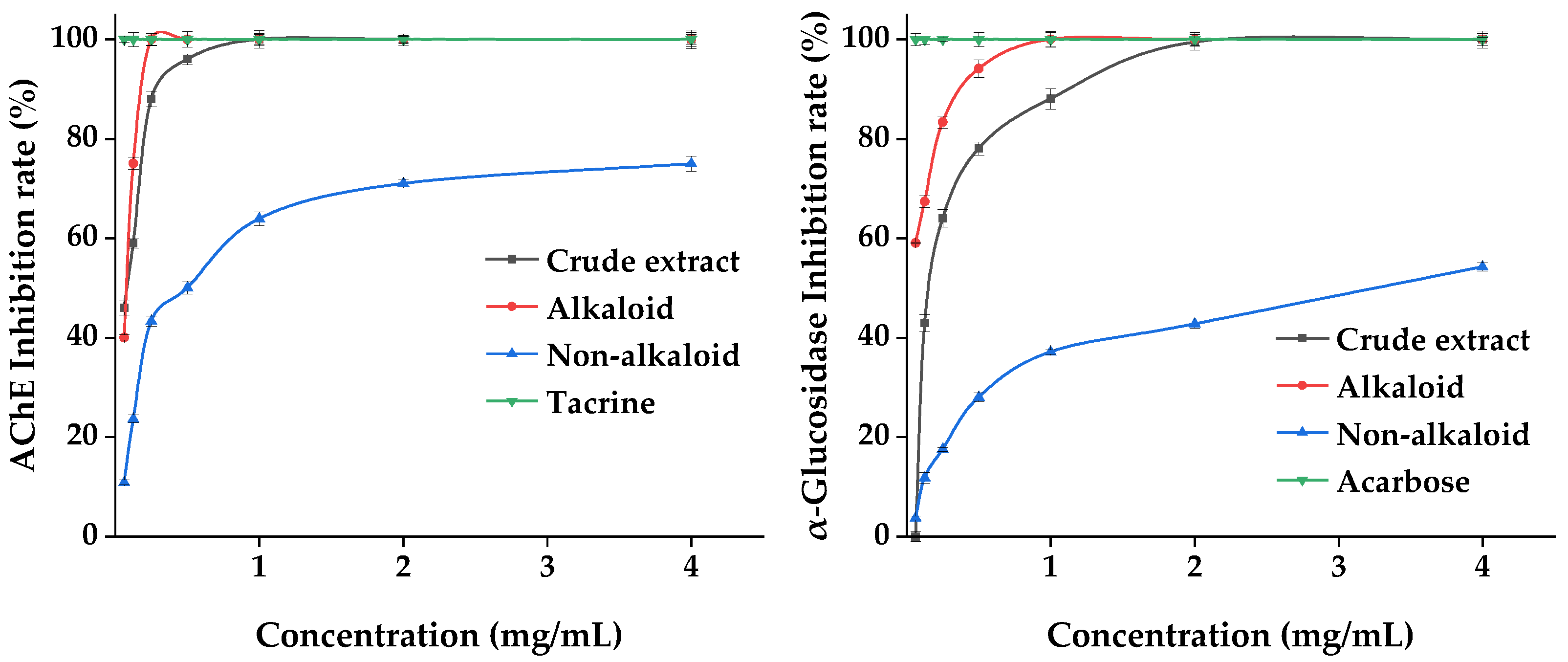
Twelve of the same components were extracted by both extraction methods (Figure 1), namely δ-3-carene, copaene, β-longipinene, β-elemene, sesquithujene, α-bergamotene, 1,9-aristoladiene, alloaromadendrene, α-bisabolene, cadina-1(10),4-diene, elemol, and guaiol. These compounds are structurally diverse and have been reported several times in the Michelia Linn. [9,19,20,21]. These results show that sesquiterpenes and their derivatives dominate the terpene composition of MDHW essential oil, while diterpenes and higher terpenes are rarely observed. This may be because Magnoliaceae is the most primitive angiosperm and therefore lacks or has lower levels of higher terpenes.
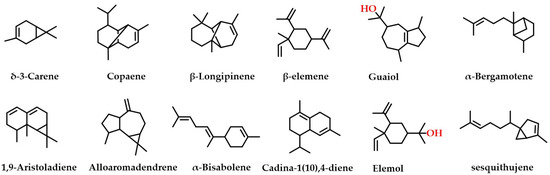
Figure 1.
The 12 same components of the two extraction methods.
β-elemene was the highest terpenoid component in SD and UE (11.88% and 8.2%, respectively), which is consistent with that reported in the literature (the content of β-elemene is as high as 14.67%) [17], indicating that this compound could be used as a potential characteristic component of MDHW essential oil. In China, β-elemene is used as a class II non-cytotoxic anti-tumor medicine since it is primarily utilized in the treatment of many different types of malignant tumors without damaging liver or kidney function [22]. Guaiol (10.67%) was effective in inhibiting brown and white rot fungi [21], whilst β-eudesmol (9.68%) is one of the most studied and major biologically active sesquiterpenes with strong anti-tumor and anti-angiogenic activities [23].
According to the above analysis, MDHW has multiple types of essential oil constituents. Furthermore, major terpene constituents such as β-elemene and β-eudesmol have been used as clinical compounds, indicating that MDHW essential oil may be a new source for pharmaceuticals.
2.2. Non-Volatile Components of MDHW with UPLC-MS/MS
The non-volatile components of the ethanolic extract of MDHW were analyzed in this study using UPLC-MS, and the details of all the identified compounds are summarized in Table 2. A total of 67 compounds were identified, including 45 alkaloids, 6 lignans, 9 flavonoids, 3 terpenoids, 2 coumarins, and 2 lipids. To our knowledge, these non-volatile components have never been reported in previous MD research. Our results revealed that the major constituents of MDHW extracts were alkaloids. According to phytochemical results, MDHW extracts were abundant in different types of alkaloids, flavonoids, lignans, and coumarins. These compounds have been shown in numerous experiments to have antioxidant, antibacterial, anti-tumor, hypotensive, antiarrhythmic, and antidepressant functions [1], indicating that MDHW extracts may have a variety of bioactive properties.

Table 2.
Non-volatile components of MDHW extracts analyzed by UPLC-MS.
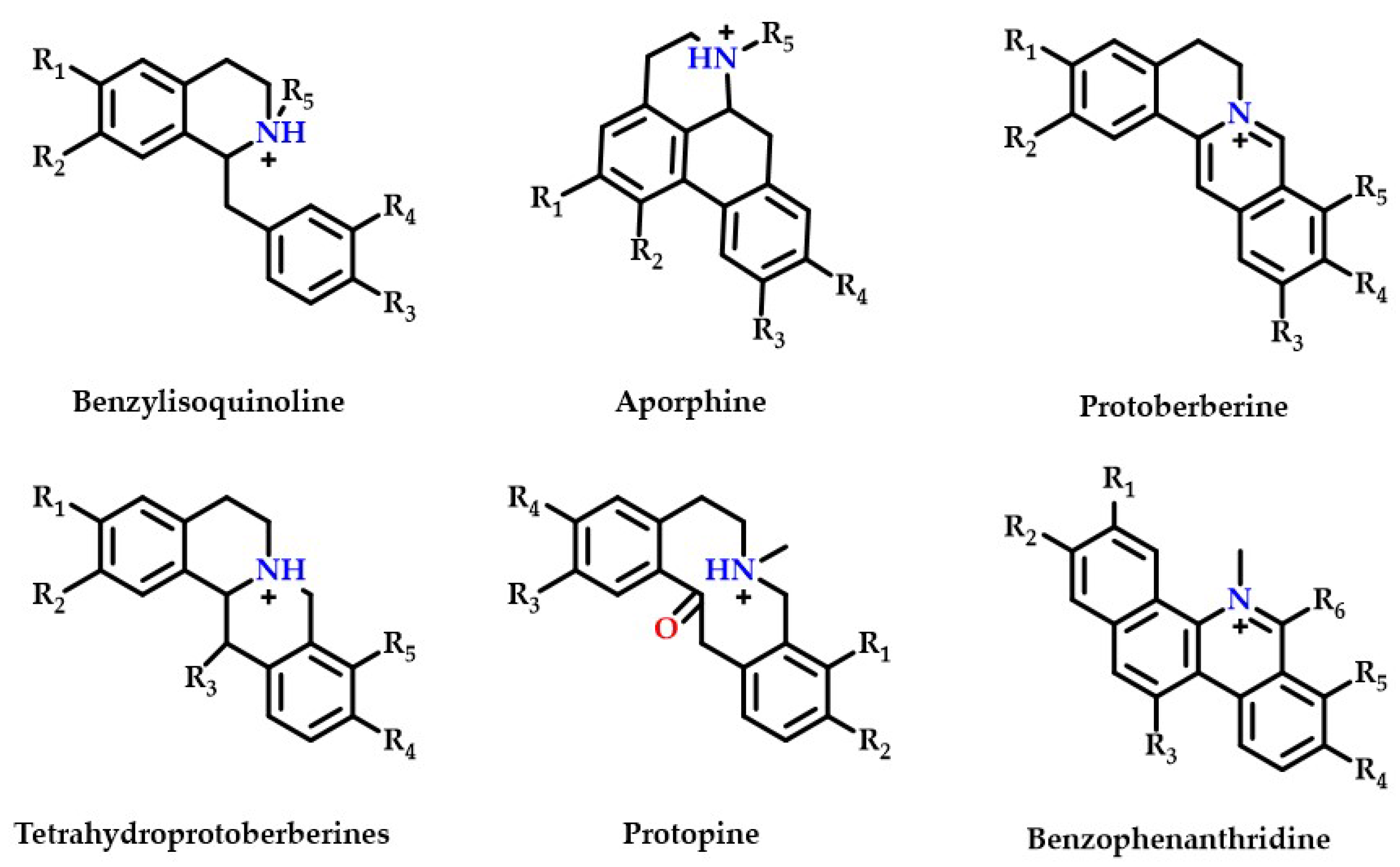
Thirty-five isoquinoline alkaloids were discovered to be the primary types of MDHW alkaloid components in the current investigation. Therefore, based on the secondary fragments, the mass spectrometric cleavage pattern of isoquinoline alkaloids and relevant literature were referred to, the compound structures of MDHW isoquinoline alkaloids were identified and the proposed fragmentation pathways of the compounds were analyzed. Based on their basic heterocyclic nuclei, these isoquinoline alkaloids can be classified into six groups, viz., benzylisoquinoline alkaloids (compounds 4, 12, 14, 15, 18, 32, 37, 39, and 43), aporphine alkaloids (compounds 3, 6, 7, 8, 10, 11, 13, 16, 17, 20, 24, 27, 31, 33, and 41), protoberberine alkaloids (compounds 21 and 25), tetrahydroprotoberberines alkaloids (compounds 2, 19, 34, and 42), protopine alkaloids (compounds 9 and 38) and benzophenanthridine alkaloids (compounds 22, 23, and 26)—the basic structures of which are shown in Figure 2. The MS/MS spectrum and proposed fragmentation pathways of 35 isoquinoline alkaloids are shown in Supplementary Figure S1.
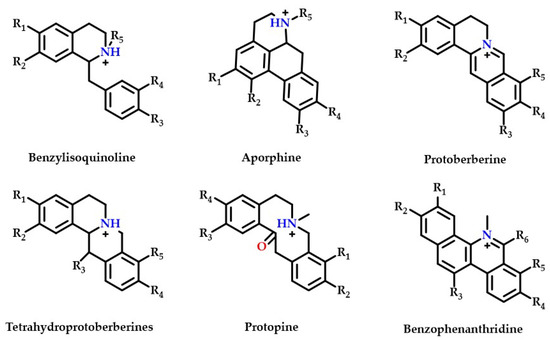
Figure 2.
The basic structure of six isoquinoline alkaloids.
Most isoquinoline alkaloids are synthesized from benzyltetrahydroisoquinoline alkaloids, which can then be converted into tetrahydroproberberberine alkaloids and aporphine alkaloids, proberberberine is derived from tetrahydroproberberine alkaloids, protopine alkaloids is generated from tetrahydroproberberine alkaloids and is continuously metabolized to dihydrobenzophenanthridine alkaloids and benzophenanthridine alkaloids [24]. The MDHW alkaloids are dominated by simply structured aporphine alkaloids, which may be because MD belongs to the Magnoliaceae, which are the most primitive angiosperms.
2.2.1. Benzylisoquinoline Alkaloids
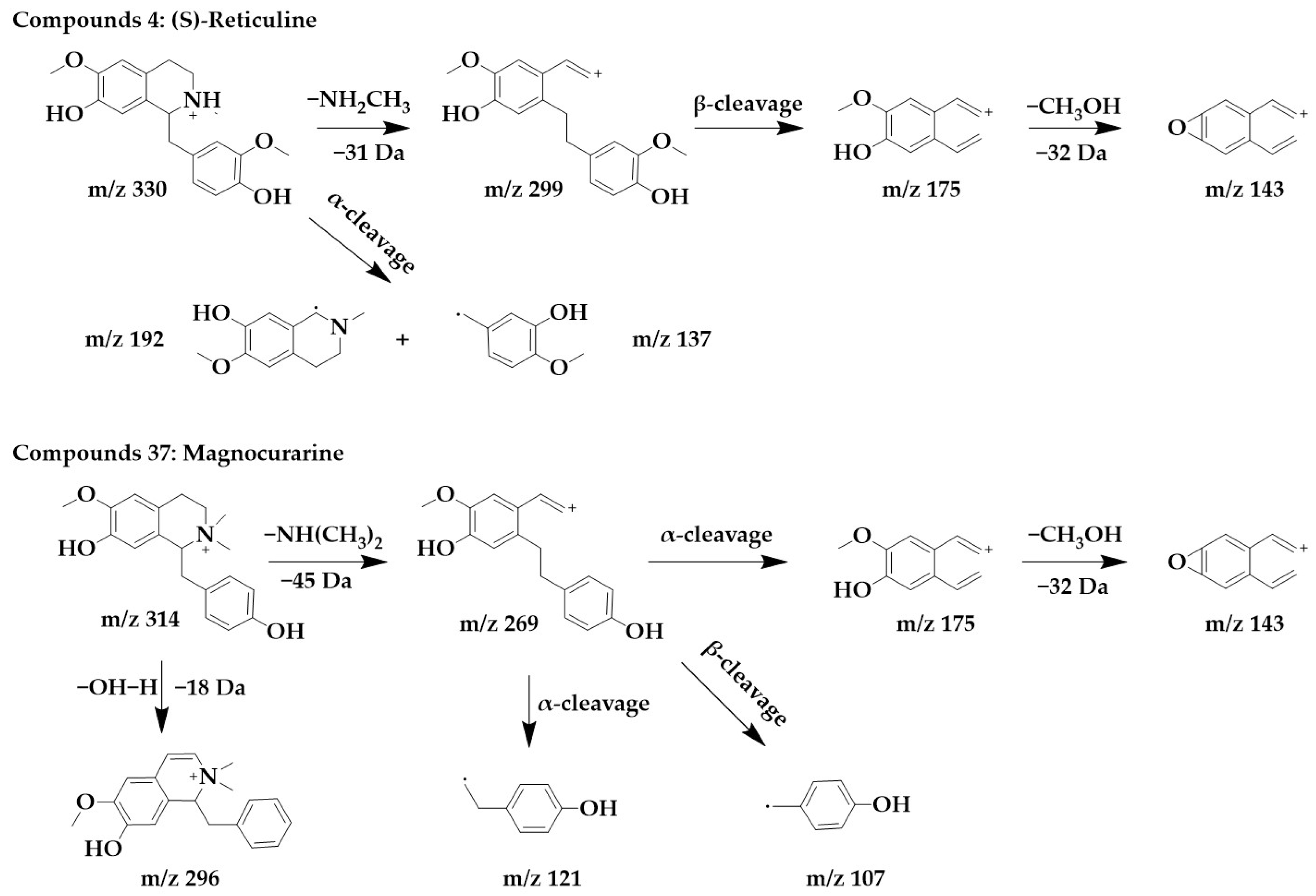
Benzylisoquinoline alkaloids would lose their nitrogenous side chains and form fragmented peaks such as m/z 192, m/z 175, m/z 143, and m/z 137 [25]. The excimer ion peak in the positive ion mode of compound 4 was m/z 330 [M+H]+, and molecular formula was probably C19H23NO4. The fragment ions with higher abundance in the mass spectra all appeared below m/z 230, and the fragments with higher abundance were m/z 192 and m/z 137. These fragmentation behaviors are consistent with the mass spectral cleavage pattern of benzylisoquinoline alkaloids. m/z 299 was formed by the loss of the nitrogen-containing side chain [M+H-NH2CH3]+ from the parent structure. m/z 299 underwent β-cleavage to form m/z 175, and m/z 175 underwent further side chain breakage and loss of CH3OH fragments to form m/z 143. These fragmentation behaviors were consistent with those reported in the literature [26], thus, compound 4 was identified as (S)-reticuline. Interestingly, (S)-reticuline is an important intermediate compound in the metabolic pathway of many isoquinoline alkaloids, from which most benzylisoquinolines alkaloids, aporphine alkaloids, proberberberine alkaloids, and benzophenanthridine alkaloids are converted [27].
The excimer ion peak of compound 37 was m/z 314 [M+H]+, the possible molecular formula was C19H23NO3, and the presence of the fragments m/z 175, m/z 143, and m/z 137 in the mass spectra indicated that it was most likely a benzylisoquinoline alkaloid, and based on the existing reports of the chemical composition of Magnocurarinaceae [28], compounds 37 was identified as magnocurarine. The excimer ion peak of compound 32 was m/z 314 [M+H]+, which has a similar MS/MS spectrum to compound 37 (Supplementary Figure S1), but both were isomers, so we presumed compound 32 to be armepavine based on the available literature [26]. The nitrogen atom on the parent structure of magnocurarine is attached to two methyls, while armepavine is attached to only one methyl. The two can be distinguished based on the pattern that benzylisoquinoline alkaloids readily lost NH3, CH3NH2, or (CH3)2NH. Magnocurarine lost (CH3)2NH to form the fragment m/z 269, and armepavine lost CH3NH2 to form the fragment m/z 283, and the breakage of the side chain would not form m/z 269. The chemical structure and proposed fragmentation pathways of compounds 4 and 37 are shown in Figure 3.
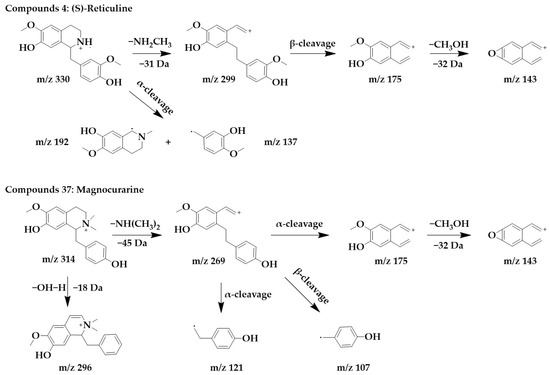
Figure 3.
Chemical structure and proposed fragmentation pathways of compounds 4 and 37.
According to the similarity pattern, in combination with secondary fragments the and related literature [29], five benzylisoquinoline alkaloids were also observed in this study. Compounds 12, 14, 15, 18, 39, and 43 were identified as norarmepavine, isococlaurine, (S)-coclaurine, O-methylarmepavine, laudanosine, and protosinomenine, respectively. Their MS/MS spectrum and possible fragmentation pathways are shown in Supplementary Figure S1.
2.2.2. Aporphine Alkaloids
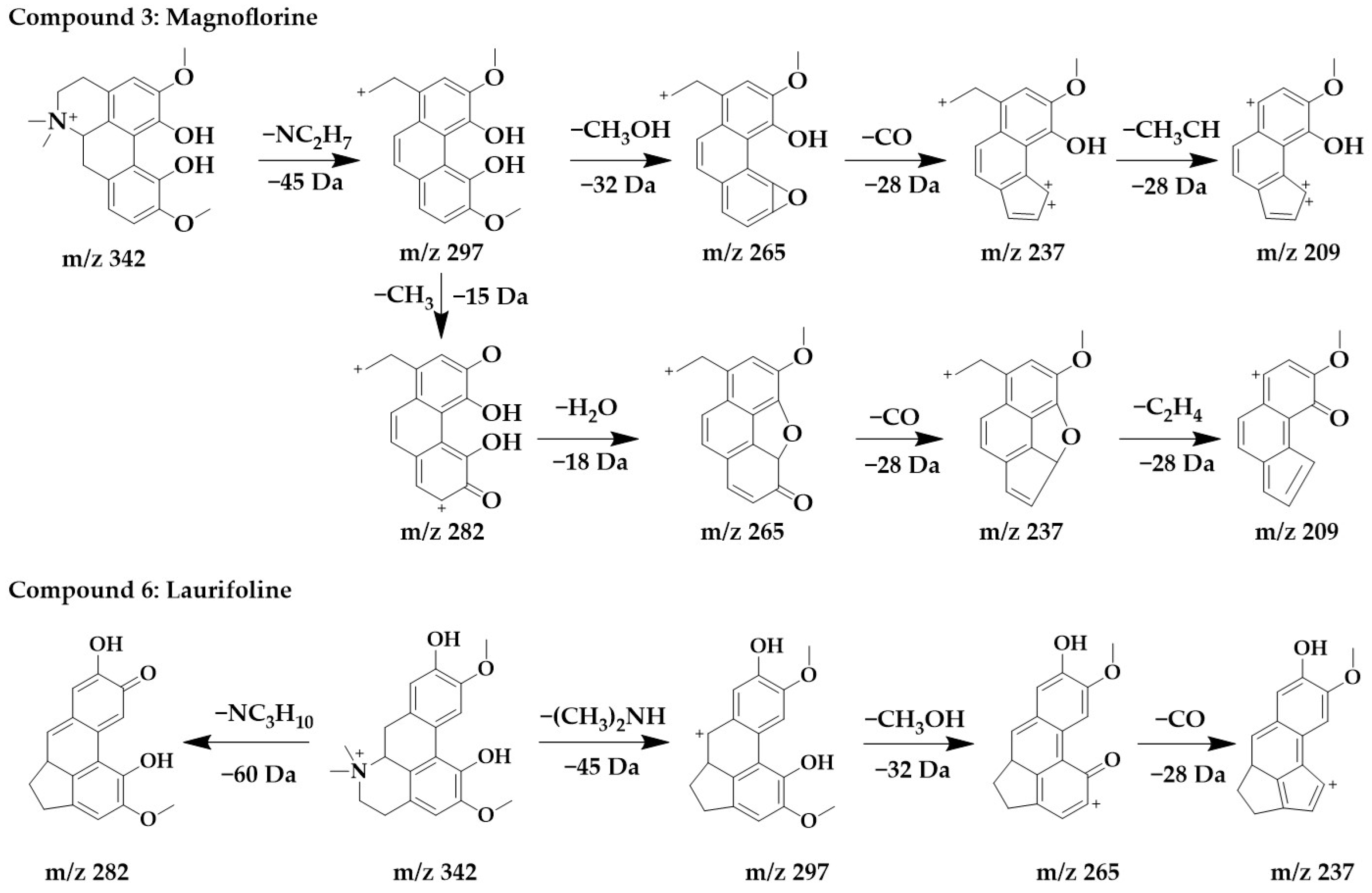
The excimer ion peak of compound 3 was m/z 342 [M+H]+, and the possible molecular formula was C20H24NO4. The fragment ions with a higher abundance in the mass spectra all appeared above m/z 210, indicating that no cleavage of the parent structure occurred. Based on the cleavage pattern of isoquinoline alkaloids [25], compound 3 can be presumed to be benzophenanthridine or aporphine alkaloids. The fragment m/z 297 was formed when the parent ion loses nitrogen methyl [(CH3)2NH]+, after which m/z 265 was obtained by the continued loss of the side chain CH3OH to form a stable ternary oxygen ring, and then a molecule of CO was lost to form a five-membered ring of the parent structure to obtain m/z 237, and the side chain CH3CH of m/z 237 breaks to form m/z 209. Another cleavage pathway for m/z 297 was the loss of a molecule of methyl to form a carbonyl group to obtain m/z 282, continued with the loss of two hydroxyls to form a parent structurally stable five-membered ring to form m/z 265, followed by the loss of one molecule of CO to form a five-membered ring to form m/z 237, and finally the loss of the side chain CH3CH to form m/z 209. In summary, compound 3 was identified as magnoflorine in comparison with the literature [30].
The excimer Ion peak of compound 6 was m/z 342 [M+H]+, and the major fragment peaks and ion abundances in its mass spectra were similar to compound 3 (Supplementary Figure S1), so they were isomers. Combined with the literature [29], compound 6 was identified as laurifoline. Due to the different positions of the hydroxyl on the D-ring, they can be distinguished by the presence of an ionic abundance of magnoflorine at m/z 219, while laurifoline was not. The chemical structure and proposed fragmentation pathways of compounds 3 and 6 are shown in Figure 4.
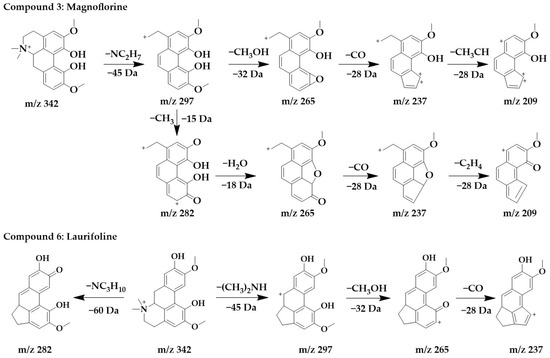
Figure 4.
Chemical structure and proposed fragmentation pathways of compounds 3 and 6.
Based on the similarity pattern and combined with the accurate parent ion molecular weights, secondary mass spectrometry information, and relevant literature [29,31,32], compounds 7, 8, 10, 11, 13, 16, 17, 20, 24, 27, 31, 33, and 41 were identified as corytuberine, N, N-dimethylglaucine, bulbocapnine, apomorphine, N-methylasimilobine, asimilobine, ushinsunine, menisperine, anolobine, roemerine, xanthoplanine, michelalbine, and anonaine, and their chemical structures and proposed fragmentation pathways are shown in Supplementary Figure S1. Among them, compounds 3, 11, 16, 17, 24, 27, 33, and 41 have been isolated from Magnoliaceae [31,33,34].
2.2.3. Protoberberine Alkaloids
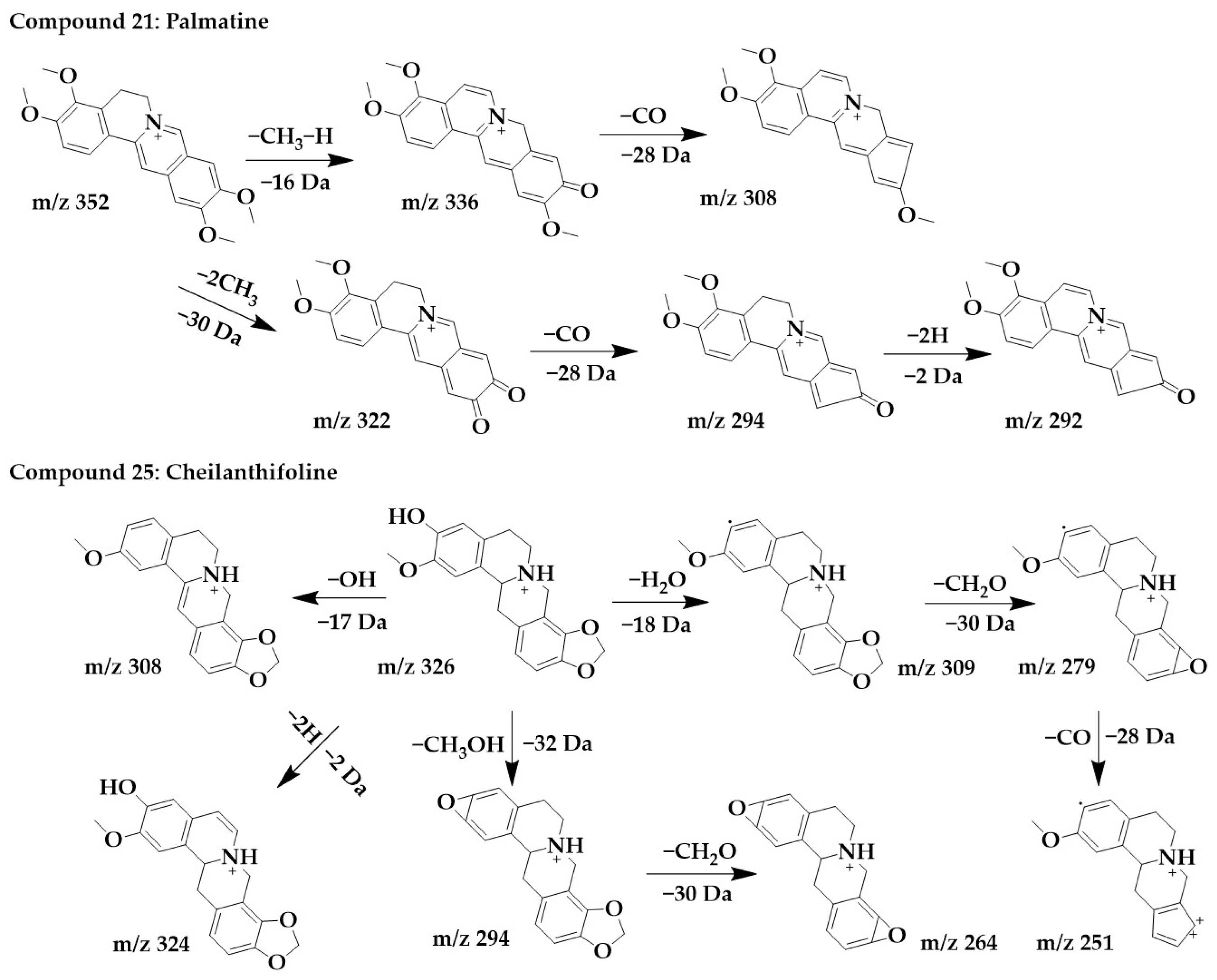
The excimer ion peak of compound 21 was m/z 352 [M+H]+, and the possible molecular formula was C21H21NO4. After the successive losses of one molecule of methyl and one H, the parent structure formed a carbonyl and obtained the fragment m/z 336, immediately followed by the continued loss of one molecule of CO to form m/z 308. Another cleavage pathway was the formation of m/z 322 and m/z 294 after the successive loss of molecules of CH3 and CO. Finally, due to the presence of carbon–carbon single bonds at the C-5,6 positions, the parent structure lost 2 molecules of H to form a stable conjugate system and obtained m/z 292 with higher abundance. These followed the cleavage pattern of protoberberine alkaloids [25], and combined with the literature [33], compound 21 was identified as palmatine.
The excimer ion peak of compound 25 was m/z 326 [M+H]+. m/z 321, m/z 307, and m/z 308 were formed by parent ions through the loss of one molecule of OH and H2O, respectively. m/z 309 formed a ternary oxygen ring after the loss of a methylenedioxy and successively lost a molecule of CO to form fragments m/z 279 and m/z 251. In addition, the parent ion can lose two hydrogens to form m/z 324. The parent ion can also lose both a molecule of CH3 and a molecule of OH to form fragmentation m/z 294, immediately followed by the continued loss of a molecule of CH2O to form the more stable fragmentation ion peak m/z 264. Combined with the cleavage pattern of protoberberine alkaloids [25], compound 25 was identified as cheilanthifoline. The chemical structure and proposed fragmentation pathways of compounds 21 and 25 are shown in Figure 5.
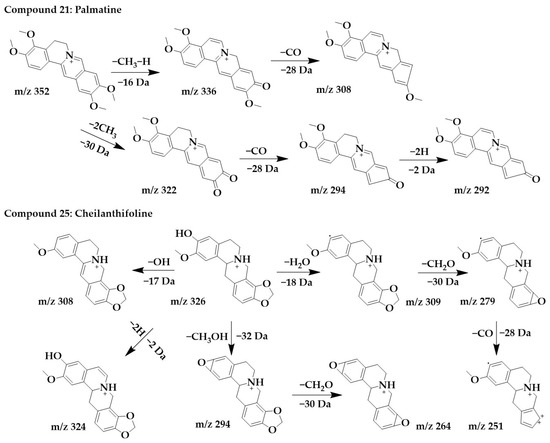
Figure 5.
Chemical structure and proposed fragmentation pathways of compounds 21 and 25.
2.2.4. Tetrahydroprotoberberine Alkaloids
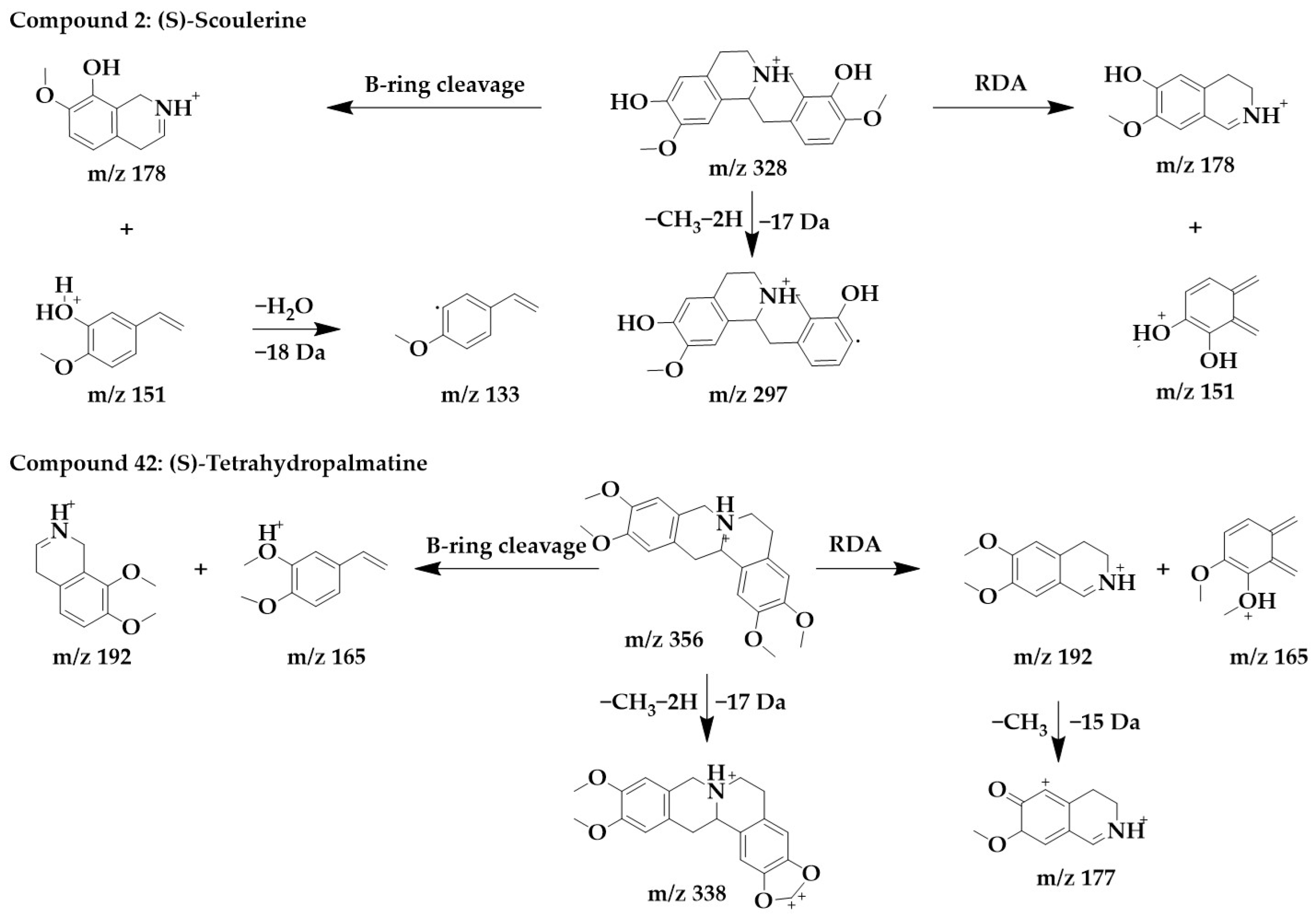
Tetrahydroproberberine alkaloids frequently undergo a retro Diels–Alder (RDA) reaction at the C-ring, with fragments m/z 206, m/z 192, and m/z 165. These common fragments are used as diagnostic ions for the structural characterization of tetrahydroprotoberberine alkaloids [25]. In addition, if the substituent at the 13th position is methyl, the RDA reaction will most likely also occur in the B-ring.
The MS/MS spectra of compounds 2, 19, 34, and 42 (Supplementary Figure S1) showed similar RDA fragmentation behaviors. The main fragments of compound 2 were m/z 178 and m/z 151. m/z 178 and m/z 151 can be formed not only by RDA reaction through the B-ring of the parent structure but also by direct RDA reaction through the C-ring. Another characteristic ion, m/z 133, was formed by the loss of one molecule of water from m/z 151. Compound 2 was identified as (S)-scoulerine in light of the literature [26].
Similarly, m/z 192 and m/z 165 were the dominant ions in compound 42, which can originate from both RDA cleavage occurring in the C-ring of the parent structure and from B-ring cleavage formation. m/z 192 further lost a molecule of methyl to form fragments m/z 177. To form a stable conjugated system, the parent structure compound 42 first lost two hydrogens and subsequently lost a molecule of methyl to form m/z 338. Compound 42 was identified as (S)-tetrahydropalmatine [33]. The chemical structure and proposed fragmentation pathways of compounds 2 and 42 are shown in Figure 6.
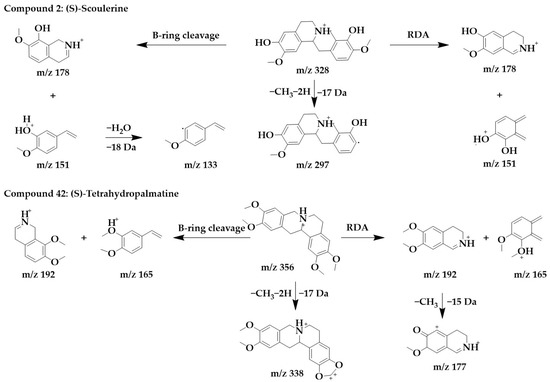
Figure 6.
Chemical structure and proposed fragmentation pathways of compounds 2 and 42.
The C-ring on the parent structure of compound 19 underwent RDA reactions to form m/z 192 and m/z 179, the B-ring underwent RDA to form m/z 205 and m/z 165, and compound 19 was identified as corydaline [34]. Similarly, compound 34 was identified as tetrahydrocolumbamine [35]. The chemical structures and proposed fragmentation pathways of compounds 19 and 34 are shown in Supplementary Figure S1.
2.2.5. Protopine Alkaloids
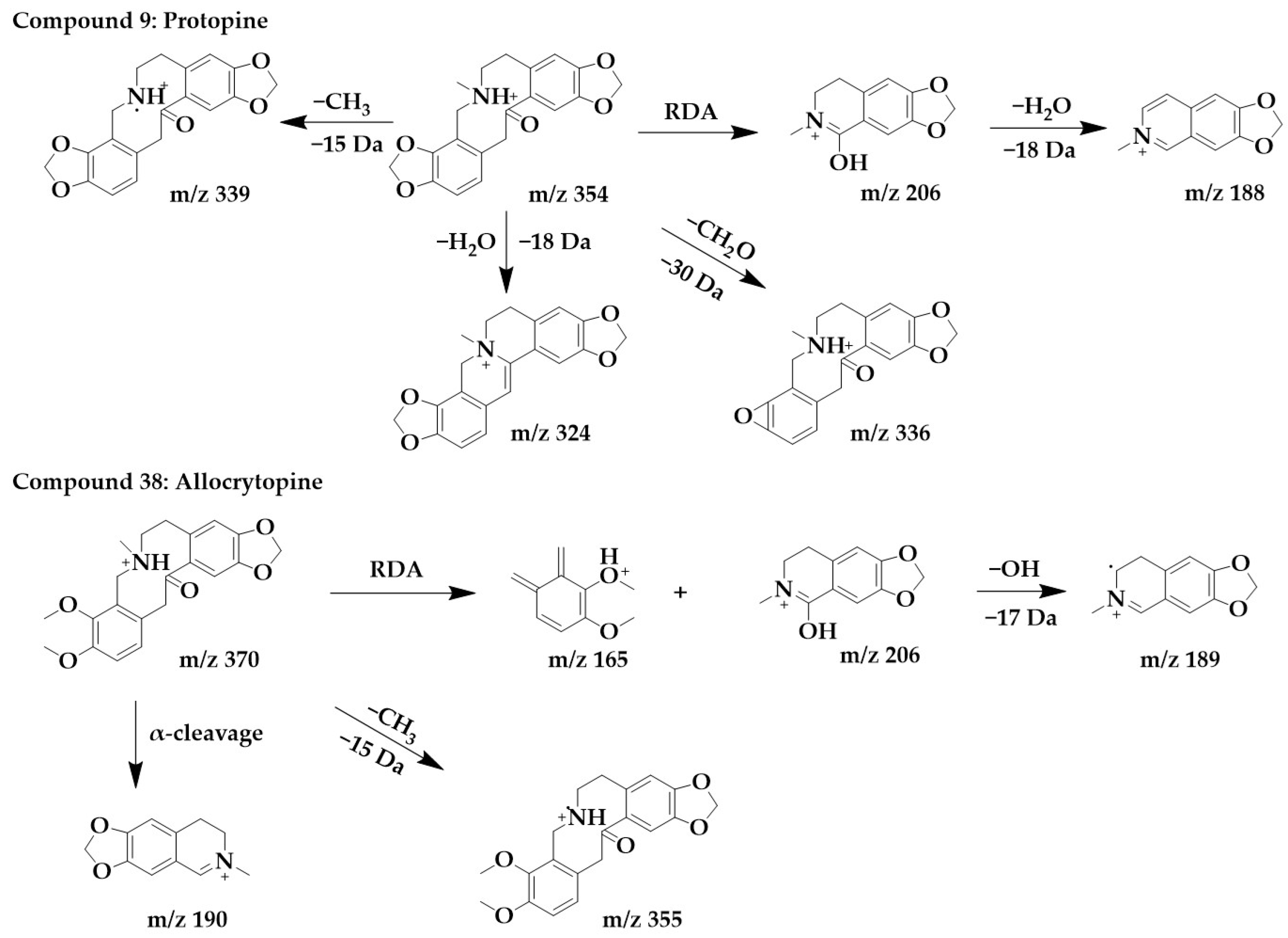
The excimer ion peak of compound 9 was m/z 354 [M+H]+, and the possible molecular formula was C20H19NO5. The fragment ion with the higher abundance in the mass spectra was m/z 206, indicating that the parent structure underwent cleavage. Fragment m/z 206 was usually formed by the RDA cleavage of the alkaloid parent structure, and m/z 206 further lost a molecule of water to form fragment m/z 188. Another possible cleavage pathway for compound 9 was the loss of methyl from the parent structure to form m/z 339, followed by the removal of a molecule of H2O to form fragment m/z 336, removal of methylenedioxy to form a ternary ring, and finally the removal of a methyl group to form m/z 324. These above processes were consistent with the cleavage pattern of protopine alkaloids [25], and then by comparing with the literature [26], compound 9 was identified as protopine.
The excimer ion peak in the positive ion mode of compound 38 was m/z 370 [M+H]+, and the molecular formula was probably C21H23NO5. The parent structure lost one CH3 to form fragment m/z 355, followed by α-cleavage to obtain fragment m/z 190. The parent structure underwent RDA cleavage to form the fragments m/z 206 and m/z 165, and m/z 206 continued to lose a hydroxyl to form m/z 189. Compound 38 was identified as allocrytopine in combination with the literature [26]. The chemical structure and proposed fragmentation pathways of compounds 9 and 38 are shown in Figure 7.
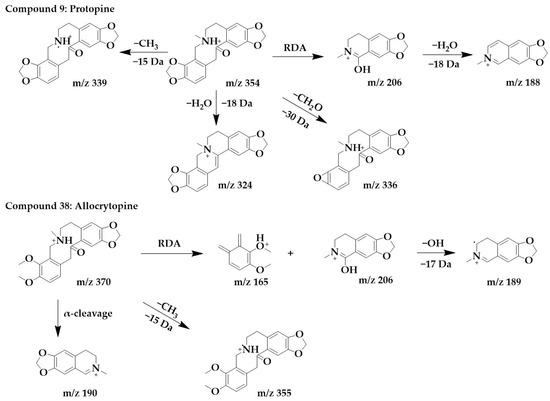
Figure 7.
Chemical structure and proposed fragmentation pathways of compounds 9 and 38.
2.2.6. Benzophenanthridine Alkaloids
The fragments with higher abundance in compounds 22, 23, and 26 were all greater than m/z 200, indicating that the parent structure was not cleaved and therefore presumed to be benzophenanthridine alkaloids [25].
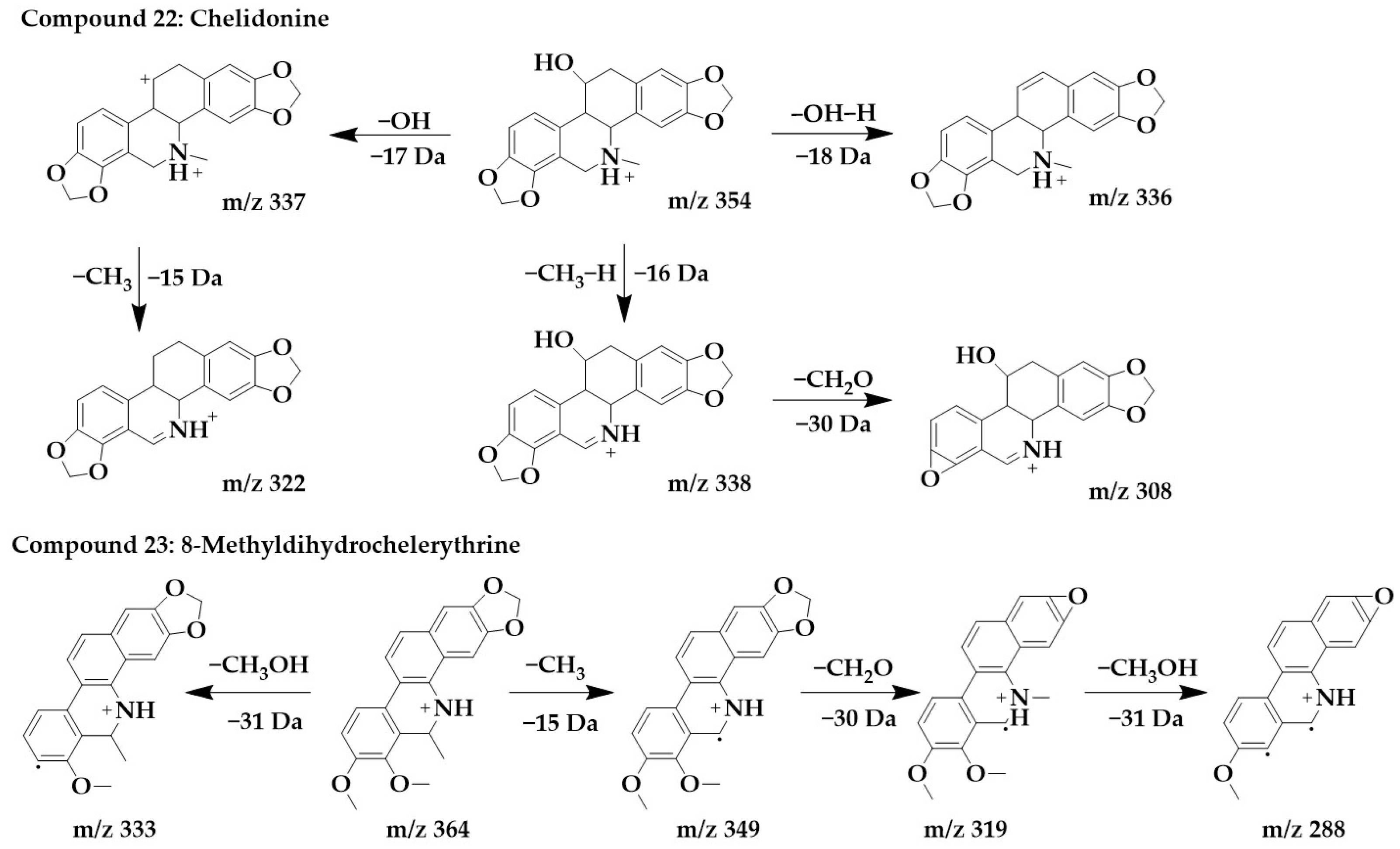
The excimer ion peak of compound 22 was m/z 354 [M+H]+, and the possible molecular formula was C20H19NO5. Based on the presence of m/z 337 and m/z 338, it can be presumed that the side chain of compound 22 contains hydroxyl, and these two fragments originated from the loss of hydroxyl or one molecule of water from a parent structure to form m/z 337, which continued with the loss of one molecule of methyl to form m/z 322. With the successive loss of CH3, H, and CH2O molecules from the parent structure, fragments m/z 338 and m/z 308 were formed, and compound 22 was identified as chelidonine [24,36].
The excimer ion peak of compound 23 was m/z 364 [M+H]+, and the possible molecular formula was C22H21NO4. The presence of methoxy in the parent ion was inferred from the fragment m/z 333 [M+H-CH3OH]+. m/z 349 was formed by the loss of one molecule of methyl from the parent structure, and then, it was presumed that the parent structure contained methylenedioxy based on m/z 349 and m/z 319, so m/z 319 was formed by the loss of one molecule of CH2O from m/z 349. Then, m/z 319 went on to lose one molecule of methoxy to form m/z 288, and compound 23 was identified as 8-methyldihydrochelerythrine. The chemical structures and proposed fragmentation pathways of compounds 22 and 23 were shown in Figure 8. Similarly, compound 26 was identified as chelerythrine [24,36]. The chemical structure and proposed fragmentation pathways of compound 26 was shown in Supplementary Figure S1.
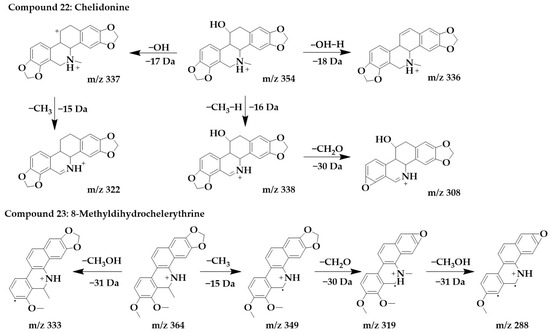
Figure 8.
Chemical structure and proposed fragmentation pathways of compounds 22 and 23.
2.3. Biological Activity of the MDHW Extract
The results of the UPLC-MS/MS analysis showed that MDHW was dominated by alkaloid components. Alkaloids have been attracting attention for their analgesic, anti-inflammatory, antibacterial, antioxidant, and other pharmacological effects [1], Therefore, in this experiment, the alkaloid fraction of MDHW was enriched and a variety of in vitro bioactivity experiments were performed on the crude extract, alkaloid, and non-alkaloid fractions to evaluate the medicinal value of MDHW, and the results are shown in Table 3.

Table 3.
In vitro bioactivity IC50 values of MDHW extracts.
The experimental results show that all MDHW extracts have certain pharmacological activities, and previous studies also showed that Magnoliaceae extracts have good antioxidant, anti-tumor, and other pharmacological activities [10]. The pharmacological activities of the crude extract and alkaloid fraction were significantly stronger than those of the non-alkaloid fraction. The alkaloid fraction was comparable to the crude extract in terms of antioxidant capacity and anti-acetylcholinesterase capacity, and significantly better than the crude extract in terms of anti-α-glucosidase, with an IC50 value of 0.04 mg/mL. The results of the present study fill the gap in the pharmacological activity of the MDHW extract and confirmed that alkaloids are the main source of active ingredient.
2.3.1. Antioxidant Properties
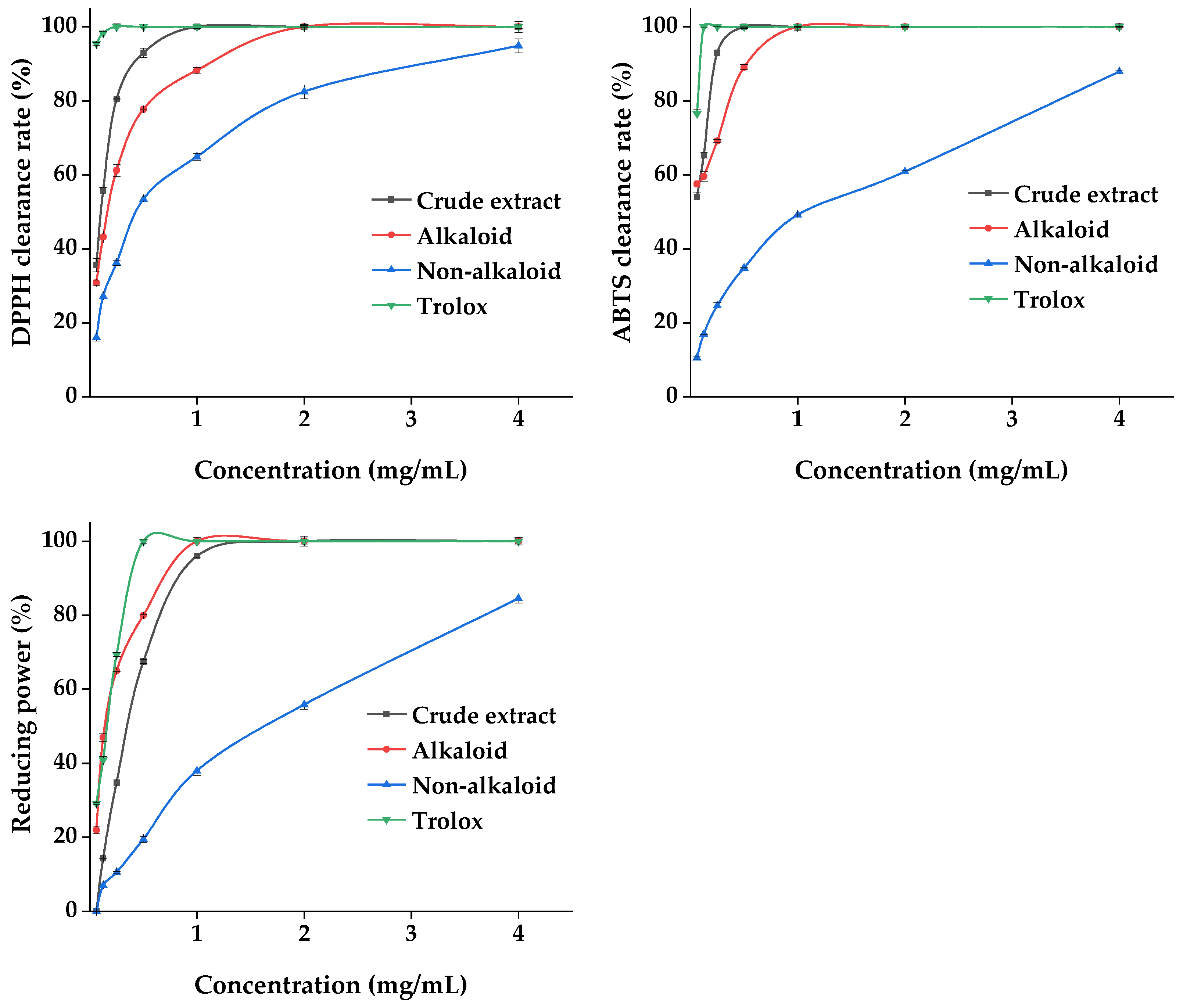
Plant materials are excellent sources of natural antioxidants. The antioxidant activity of plant extracts cannot be assessed by a single approach due to the complexity of the phytochemical composition and different mechanisms of antioxidant reactions. Three methods, namely DPPH, ABTS, and the total reducing power, were used to comprehensively evaluate the antioxidant capacity of the MDHW extracts. The analyses of the antioxidant activity of the MDHW extract and the positive control trolox were shown in Figure 9. As shown in Figure 9, the positive control trolox achieves 100% DPPH and ABTS radical scavenging as well as 100% total reducing power before 0.5 mg/mL. The crude extract and alkaloid fractions showed a dose-dependent antioxidant activity between 0.06 and 2 mg/mL, and the non-alkaloid fraction showed a weaker overall antioxidant activity but also showed some dose dependence (0.06–4.00 mg/mL). The DPPH radical scavenging rate: crude extract > alkaloid fraction > non-alkaloid fraction; ABTS radical scavenging rate: crude extract > alkaloid fraction > non-alkaloid fraction; total reducing power: alkaloid > crude extract > non-alkaloid fraction. This shows that the antioxidant capacity of the alkaloid fraction is comparable to or even better than that of the crude extract. It was noteworthy that the total reducing power of the crude extract was weaker than that of the alkaloids, probably due to the antagonistic effect of the components present in the crude extract in terms of total reducing power. It also cannot be excluded that the amount and proportion of each metabolite in the crude extract lead to a decrease in the total reducing power. Similarly to our results, the antioxidant activity of the total alkaloids was comparable to the positive standard a-tocopherol with an ABTS value of 0.5 mg/mL and a FRAP value of 658 mg/100 g [37], and the purified flavonoids were higher than the crude extracts in terms of antioxidant capacity [38].
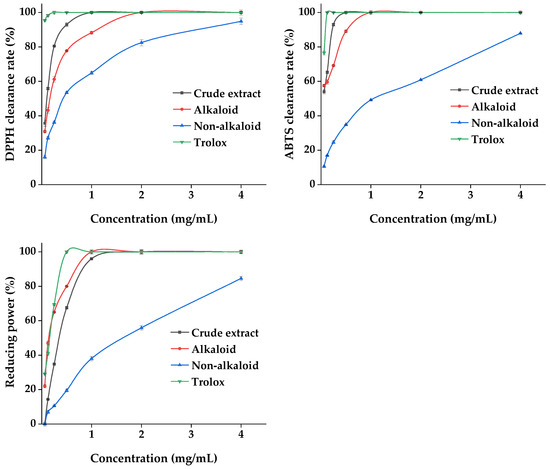
Figure 9.
Antioxidant properties of the MDHW extract.
UPLC-MS/MS analysis revealed that the non-volatile components of MDHW were dominated by isoquinoline alkaloids. Isoquinoline alkaloids have a wide plant distribution and rich chemical structure types, and their unique pharmacological activities are one of the main reasons for interest and research [39]. Aporphine alkaloids were the primary structural type in the current investigation, and prior research indicated that they were a potential class of plant metabolites with a variety of biological and antioxidant activities [40]. Magnoflorine and apomorphine can exert antioxidant activity by scavenging ROS and free radicals and blocking the hypoxanthine–xanthine oxidase system [30]. Liu et al. extracted and isolated 15 aporphine alkaloids, including anonaine, asimilobine, and roemerine, from the lotus flower, and found that all of them had good antioxidant activity by scavenging free radicals, metal complexation, and iron reduction [41]. The antioxidant mechanism of protopine was to boost the activities of catalase, glutathione peroxidase, and superoxide dismutase while inhibiting the growth of intracellular Ca2+, the expression of caspase-3, and H2O2-induced apoptosis [42]. Combining previous studies with our results, it can be speculated that isoquinoline alkaloids are the main contributors to the antioxidant capacity of MDHW extracts.
Overall, the antioxidant capacity of all MDHW extracts was present, proving that they can also be a great source of natural antioxidants. Alkaloids are the active ingredients, the rich isoquinoline alkaloid component may be responsible for the good antioxidant capacity.
2.3.2. Enzyme Inhibition Effects
Acetylcholinesterase (AChE) and α-glucosidase are the key enzymes inhibiting AD and DM. We evaluated the effect of the MDHW extract on the inhibition of these two enzymes, and the results are shown in Figure 10. The positive control tacrine and acarbose reached 100% inhibition at the lowest concentration of 0.06 mg/mL. The crude extract and alkaloid fractions showed dose-dependent enzyme inhibition activity between 0.06 and 2 mg/mL, and the non-alkaloid fraction showed weaker inhibition overall but also showed a certain dose dependence (0.06–4.00 mg/mL). The inhibitory capacity of both enzymes was alkaloid fraction > crude extract > non-alkaloid fraction, indicating that the alkaloid fraction was significantly effective in inhibiting AChE and α-glucosidase. The lower inhibitory effect of the crude extracts than the alkaloid fraction can be explained by complex interactions (synergistic or antagonistic) among phytochemicals.
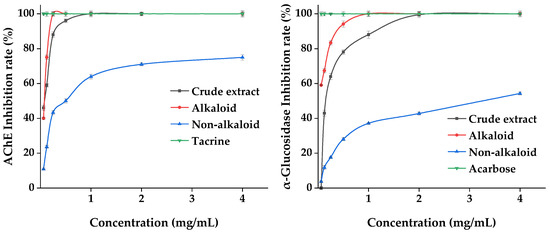
Figure 10.
Enzyme Inhibition Effects of the MDHW Extract.
Many acetylcholinesterase inhibitors were isolated from natural products, and most compounds have been shown to have some inhibitory activity, but alkaloids are considered to be the most promising natural products for the treatment of AD due to their complex N-containing structures [43]. As the structural type of this most abundant compound, aporphine alkaloids not only show excellent activity in antioxidants but also effectively inhibit AChE [40]. Protopine inhibits AChE in a dose-dependent manner and has been shown to be reversible, specific, and competitive [42], and it has also been shown to have almost the same efficacy as velnacrine, the most commonly used tacrine derivative on the market for the treatment of AD [44].
In addition, other alkaloids found in MDHW were also shown to be effective in inhibiting the AChE activity, such as allocryptopine with an IC50 value of 250 μM against AChE [45]. Bamatin has a strong inhibitory activity against AChE with an IC50 value <10 μM [46]. The inhibitory effect of asimilobine on AChE is comparable to that of the standard galanthamine and is time- and concentration-dependent [44]. Michelalbine has a good binding affinity for potential targets for the treatment of diabetes, namely human amylin peptide and dipeptidyl peptidase-4, and can effectively control blood glucose [47]. In addition, magnoflorine, anonaine, apomorphine, and roemerine also have significant efficacy in insulin resistance and obesity suppression [30,48].
Overall, the MDHW extract showed significant effects on AChE and α-glucosidase inhibition, indicating its great developmental value in delaying Alzheimer’s disease and hypoglycemic products. Alkaloids are among the active ingredients, and the isoquinoline alkaloid-rich composition may be responsible for the good AChE and α-glucosidase activity inhibition ability.
2.3.3. Antimicrobial Activities
To investigate the bacterial inhibitory potential of MDHW extracts, two gram-positive bacteria, Staphylococcus aureus (S. aureus) and Bacillus subtilis (B. subtilis), as well as two gram-negative bacteria, Escherichia coli (E. coli) and Erwinia carotovora (E. carotovora), were subjected to minimum inhibitory concentration (MIC) determination. The results of the MIC determination of the three extracts against the four pathogenic bacteria are shown in Table 4. The positive control 100 ug/mL kanamycin completely inhibited the growth of all strains, while the negative control 50% methanol had no inhibitory ability for all strains. The MIC values of these extracts against the four pathogenic bacteria ranged from 0.125 to 2 mg/mL, indicating that they had a certain inhibitory effect on all the test strains. The enriched alkaloid fraction had stronger bacterial inhibitory effects compared to the crude extract and the non-alkaloid fraction. This may be due to the higher content of alkaloids in the purified extract than in the crude extract, and alkaloids are considered to have antibacterial activity [49]. The non-alkaloid fraction had a weak antibacterial ability with MIC values greater than 1 mg/mL, while the crude extract and alkaloid fraction showed great potential for bacterial inhibition against all tested strains.

Table 4.
The MIC of MDHW extracts against four pathogenic bacteria.
The gram-positive and gram-negative bacteria are Class II dangerous biological agents, according to the Advisory Committee on Dangerous Pathogens (ACDP), since they can cause human sickness and perhaps endanger life [7]. These strains are often found in household contaminants such as cereals, meat, eggs, and water contamination, so it is important to inhibit their growth [50]. The microbe most sensitive to all extracts was S. aureus, and the MIC values of crude extract, alkaloid, and non-alkaloid parts were 0.25, 0.125, and 0.5 mg/mL, respectively. All extracts showed stronger inhibition against gram-positive bacteria than gram-negative bacteria. This phenomenon can be explained by the fact that gram-positive bacteria are to some extent more sensitive to drugs than gram-negative bacteria [51].
Many studies have shown that alkaloids, flavonoids, and lignans have antibacterial effects on many pathogenic bacteria [38,52,53]. Combined with the UPLC-MS, the analysis of MDHW showed that MDHW contained effective active antibacterial components such as alkaloids and flavonoids and lignans, especially isoquinoline alkaloids. There are various types of alkaloid structures among which isoquinoline alkaloids and indole alkaloids are the main types of compounds with antibacterial activity [49]. Moreover, 1 μg/mL roemerine significantly inhibited biofilm formation and prevented the transition of Candida albicans yeast to mycelium transformation [54]. In previous studies, magnoflorine, palmatine, berberine, allocryptopine, chelidonine, chelerythrine, and anonaine [30,36,53,55] have been shown to have strong antibacterial abilities, and these compounds are found in the present study. In addition to the alkaloids, the flavonoids (kaempferol [56], luteolin [57]) and lignin (podophyllotoxin [58]) found in this study have also been shown to have good antibacterial potential.
The above analysis indicates that MDHW can be a potential source of natural antimicrobial agents, in which alkaloids are the main active ingredient, and the enrichment of isoquinoline alkaloid components may be responsible for the significant antimicrobial activity.
3. Materials and Methods
3.1. Plant Material
Three plants of Michelia macclurei Dandy were collected in May 2021 in Nanning, Guangxi, China, and identified as Michelia macclurei Dandy by the Guangxi University Testing Centre. After the removal of the bark and sapwood, the heartwood (Supplementary Figure S2) was naturally dried, crushed, and filtered through a 40-mesh sieve, and then sealed and dried for storage.
3.2. Preparation of Plant Extracts
3.2.1. Volatile Compounds
Steam distillation: extraction of essential oils by SD was carried out according to the literature with slight modifications [59]. Briefly, the volatile oil was extracted by adding 130 g of heartwood powder to 850 mL of water, connecting the volatile oil extractor, and adding 5 mL of ether to the upper layer, heating in an electric heating jacket, and refluxing for 10 h. After cooling, the ether layer was collected to obtain the volatile oil. The volatile oil was dissolved in chromatographic grade n-hexane and analyzed by GC-MS.
Ultrasonic extraction accurately weighs 0.100 g of heartwood powder dissolved in 5 mL of methanol and extracted with ultrasound for 1h. Then, 1 mL of supernatant was evaporated and dissolved in chromatographic grade hexane and passed through a 0.22 μm filter membrane.
3.2.2. Non-Volatile Compounds
Extraction steps were slightly modified from the literature [60]. A total of 100 g of heartwood powder was dissolved in 1000 mL of ethanol (95%, v/v) and extracted for 2 h. Extracted twice, the filtrate was concentrated and dried to obtain the crude extract. The crude extract was added to distilled water, adjusted to pH 7.0, and filtered, and the filtrate was the non-alkaloid fraction. The pH of the acid water layer was adjusted to 9.0–10 with 2% NaOH, and chloroform extraction was performed to obtain the chloroform layer and the alkaline water layer. The chloroform layer was recovered and the lipid-soluble alkaloids were obtained. An appropriate amount of n-butanol was added to the alkaline aqueous layer, eluted, and dried to constant weight to obtain water-soluble alkaloids. The lipid-soluble alkaloids and the water-soluble alkaloids were combined to obtain the total alkaloid fraction. All extracts were stored in the refrigerator at −4 °C until use.
3.3. GC-MS Analysis
3.3.1. Instrument Condition
The analysis was performed using the Thermo Scientific TRACE 1300-TSQ 9000 chromatograph with the TG-5SILMS column (30 m × 0.25 mm × 0.25 μm), injection port temperature of 280 °C, injection volume of 1 μL, split mode (split ratio 10:1), and a flow rate of 1.0 mL/min. The ramp-up procedure was an initial temperature of 40 °C (hold for 2 min), and ramped up to 280 °C at 5 °C/min (hold for 5 min). Mass spectrometry conditions: scan range 30–700 m/z, solvent delay 3.0 min, transmission line temperature 28 °C, ion source EI, ion source temperature 300 °C, and high purity helium as the carrier gas.
3.3.2. Component Identification
The MS/MS spectra were automatically searched using the Thermo Fisher mass spectrometry database and derived. The retention index RI was calculated in combination with the n-alkane peak exit time. Based on the RI, MS/MS spectra, the latest NIST library to confirm the structure of each chemical component, and the relative percentages of each chemical component were determined using the peak area normalization method.
3.4. UPLC-MS Analysis
3.4.1. Instrument Condition
Q-EXACTIVE-MS (Thermo Scientific, Waltham, MA, USA) was used for the analysis. The chromatographic column was ACQUITY UPLCBEHC18 (2.1 mm × 50 mm × 1.7 μm) with a mobile phase of 0.1% formic acid-water in A and acetonitrile in B. The column temperature was 30 °C, the injection volume was 1 μL, and the flow rate was 0.8 mL/min. The column temperature was 30 °C, the injection volume was 1 μL, and the flow rate was 0.8 mL/min. The gradient conditions were 0–3 min, 5–10% B; 3–6 min, 10–30%; 6–15 min, 30–70% B; 15–20 min, 70–95% B; 18–23 min, 95% B; 23–25 min, 95–5% B. The mass spectrometry conditions were electrospray ionization (ESI), an ion source temperature of 300 °C, auxiliary gas flow rate of 69 kPa, scan modes of full MS and full MS/dd-MS2, a mass range of 100–1,000 Da, and primary and secondary scan resolutions of 70,000 and 17,500, respectively.
3.4.2. Component Identification
The identification of compounds based on accurate masses, elemental compositions, and secondary mass spectral fragments provided by the mzCloud Mass Spectral Library database. The accurate mass measurements of ions and major characteristic ions were controlled to within ±5.0 ppm of their expected elemental composition.
3.5. Biological Activity
The extracts (crude extract, alkaloid, and non-alkaloid fractions) were prepared at concentrations of 0.0625, 0.125, 0.25, 0.5, 1, 2, and 4 mg/mL, and all biological activity tests were carried out using this series of concentrations.
3.5.1. Antioxidant Assays
In this work, three different methods were used to evaluate the antioxidant properties of MDHW extracts. Trolox (for free radical scavenging and the total reducing power) was used as a positive control.
The DPPH assay was carried out according to the method described in the literature with minor modifications [61]. Briefly, a 2.5 mL aliquot of DPPH ethanol solution (0.2 mM) was mixed with the 2.5 mL sample solution. The absorbance was measured at 517 nm after 30 min at room temperature and protected from light.
The ABTS assay was carried out according to the method described in the literature with minor modifications [3]. ABTS (7 mM) and aqueous potassium persulphate (2.45 mM) were mixed in equal volumes and left to stand overnight at room temperature and protected from light to form the ABTS+ solution. The absorbance at 734 nm was adjusted with ethanol to 0.700 ± 0.005 before use. A total of 0.1 mL of the sample solution and 2 mL of ABTS+ solution were mixed. The absorbance at 734 nm was measured after 30 min at room temperature and protected from the light.
The total reducing power determination was carried out according to the method described in the literature with minor modifications [3]. 800 μL of PBS solution (0.2 M, PH 6.6), 1 mL of K3Fe (1%) solution, and 100 μL of sample solution were mixed and incubated at 50 °C for 20 min; then, 1 mL of trichloroacetic acid was added (10%), and the mixture was centrifuged and took 1 mL of supernatant; finally, 1 mL of distilled water and 200 μL of FeCl3 (0.1%) were added and the absorbance was measured at 700 nm after 10 min.
3.5.2. Enzyme Inhibitory Assays
The AChE inhibition assay was performed according to the method described in the literature with slight modifications [3]. As such, 3 mL of PBS solution (0.1M, PH 8.0), 20 μL AChE solution (0.5 U/mL), and 100 μL sample solution were mixed and incubated for 2 min at 37 °C. The reaction was terminated by the addition of 1 mL of SDS (4%) and the absorbance was quickly measured at 412 nm.
The α-glucosidase inhibition assay was performed according to the literature with slight modifications [3]. As such, 1.5 mL PBS solution (0.1 M, PH 6.8), 200 μL α-glucosidase (0.6 U/mL), and 10 μL sample solution were mixed and incubated for 10 min at 37 °C; then, 160 μL PNPG (2.5 mM) was added and the mixture was incubated for 20 min at 37 °C. The reaction was terminated by the addition of 1 mL of Na2CO3 (0.2 M) and the absorbance was quickly measured at 405 nm.
3.5.3. Antimicrobial Assay
Four-gram pathogenic bacteria (S. aureus, B. subtilis, E. coli, and E. carotovora) were subcultured twice before use. Then, 108 CFU/mL of bacterial broth was prepared by autoclaving a liquid medium (containing 0.9% NaCl). The MIC was determined using a 96-well plate microdilution method with reference to the literature [62]. 50 μL of liquid medium, 50 μL of sample solution, and 50 μL of bacterial solution (108 CFU/mL) were added to each well and incubated for 20 h at 37 °C; then, 20 μL of resazurin solution (0.3 mg/mL) was added and the mixture underwent further incubation for 4 h. The concentration that can cause any color change to the solution was defined as MIC. Kanamycin (100 μg/mL) was used as a positive control and methanol (50%) as a negative control.
3.6. Data Analysis
Compound Discoverer 3.1 was used for the mass spectrometry data analysis, and compound structures were drawn using ChemDraw 20.0. Three replicates were set up for the bioactivity experiments and data were reported as the mean ± standard deviation (SD). One-way ANOVA IC50 values were calculated using IBM SPSS Statistics 19. Origin 2023 was used for data plotting.
4. Conclusions
In this study, a comprehensive analysis of the MDHW extract was conducted for the first time using GC-MS and UPLC-MS. The results show that the MDHW chemical components were of various structural types, with volatile components dominated by sesquiterpenes and their derivatives and fatty compounds, and non-volatile components including alkaloids, lignans, flavonoids, terpenoids, etc., among which isoquinoline alkaloids were the main component types. In vitro bioactivity experiments showed that the MDHW extract has excellent antioxidant, anti-acetylcholinesterase, anti-α-glucosidase activities, as well as antibacterial ability. MDHW can be used as a potential source of bioactive substances that can be developed in natural antioxidants, delay Alzheimer’s disease, hypoglycemic products, and antibacterial agents. Alkaloids are the main active ingredients of MDHW, and the main type of isoquinoline alkaloids may be the main reason for their excellent biological activity. However, the antioxidant inhibiting AD and DM and the antibacterial mechanisms of the alkaloids as well as important polyphenolic compounds in MDHW still need further study. Our research provides helpful information for the further rational exploitation of MD plant resources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24097972/s1.
Author Contributions
Conceptualization, Investigation, S.C.; Methodology, Validation, S.C. and B.W.; Data analysis and Visualization, S.C.; Writing—original draft preparation, S.C.; Writing—review and editing, Y.F.; Resources, Supervision, and Funding acquisition, Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32171702).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sut, S.; Baldan, V.; Faggian, M.; Peron, G.; Dall, A.S. Nutraceuticals, a new challenge for medicinal chemistry. Curr. Med. Chem. 2016, 23, 3198–3223. [Google Scholar] [CrossRef]
- Yingming, P.; Ying, L.; Hengshan, W.; Min, L. Antioxidant activities of several chinese medicine herbs. Food Chem. 2004, 88, 347–350. [Google Scholar] [CrossRef]
- Ma, S.; Qiao, M.; Fu, Y.; Wei, P.; Li, Y.; Liu, Z. Comparative analysis of biological activity of artificial and wild agarwood. Forests 2021, 12, 1532. [Google Scholar] [CrossRef]
- Nouri, L.; Nafchi, A.M.; Karim, A.A. Phytochemical, antioxidant, antibacterial, and α-amylase inhibitory properties of different extracts from betel leaves. Ind. Crop. Prod. 2014, 62, 47–52. [Google Scholar] [CrossRef]
- Bekir, J.; Cazaux, S.; Mars, M.; Bouajila, J. In vitro anti-cholinesterase and anti-hyperglycemic activities of flowers extracts from seven pomegranate varieties. Ind. Crop. Prod. 2016, 81, 176–179. [Google Scholar] [CrossRef]
- Luo, X.; Zeng, L.; Li, Q.; Wang, Z.; Kong, F.; Bi, Y. β-cyclodextrin inclusion complex containing essential oil from wampee [Clausena lansium (lour.) Skeels] fruit pericarp: Synthesis, characterization, and evaluation of antioxidant activity. J. Mol. Struct. 2022, 1266, 133525. [Google Scholar] [CrossRef]
- Shakeri, A.; Khakdan, F.; Soheili, V.; Sahebkar, A.; Shaddel, R.; Asili, J. Volatile composition, antimicrobial, cytotoxic and antioxidant evaluation of the essential oil from Nepeta sintenisii bornm. Ind. Crop. Prod. 2016, 84, 224–229. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Nadri, M.H.; Othman, N.Z.; Rashid, S.N.A.A.; Lim, Y.; Leong, H. Phytochemistry, bioactivities and traditional uses of Michelia × alba. Molecules 2022, 27, 3450. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Q.; Chen, Y.; Zhong, C.; Zhang, Y.; Chen, Z.; Pinyopusarerk, K.; Bush, D. Arbuscular mycorrhizal fungi enhanced growth of Magnolia macclurei (dandy) figlar seedlings grown under glasshouse conditions. For. Sci. 2017, 63, 441–448. [Google Scholar] [CrossRef]
- Niu, D.; Wang, S.; Ouyang, Z. Comparisons of carbon storages in Cunninghamia lanceolata and Michelia macclurei plantations during a 22-year period in southern china. J. Environ. Sci. China 2009, 21, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, S.; Zhong, M. Ecosystem carbon storage and soil organic carbon stability in pure and mixed stands of Cunninghamia lanceolata and michelia macclurei. Plant Soil 2013, 370, 295–304. [Google Scholar] [CrossRef]
- Hui-Fen, M.A.; Yong-Kang, S.; Jia-Bo, H.; Shao-Yu, C.; Ming-Yue, H.; Dan, L.I.; Liang, X.U.; Bin, Z.; Yong, C. Study on chemical components in the volatile oils from Michelia polyneura c.y.wu ex law et y.f.wu.and michelia macclurei dandy. Guangdong Agric. Sci. 2011, 38, 110–113. [Google Scholar]
- Xiao-Kai, S.; Chun-Liang, L.U.; Kun, H.U.; Xiao-Yan, W.; Hai-Yang, J.; Sai-Juan, C. Gc-ms analysis of volatile components from barks of Michelia macclurei and their inhibition on in vitro growth of hepg2 cells. Chin. Tradit. Herb. Drugs 2011, 42, 2213–2215. [Google Scholar]
- Moniodis, J.; Jones, C.G.; Barbour, E.L.; Plummer, J.A.; Ghisalberti, E.L.; Bohlmann, J. The transcriptome of sesquiterpenoid biosynthesis in heartwood xylem of western australian sandalwood (Santalum spicatum). Phytochemistry 2015, 113, 79–86. [Google Scholar] [CrossRef]
- Ma, R.; Luo, J.; Qiao, M.; Fu, Y.; Zhu, P.; Wei, P.; Liu, Z. Chemical composition of extracts from Dalbergia odorifera heartwood and its correlation with color. Ind. Crop. Prod. 2022, 180, 114728. [Google Scholar] [CrossRef]
- Xiaokai, S.; Zhiling, C.; Lei, G.; Zhihua, L.I. GC-MS analysis of volatile components from trunk of michelia macclurei dandy.and the inhibition of etmmd on growth of mda-mb-231 cell lines and its apoptosis-inducing. Chin. J. Mod. Appl. Pharm. 2014, 31, 911–915. [Google Scholar]
- Fukushima, S.; Cohen, S.M.; Eisenbrand, G.; Gooderham, N.J.; Taylor, S.V. Fema gras assessment of natural flavor complexes: Lavender, guaiac coriander-derived and related flavoring ingredients. Food Chem. Toxicol. 2020, 145, 111584. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, S.; Taprial, S.; Kashyap, D.; Kumar, A.; Prakash, O. A review of chemical and biological profile of genus Michelia. Chin. J. Integr. Med. 2012, 10, 1336–1341. [Google Scholar] [CrossRef]
- Sua, Y.; Hsub, K.; Wang, E.I.; Hob, C. Chemical composition and anti-mildew activities of essential oils from different parts of Michelia compressa var. Formosana. Nat. Prod. Commun. 2015, 10, 665–668. [Google Scholar] [CrossRef]
- Wu, C.; Huang, S.; Ko, C.; Chang, H. Antifungal sesquiterpenoids from Michelia formosana leaf essential oil against wood-rotting fungi. Molecules 2022, 27, 2136. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Zeng, Y.; Zeng, Z.; Zhang, N.; Li, C.; Zeng, Y.; You, Y.; Wang, S.; Chen, X.; Sui, X.; et al. Drug delivery systems for elemene, its main active ingredient β-elemene, and its derivatives in cancer therapy. Int. J. Nanomed. 2018, 13, 6279–6296. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Chaijaroenkul, W.; Na Bangchang, K. Therapeutic potential and pharmacological activities of β-eudesmol. Chem. Biol. Drug Des. 2021, 97, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Yan, F.; Huang, P.; Zeng, J. Establishing the metabolic network of isoquinoline alkaloids from the Macleaya genus. Phytochemistry 2021, 185, 112696. [Google Scholar] [CrossRef]
- Qing, Z.; Cheng, P.; Liu, X.; Liu, Y.; Zeng, J. Systematic identification of alkaloids in Macleaya microcarpa fruits by liquid chromatography tandem mass spectrometry combined with the isoquinoline alkaloids biosynthetic pathway. J. Pharm. Biomed. Anal. 2015, 103, 26–34. [Google Scholar] [CrossRef]
- Jeong, E.; Lee, S.Y.; Yu, S.M.; Park, N.H.; Lee, H.; Yim, Y.; Hwang, G.; Cheong, C.; Jung, J.H.; Hong, J. Identification of structurally diverse alkaloids in Corydalis species by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1661–1674. [Google Scholar] [CrossRef]
- Menendez-Perdomo, I.M.; Facchini, P.J. Isolation and characterization of two o-methyltransferases involved in benzylisoquinoline alkaloid biosynthesis in sacred lotus (Nelumbo nucifera). J. Biol. Chem. 2020, 295, 1598–1612. [Google Scholar] [CrossRef]
- Guo, K.; Tong, C.; Fu, Q.; Xu, J.; Shi, S.; Xiao, Y. Identification of minor lignans, alkaloids, and phenylpropanoid glycosides in Magnolia officinalis by hplcnulldadnullqtof-ms/ms. J. Pharm. Biomed. Anal. 2019, 170, 153–160. [Google Scholar] [CrossRef]
- Sim, H.; Yoon, S.H.; Kim, M.S.; Kim, B.; Park, H.; Hong, J. Identification of alkaloid constituents from Fangchi species using ph control liquid-liquid extraction and liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. RCM 2015, 29, 837–854. [Google Scholar] [CrossRef]
- Okon, E.; Kukula-Koch, W.; Jarzab, A.; Halasa, M.; Stepulak, A.; Wawruszak, A. Advances in chemistry and bioactivity of magnoflorine and magnoflorine-containing extracts. Int. J. Mol. Sci. 2020, 21, 1330. [Google Scholar] [CrossRef]
- Yeha, Y.; Huangb, J.; Kuoc, P.; Chena, C. Bioactive constituents from Michelia champaca. Nat. Prod. Commun. 2011, 9, 1251–1252. [Google Scholar]
- De Lima, B.; Da Silva, F.; Soares, E.; de Almeida, R.; Da Silva-Filho, F.; Barison, A.; Costa, E.; Koolen, H.; de Souza, A.; Pinheiro, M.L. Integrative approach based on leaf spray mass spectrometry, hplc-dad-ms/ms, and nmr for comprehensive characterization of isoquinoline-derived alkaloids in leaves of Onychopetalum amazonicum r. E. Fr. J. Braz. Chem. Soc. 2020, 31, 79–89. [Google Scholar] [CrossRef]
- Deng, Y.; Liao, Q.; Li, S.; Bi, K.; Pan, B.; Xie, Z. Simultaneous determination of berberine, palmatine and jatrorrhizine by liquid chromatography–tandem mass spectrometry in rat plasma and its application in a pharmacokinetic study after oral administration of coptis–evodia herb couple. J. Chromatogr. B 2008, 863, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, J.; Lin, C.; Miao, L.; Lin, L. Alkaloid profiling of the traditional chinese medicine rhizoma corydalis using high performance liquid chromatography-tandem quadrupole time-of-flight mass spectrometry. Acta Pharm. Sin. B 2014, 4, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Le, P.M.; Mccooeye, M.; Windust, A. Application of uplc-qtof-ms in mse mode for the rapid and precise identification of alkaloids in goldenseal (Hydrastis canadensis). Anal. Bioanal. Chem. 2014, 406, 1739–1749. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Fornal, E.; Jesionek, W.; Majer-Dziedzic, B.; Choma, I.M. Effect-directed isolation and identification of antibacterial Chelidonium majus l. Alkaloids. Chromatographia 2015, 78, 707–716. [Google Scholar] [CrossRef]
- Ruan, X.; Cui, W.; Yang, L.; Li, Z.; Liu, B.; Wang, Q. Extraction of total alkaloids, peimine and peiminine from the flower of Fritillaria thunbergii miq using supercritical carbon dioxide. J. CO2 Util. 2017, 18, 283–293. [Google Scholar] [CrossRef]
- Wu, M.; Xu, J.; Zhang, H.; Xia, W.; Li, W.; Zhang, W. Purification and identification of flavonoid molecules from Rosa setate x rosa rugosa waste extracts and evaluation of antioxidant, antiproliferative and antimicrobial activities. Molecules 2022, 27, 4379. [Google Scholar] [CrossRef]
- Hagel, J.M.; Facchini, P.J. Benzylisoquinoline alkaloid metabolism: A century of discovery and a brave new world. Plant Cell Physiol. 2013, 54, 647–672. [Google Scholar] [CrossRef]
- Khizrievaa, S.S.; Borisenkoa, S.N.; Maksimenkoa, E.V.; Vetrovaa, E.V.; Borisenkoa, A.V.I.M.N.I. Antioxidant properties and effects of aporphine alkaloids and their phenanthrene seco-isomers on acetylcholinesterase activity. Russ. J. Bioorg. Chem. 2022, 48, 237–246. [Google Scholar]
- Liu, C.; Kao, C.; Wu, H.; Li, W.; Huang, C.; Li, H.; Chen, C. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera gaertn. Cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Kong, L.; Cao, Y.; Yan, L. Identification and quantification, metabolism and pharmacokinetics, pharmacological activities, and botanical preparations of protopine: A review. Molecules 2022, 27, 215. [Google Scholar] [CrossRef]
- Pereira, D.M.; Ferreres, F.; Oliveira, J.M.A.; Gaspar, L.; Faria, J.; Valentão, P.; Sottomayor, M.; Andrade, P.B. Pharmacological effects of Catharanthus roseus root alkaloids in acetylcholinesterase inhibition and cholinergic neurotransmission. Phytomedicine 2010, 17, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Satheeshkumar, N.; Venkatesh, P.; Venkatesh, M. Lead finding for acetyl cholinesterase inhibitors from natural origin: Structure activity relationship and scope. Mini Rev. Med. Chem. 2011, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Siatka, T.; Adamcová, M.; Opletal, L.; Cahlíková, L.; Jun, D.; Hrabinová, M.; Kuneš, J.; Chlebek, J. Cholinesterase and prolyl oligopeptidase inhibitory activities of alkaloids from Argemone platyceras (papaveraceae). Molecules 2017, 22, 1181. [Google Scholar] [CrossRef]
- Huang, Q.; Bi, J.; Sun, Q.; Yang, F.; Wang, H.; Tang, G.; Zhao, F.; Huanwang, J.X.E.J. Bioactive isoquinoline alkaloids from Corydalis saxicola. Planta Med. 2012, 1, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kausar, M.A.; Anwar, S.; Eltayb, W.A.; Kuddus, M.; Khatoon, F.; El-Arabey, A.A.; Khalifa, A.M.; Rizvi, M.R.; Najm, M.Z.; Thakur, L.; et al. Md simulation studies for selective phytochemicals as potential inhibitors against major biological targets of diabetic nephropathy. Molecules 2022, 27, 4980. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, N.; Zhou, F.; Lin, D. Natural aporphine alkaloids with potential to impact metabolic syndrome. Molecules 2021, 26, 6117. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media for the Examination of Food; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol. 2006, 108, 1–9. [Google Scholar] [CrossRef]
- Agüero, M.B.; Svetaz, L.; Baroni, V.; Lima, B.; Luna, L.; Zacchino, S.; Saavedra, P.; Wunderlin, D.; Feresin, G.E.; Tapia, A. Urban propolis from san juan province (argentina): Ethnopharmacological uses and antifungal activity against Candida and dermatophytes. Ind. Crop. Prod. 2014, 57, 166–173. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Du, F.; Yan, L.; He, G.; He, J.; Wang, C.; Rao, G.; Jiang, Y.; Xu, G. Potent activities of roemerine against Candida albicans and the underlying mechanisms. Molecules 2015, 20, 17913–17928. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, T.; Tseng, W.; Lu, F.; Hung, R.; Chen, C.; Chen, C. (−)-anonaine induces apoptosis through bax- and caspase-dependent pathways in human cervical cancer (hela) cells. Food Chem. Toxicol. 2008, 46, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Ben Hsouna, A.; Michalak, M.; Ben Akacha, B.; Dhifi, W.; Ben Saad, R.; Brini, F.; Mnif, W. Assessment of the phytochemical composition, antimicrobial activity and anti-inflammatory effects of Lobularia maritima extracts on lipopolysaccharide-stimulated raw 264.7 cells and their capacity to extend the shelf life of raw minced beef. J. Funct. Food. 2022, 99, 105327. [Google Scholar] [CrossRef]
- Yin, L.; Han, H.; Zheng, X.; Wang, G.; Li, Y.; Wang, W. Flavonoids analysis and antioxidant, antimicrobial, and anti-inflammatory activities of crude and purified extracts from Veronicastrum latifolium. Ind. Crop. Prod. 2019, 137, 652–661. [Google Scholar] [CrossRef]
- Jin, L.; Song, Z.; Cai, F.; Ruan, L.; Jiang, R. Chemistry and biological activities of naturally occurring and structurally modified podophyllotoxins. Molecules 2023, 28, 302. [Google Scholar] [CrossRef] [PubMed]
- Demirpolat, A.; Akman, F.; Kazachenko, A.S. An experimental and theoretical study on essential oil of Aethionema sancakense: Characterization, molecular properties and rdg analysis. Molecules 2022, 27, 6129. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, H.; Dong, A. Systematic separation and purification of alkaloids from Euchresta tubulosa dunn. By various chromatographic methods. Processes 2019, 7, 924. [Google Scholar] [CrossRef]
- Ben Akacha, B.; Garzoli, S.; Ben Saad, R.; Brini, F.; Mnif, W.; Kačániová, M.; Ben Hsouna, A. Biopreservative effect of the tunisian halophyte Lobularia maritima flavonoid fraction, used alone and in combination with linalool in stored minced beef meat. Metabolites 2023, 13, 371. [Google Scholar] [CrossRef]
- Ben Akacha, B.; Avarc-Gajić, J.; Elhadef, K.; Ben Saad, R.; Brini, F.; Mnif, W.; Smaoui, S.; Ben Hsouna, A. The essential oil of tunisian halophyte Lobularia maritima: A natural food preservative agent of ground beef meat. Life 2022, 12, 1571. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).