Poly(levodopa)-Functionalized Polysaccharide Hydrogel Enriched in Fe3O4 Particles for Multiple-Purpose Biomedical Applications
Abstract
:1. Introduction
2. Results and Discussion
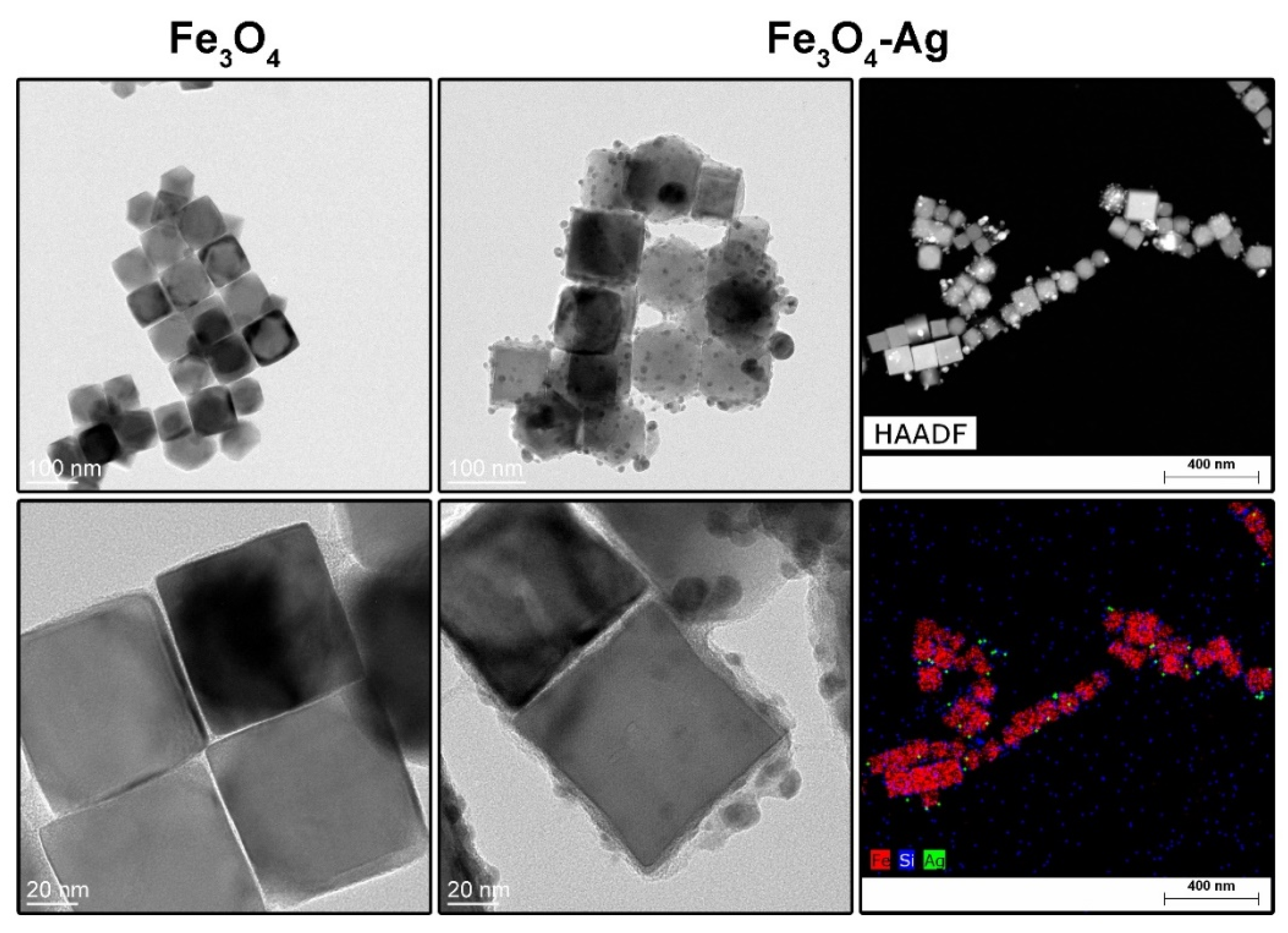
2.1. Characterization of Nanoparticles and Heterostructures
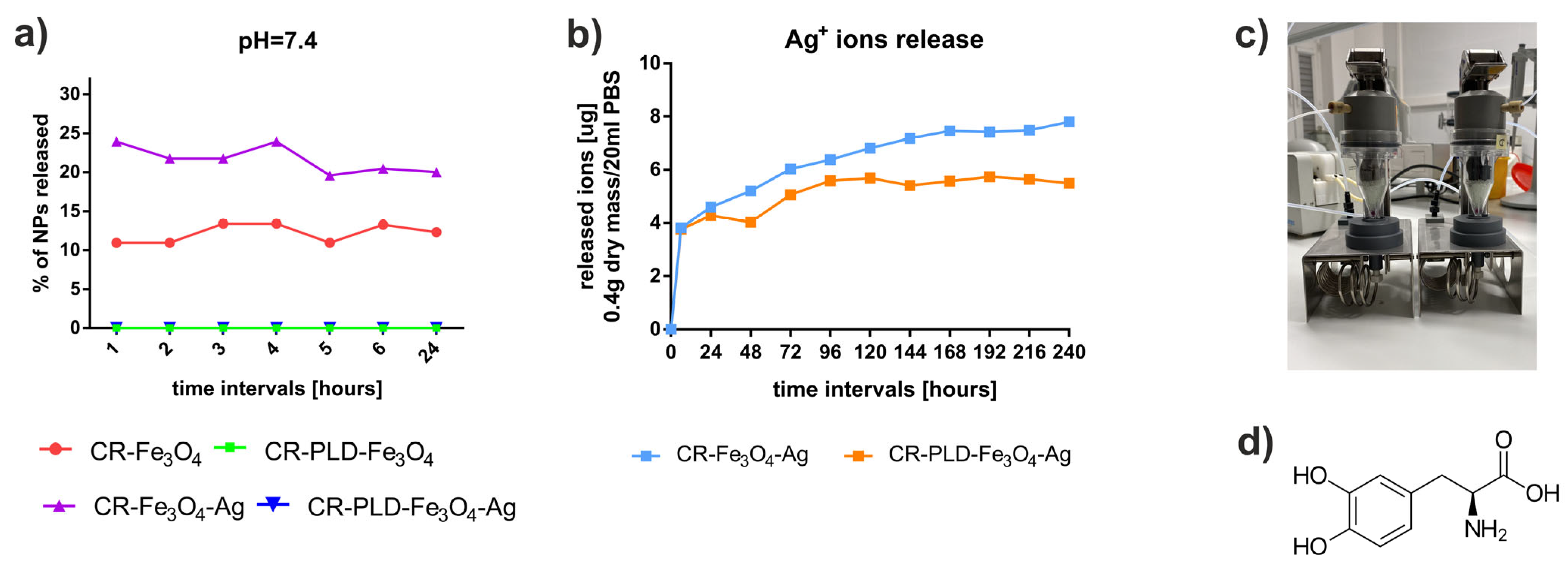
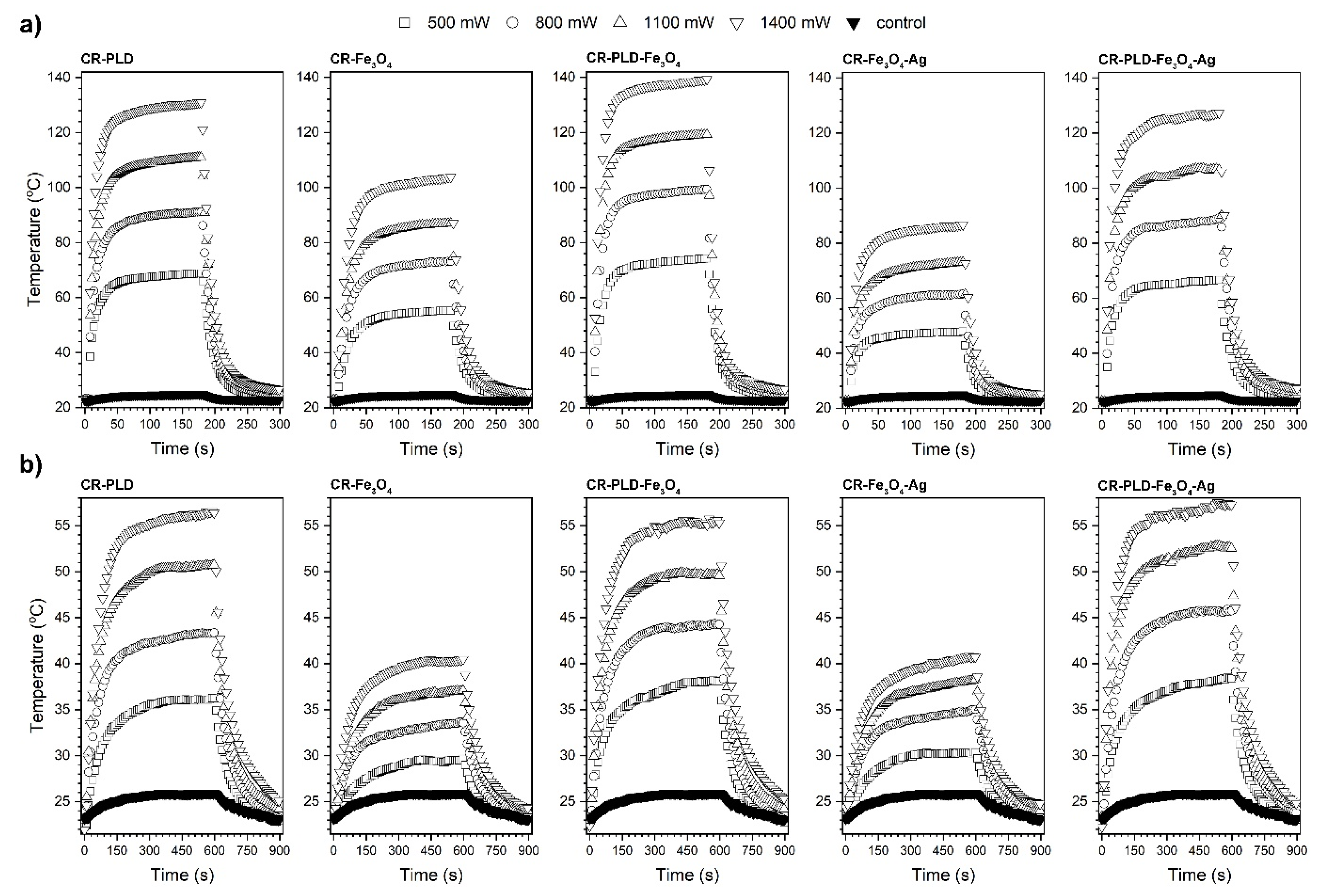
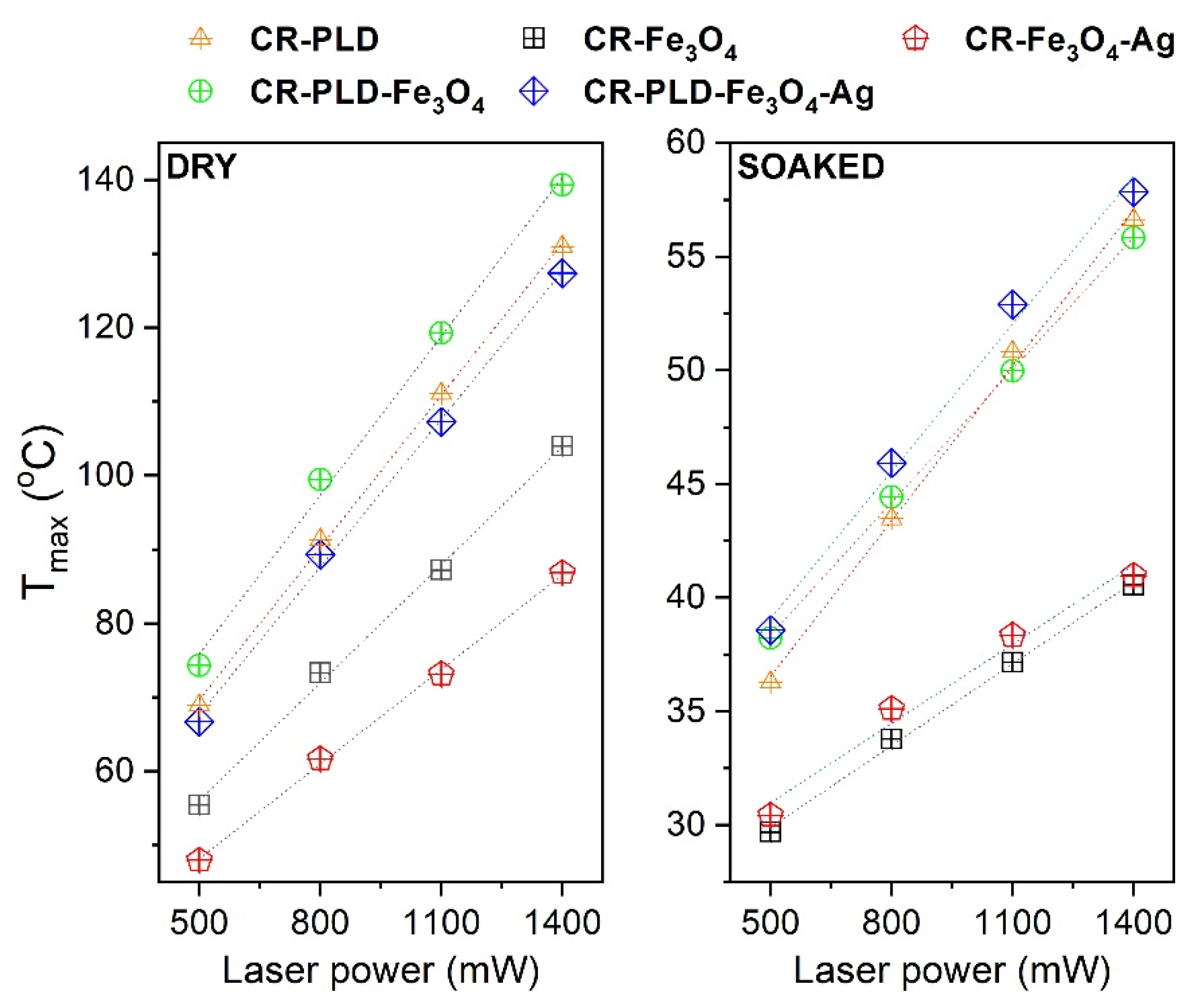
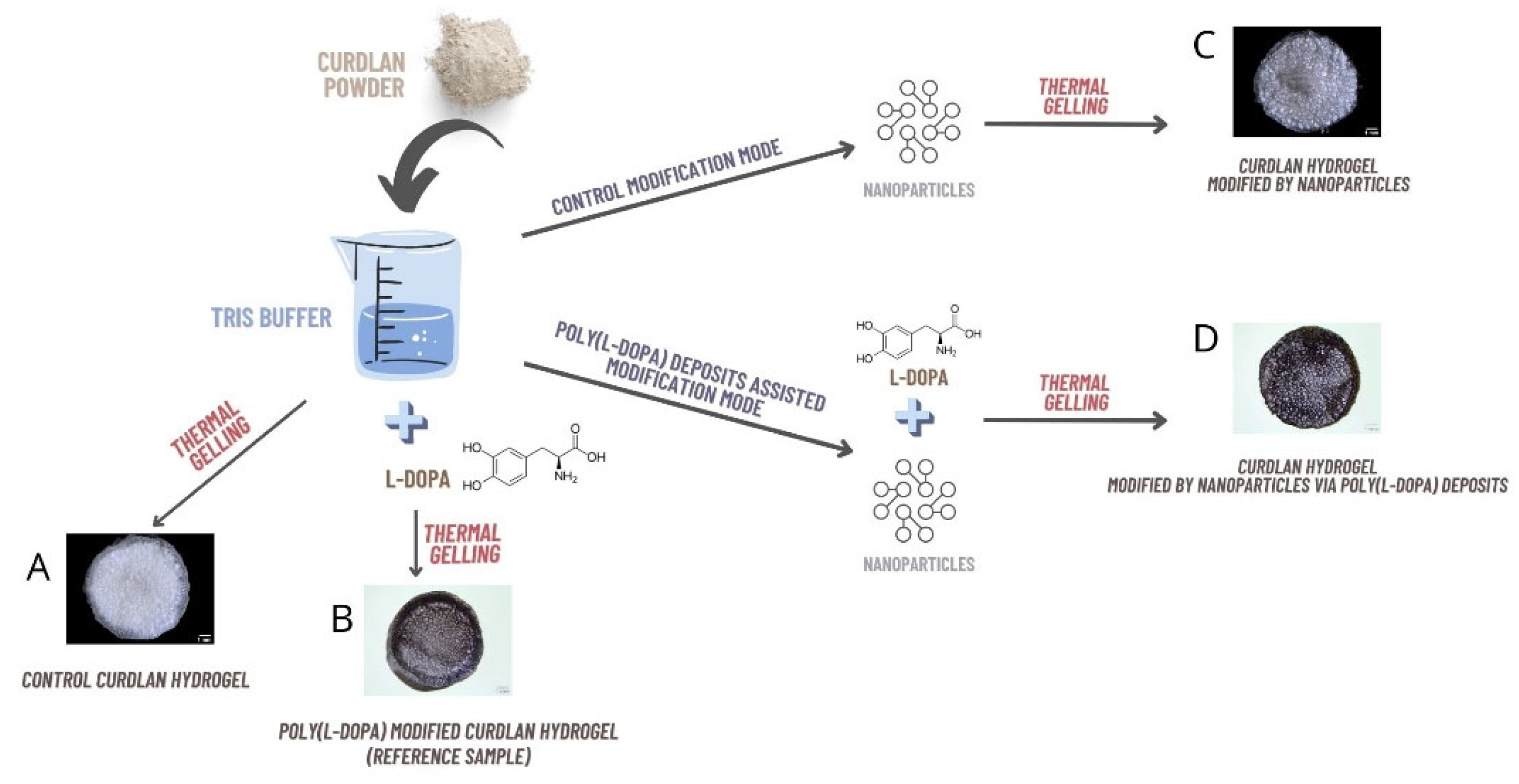
2.2. Characterization of Hydrogels (FTIR, NPs and Silver Ions Release, Heat Generation Effects, Mechanical Properties, and Wettability)
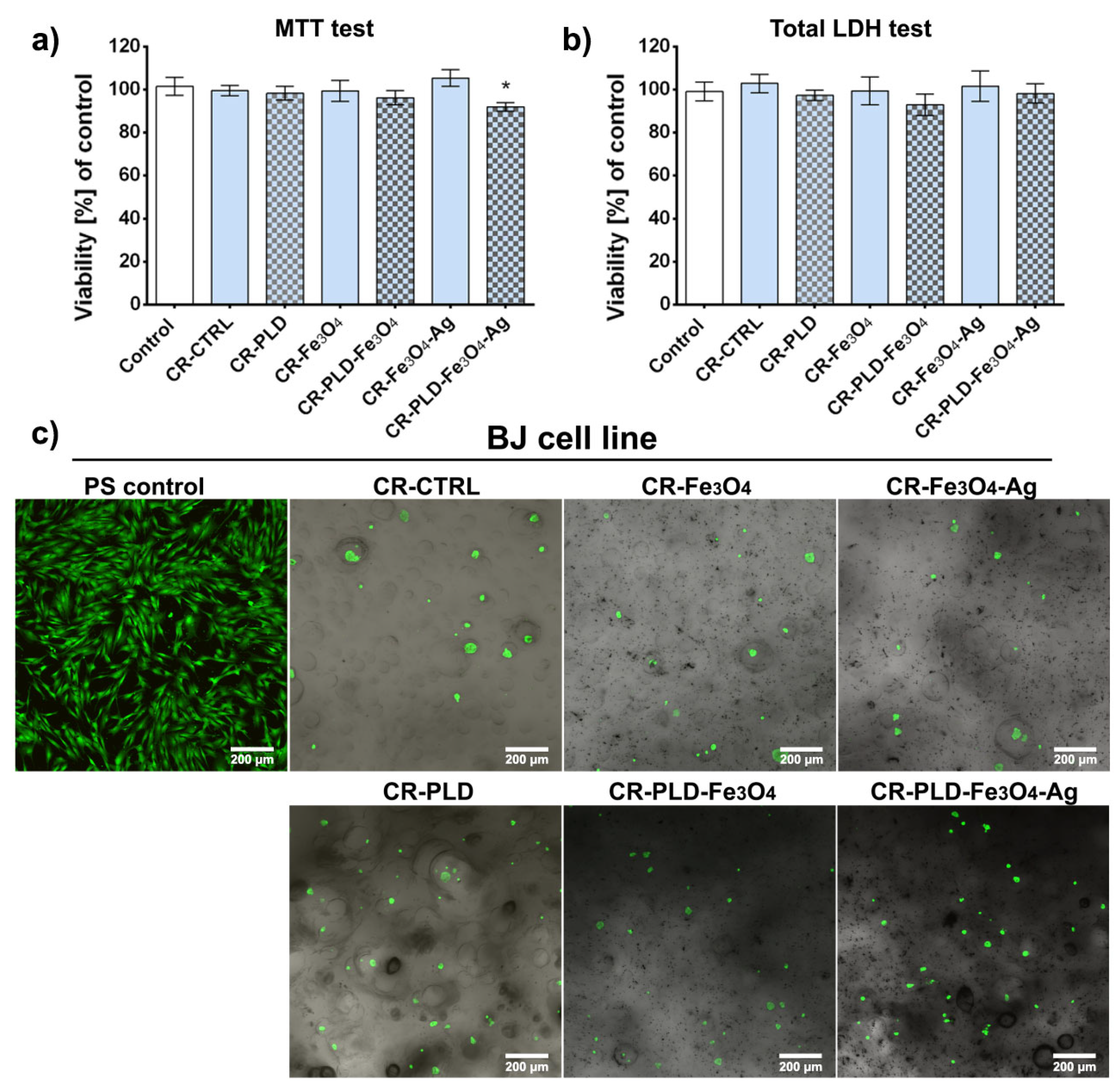
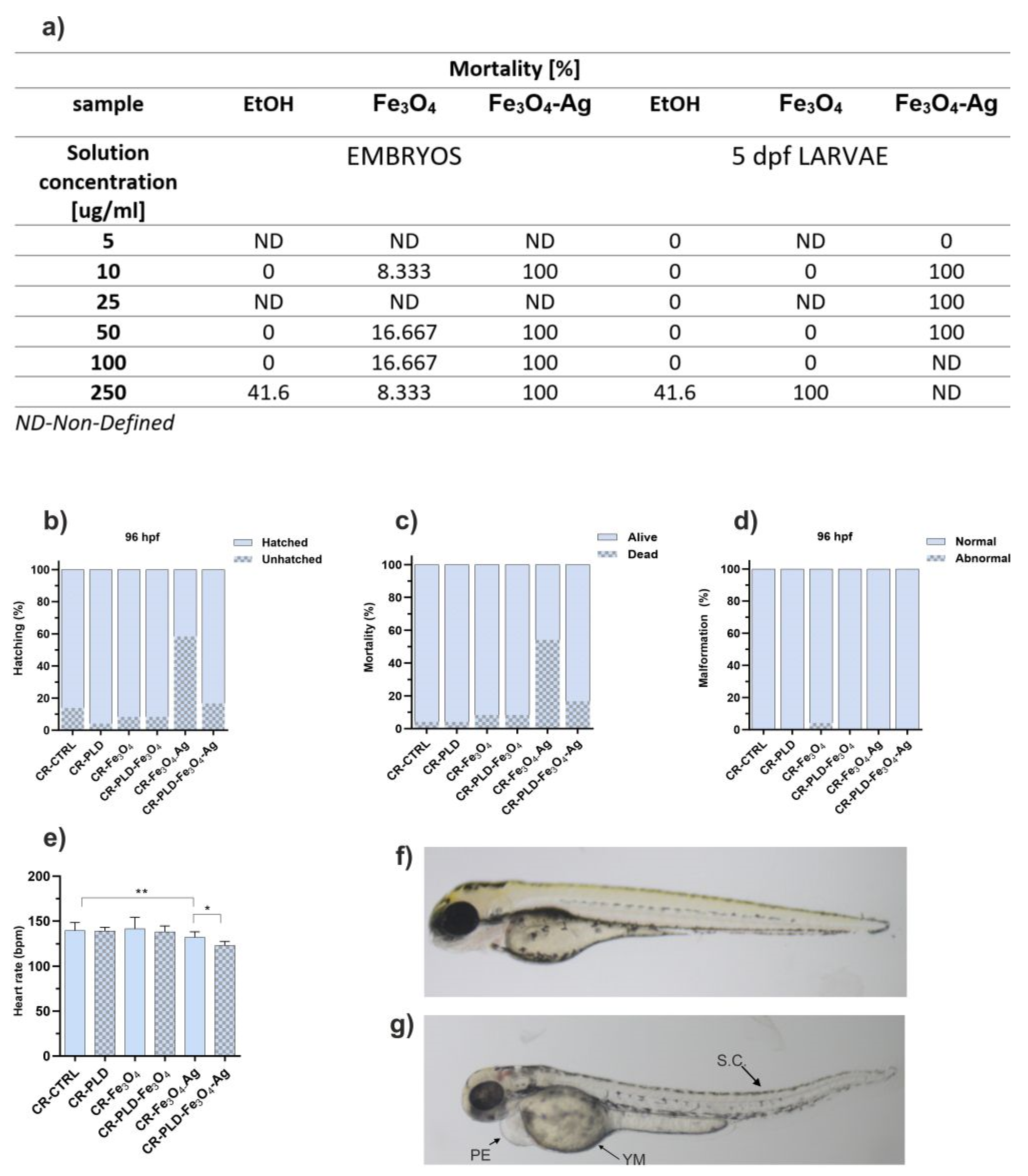
2.3. Cytotoxicity and Toxicity in Fibroblasts and Zebrafish Model
2.4. Hemocompatibility
2.5. Antibacterial Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Fabrication of the Cubic MNPs
3.2.2. Synthesis of the Fe3O4–Ag Heterostructures
3.3. Characterization of Cubic Fe3O4 Nanoparticles and Fe3O4–Ag Heterostructures
3.4. Synthesis of Hydrogel Samples
3.5. Nanoparticles and Ions Release
Ag+ Ion Concentration Measurements Methodology
3.6. Contactless Energy Conversion
3.7. Mechanical Tests
3.8. Wettability Characterization
3.9. Cell Culture Experiments
Biocompatibility Evaluation
3.10. In Vivo Experiments—Danio rerio Model
3.11. Blood Compatibility Tests
3.12. Antibacterial Activity Evaluation
3.12.1. Bacterial Strains and Maintenance
3.12.2. Antibacterial Activity Test (Based on the Standard: AATCC Test Method 100-2004)
3.12.3. Bacterial Adhesion Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Wang, F. Review on the preparation, biological activities and applications of curdlan and its derivatives. Eur. Polym. J. 2020, 141, 110096. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H. Recent progress on curdlan provided by functionalization strategies. Food Hydrocoll. 2017, 68, 128–135. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiao, J.; Guan, S.; Geng, Z.; Zhao, R.; Gao, B. A hydrogen-bonded antibacterial curdlan-tannic acid hydrogel with an antioxidant and hemostatic function for wound healing. Carbohydr. Polym. 2022, 285, 119235. [Google Scholar] [CrossRef] [PubMed]
- Michalicha, A.; Roguska, A.; Przekora, A.; Budzyńska, B.; Belcarz, A. Poly(levodopa)-modified β-glucan as a candidate for wound dressings. Carbohydr. Polym. 2021, 272, 118485. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Gao, P.; Li, Y.; Qi, P.; Liu, J.; Shen, R.; Wang, L.; Huang, N.; Xiong, K.; Tian, W.; et al. Poly-dopamine, poly-levodopa, and poly-norepinephrine coatings: Comparison of physico-chemical and biological properties with focus on the application for blood-contacting devices. Bioact. Mater. 2021, 6, 285–296. [Google Scholar] [CrossRef]
- Xu, L.Q.; Yang, W.J.; Neoh, K.G.; Kang, E.T.; Fu, G.D. Dopamine-Induced Reduction and Functionalization of Graphene Oxide Nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar] [CrossRef]
- Teixeira, B.N.; Aprile, P.; Mendonça, R.H.; Kelly, D.J.; Thiré, R.M. da S.M. Evaluation of bone marrow stem cell response to PLA scaffolds manufactured by 3D printing and coated with polydopamine and type I collagen. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2019, 107, 37–49. [Google Scholar] [CrossRef]
- Hertault, A.; Chai, F.; Maton, M.; Sobocinski, J.; Woisel, P.; Maurel, B.; Lyskawa, J.; Blanchemain, N. In vivo evaluation of a pro-healing polydopamine coated stent through an in-stent restenosis rat model. Biomater. Sci. 2021, 9, 212–220. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, Z.; Liu, Y.; Wang, J.; Maitz, M.F.; Wang, Y.; Liu, S.; Chen, J.; Huang, N. Surface modification with dopamine and heparin/poly-l-lysine nanoparticles provides a favorable release behavior for the healing of vascular stent lesions. ACS Appl. Mater. Interfaces 2014, 6, 8729–8743. [Google Scholar] [CrossRef]
- Burzio, L.A.; Waite, J.H. Cross-linking in adhesive quinoproteins: Studies with model decapeptides. Biochemistry 2000, 39, 11147–11153. [Google Scholar] [CrossRef] [PubMed]
- LaVoie, M.J.; Ostaszewski, B.L.; Weihofen, A.; Schlossmacher, M.G.; Selkoe, D.J. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 2005, 11, 1214–1221. [Google Scholar] [CrossRef]
- Michalicha, A.; Pałka, K.; Roguska, A.; Pisarek, M.; Belcarz, A. Polydopamine-coated curdlan hydrogel as a potential carrier of free amino group-containing molecules. Carbohydr. Polym. 2021, 256, 117524. [Google Scholar] [CrossRef]
- Naghdi, M.; Ghovvati, M.; Rabiee, N.; Ahmadi, S.; Abbariki, N.; Sojdeh, S.; Ojaghi, A.; Bagherzadeh, M.; Akhavan, O.; Sharifi, E.; et al. Magnetic nanocomposites for biomedical applications. Adv. Colloid Interface Sci. 2022, 308, 102771. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Roy, I.; Gandhi, S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry 2022, 8, 107. [Google Scholar] [CrossRef]
- Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Le, M.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F. Cooperative Organization in Iron Oxide Multi-Core Nanoparticles Potentiates Their Efficiency as Heating Mediators and MRI Contrast Agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Martínez Maestro, L.; Del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Ortgies, D.H.; Teran, F.J.; Rocha, U.; de la Cueva, L.; Salas, G.; Cabrera, D.; Vanetsev, A.S.; Rähn, M.; Sammelselg, V.; Orlovskii, Y.V.; et al. Optomagnetic Nanoplatforms for In Situ Controlled Hyperthermia. Adv. Funct. Mater. 2018, 28, 1704434. [Google Scholar] [CrossRef]
- Kulpa-Greszta, M.; Wnuk, M.; Tomaszewska, A.; Adamczyk-Grochala, J.; Dziedzic, A.; Rzeszutek, I.; Zarychta, B.; Błoniarz, D.; Lewińska, A.; Pązik, R. Synergic Temperature Effect of Star-like Monodisperse Iron Oxide Nanoparticles and Their Related Responses in Normal and Cancer Cells. J. Phys. Chem. B 2022, 126, 8515–8531. [Google Scholar] [CrossRef]
- Kulpa-Greszta, M.; Pązik, R.; Kłoda, P.; Tomaszewska, A.; Zachanowicz, E.; Pałka, K.; Ginalska, G.; Belcarz, A. Efficient non-contact heat generation on flexible, ternary hydroxyapatite/curdlan/nanomagnetite hybrids for temperature controlled processes. Mater. Sci. Eng. C 2021, 118, 111360. [Google Scholar] [CrossRef]
- Liu, R.; Guo, Y.; Odusote, G.; Qu, F.; Priestley, R.D. Core-Shell Fe3O4 Polydopamine Nanoparticles Serve Multipurpose as Drug Carrier, Catalyst Support and Carbon Adsorbent. ACS Appl. Mater. Interfaces 2013, 5, 9167–9171. [Google Scholar] [CrossRef]
- Zhou, W.H.; Lu, C.H.; Guo, X.C.; Chen, F.R.; Yang, H.H.; Wang, X.R. Mussel-inspired molecularly imprinted polymer coating superparamagnetic nanoparticles for protein recognition. J. Mater. Chem. 2010, 20, 880–883. [Google Scholar] [CrossRef]
- Yang, J.C.; Chen, Y.; Li, Y.H.; Yin, X.B. Magnetic Resonance Imaging-Guided Multi-Drug Chemotherapy and Photothermal Synergistic Therapy with pH and NIR-Stimulation Release. ACS Appl. Mater. Interfaces 2017, 9, 22278–22288. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Li, B.; Li, J.; Lv, H.; Wu, L.; Zhang, H.; Yang, B.; Zhu, M.; Jiang, J. In vivo migration of Fe3O4@polydopamine nanoparticle-labeled mesenchymal stem cells to burn injury sites and their therapeutic effects in a rat model. Biomater. Sci. 2019, 7, 2861–2872. [Google Scholar] [CrossRef]
- Imran, M.; Zouli, N.; Ahamad, T.; Alshehri, S.M.; Chandan, M.R.; Hussain, S.; Aziz, A.; Dar, M.A.; Khan, A. Carbon-coated Fe3O4core-shell super-paramagnetic nanoparticle-based ferrofluid for heat transfer applications. Nanoscale Adv. 2021, 3, 1962–1975. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Alam, M.M.; Hussain, S.; Abutaleb, A.; Aziz, A.; Chandan, M.R.; Irshad, K.; Al-Hagri, A.M.A.; Bakather, O.Y.; Khan, A. Colloidal Fe3O4 nanoparticles-based oil blend ferro-nanofluid for heat transfer application. Eur. Phys. J. Plus 2021, 136, 752. [Google Scholar] [CrossRef]
- Wang, D.W.; Zhu, X.M.; Lee, S.F.; Chan, H.M.; Li, H.W.; Kong, S.K.; Yu, J.C.; Cheng, C.H.K.; Wang, Y.X.J.; Leung, K.C.F. Folate-conjugated Fe3O4@SiO2@gold nanorods@mesoporous SiO2 hybrid nanomaterial: A theranostic agent for magnetic resonance imaging and photothermal therapy. J. Mater. Chem. B 2013, 1, 2934–2942. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Andrade, G.F.; Lima, G.S.; Gastelois, P.L.; Assis Gomes, D.; Macedo, W.A.A.; de Sousa, E.M.B. Surface modification and biological evaluation of kojic acid/silica nanoparticles as platforms for biomedical systems. Int. J. Appl. Ceram. Technol. 2020, 17, 380–391. [Google Scholar] [CrossRef]
- Zhang, D.; Hegab, H.E.; Lvov, Y.; Dale Snow, L.; Palmer, J. Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. Springerplus 2016, 5, 48–68. [Google Scholar] [CrossRef]
- Ahangaran, F.; Hassanzadeh, A.; Nouri, S. Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. Int. Nano Lett. 2013, 3, 3–7. [Google Scholar] [CrossRef]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Pieta, I.S.; Nowakowski, R.; Pisarek, M.; Przekora, A. Effect of gelation temperature on the molecular structure and physicochemical properties of the curdlan matrix: Spectroscopic and microscopic analyses. Int. J. Mol. Sci. 2020, 21, 6154. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Sallem, F.; Mirjolet, C.; Nury, T.; Sahoo, S.K.; Millot, N.; Kumar, R. Polydopamine modified superparamagnetic iron oxide nanoparticles as multifunctional nanocarrier for targeted prostate cancer treatment. Nanomaterials 2019, 9, 138. [Google Scholar] [CrossRef]
- Siciliano, G.; Monteduro, A.G.; Turco, A.; Primiceri, E.; Rizzato, S.; Depalo, N.; Curri, M.L.; Maruccio, G. Polydopamine-Coated Magnetic Iron Oxide Nanoparticles: From Design to Applications. Nanomaterials 2022, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Wang, H.; Zhang, Y.; Ding, G. Preparation of polydopamine-coated magnetic nanoparticles for dispersive solid-phase extraction of water-soluble synthetic colorants in beverage samples with HPLC analysis. Talanta 2016, 149, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Fang, J.; Li, J.; Wang, C.; He, Z.; Zhu, L.; Xu, Z.; Zeng, H. Polydopamine Nanotubes Decorated with Ag Nanoparticles as Catalyst for the Reduction of Methylene Blue. ACS Appl. Nano Mater. 2020, 3, 156–164. [Google Scholar] [CrossRef]
- Kulpa-Greszta, M.; Tomaszewska, A.; Michalicha, A.; Sikora, D.; Dziedzic, A.; Wojnarowska-Nowak, R.; Belcarz, A.; Pązik, R. Alternating magnetic field and NIR energy conversion on magneto-plasmonic Fe3O4@APTES-Ag heterostructures with SERS detection capability and antimicrobial activity. RSC Adv. 2022, 12, 27396–27410. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Xu, H.; Xiao, B.; Zhou, X.; Liu, X.; Zhou, Z.; Patra, H.K.; Slater, N.; Tang, J.; Shen, Y. Single-step formulation of levodopa-based nanotheranostics-strategy for ultra-sensitive high longitudinal relaxivity MRI guided switchable therapeutics. Biomater. Sci. 2020, 8, 1615–1621. [Google Scholar] [CrossRef]
- Du, C.; Qian, J.; Zhou, L.; Su, Y.; Zhang, R.; Dong, C.M. Biopolymer-Drug Conjugate Nanotheranostics for Multimodal Imaging-Guided Synergistic Cancer Photothermal-Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 31576–31588. [Google Scholar] [CrossRef] [PubMed]
- Batul, R.; Tamanna, T.; Khaliq, A.; Yu, A. Recent progress in the biomedical applications of polydopamine nanostructures. Biomater. Sci. 2017, 5, 1204–1229. [Google Scholar] [CrossRef]
- Kang, S.; Baskaran, R.; Ozlu, B.; Davaa, E.; Kim, J.J.; Shim, B.S.; Yang, S.-G. T1-Positive Mn2+-Doped Multi-Stimuli Responsive poly(L-DOPA) Nanoparticles for Photothermal and Photodynamic Combination Cancer Therapy. Biomedicines 2020, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Yang, P.; Guo, W.; Zhang, Q.P.; Liu, X.; Li, Y. Metal ion-promoted fabrication of melanin-like poly(L-DOPA) nanoparticles for photothermal actuation. Sci. China Chem. 2020, 63, 1295–1305. [Google Scholar] [CrossRef]
- Tamilarasan, U.; Dhanasekar, S.; Raja Karthikeyan, K.; Ramesh Babu, T.S.; Gopinathan, R.; Pratheep, V.G.; Rajalakshmy, P.; Subbiah, R.; Praveen Kumar, S. Study of Mechanical Properties on Ferric Oxide Microparticles Reinforced with Polyethylene. Adv. Mater. Sci. Eng. 2022, 2022, 3077301. [Google Scholar] [CrossRef]
- Agrawal, G.; Negi, Y.S.; Pradhan, S.; Dash, M.; Samal, S.K. Wettability and Contact Angle of Polymeric Biomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081007372. [Google Scholar]
- Vivcharenko, V.; Wojcik, M.; Palka, K.; Przekora, A. Highly Porous and Superabsorbent Biomaterial Made of Marine-Derived Polysaccharides and Ascorbic Acid as an Optimal Dressing for Exuding Wound Management. Materials 2021, 14, 1211. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, L.; Xu, C.; Kai, D.; Fan, X.; You, M.; Hui, C.M.; Wu, C.; Wu, Y.L.; Li, Z. Engineered Janus amphipathic polymeric fiber films with unidirectional drainage and anti-adhesion abilities to accelerate wound healing. Chem. Eng. J. 2021, 421, 127725. [Google Scholar] [CrossRef]
- Aduba, D.C.; Yang, H. Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials. Bioengineering 2017, 4, 1. [Google Scholar] [CrossRef]
- Ankamwar, B.; Lai, T.C.; Huang, J.H.; Liu, R.S.; Hsiao, M.; Chen, C.H.; Hwu, Y.K. Biocompatibility of Fe3O4 nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancercells. Nanotechnology 2010, 21, 075102. [Google Scholar] [CrossRef]
- Basma, A.N.; Morris, E.J.; Nicklas, W.J.; Geller, H.M. L-DOPA Cytotoxicity to PC12 Cells in Culture Is via Its Autoxidation. J. Neurochem. 1995, 64, 825–832. [Google Scholar] [CrossRef]
- Hong, S.; Kim, K.Y.; Wook, H.J.; Park, S.Y.; Lee, K.D.; Lee, D.Y.; Lee, H. Attenuation of the in vivo toxicity of biomaterials by polydopamine surface modification. Nanomedicine 2011, 6, 793–801. [Google Scholar] [CrossRef]
- Rippon, M.G.; Rogers, A.A.; Ousey, K. Antimicrobial stewardship strategies in wound care: Evidence to support the use of dialkylcarbamoyl chloride (DACC)-coated wound dressings. J. Wound Care 2021, 30, 284–296. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Kavosh, M.; Horvath, R.; Ghalamboran, M.R.; Ramsden, J.J. Bacterial adsorption onto monolayer ferromagnetic nanofilms. J. Bionanosci. 2010, 4, 119–122. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Polega, E.; Thomson, K.; Bhuiyan, M.S.A.; Pinnaratip, R.; Trought, M.; Kendrick, C.; Gao, Y.; Perrine, K.A.; Pan, L.; et al. Antibacterial Properties of Mussel-Inspired Polydopamine Coatings Prepared by a Simple Two-Step Shaking-Assisted Method. Front. Chem. 2019, 7, 631. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Hovhannisyan, A.; Gevorgyan, V.; Ananyan, M.; Trchounian, A. Antibacterial effects of iron oxide (Fe3O4) nanoparticles: Distinguishing concentration-dependent effects with different bacterial cells growth and membrane-associated mechanisms. Appl. Microbiol. Biotechnol. 2019, 103, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Michalicha, A.; Przekora, A.; Stefaniuk, D.; Jaszek, M.; Matuszewska, A.; Belcarz, A. Medical Use of Polycatecholamines + Oxidoreductases-Modified Curdlan Hydrogels—Perspectives. Int. J. Mol. Sci. 2022, 23, 10084. [Google Scholar] [CrossRef]
- Przekora, A.; Czechowska, J.; Pijocha, D.; Ślósarczyk, A.; Ginalska, G. Do novel cement-type biomaterials reveal ion reactivity that affects cell viability in vitro? Cent. Eur. J. Biol. 2014, 9, 277–289. [Google Scholar] [CrossRef]




| Biomaterial Designation | Contact Angle [°] |
|---|---|
| CR–CTRL CR–PLD CR–Fe3O4 CR–PLD–Fe3O4 CR–Fe3O4–Ag CR–PLD–Fe3O4–Ag | 77.22 ± 8.24 58.38 ± 9.07 57.04 ± 5.49 51.90 ± 5.23 59.27 ± 11.43 48.78 ± 9.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalicha, A.; Tomaszewska, A.; Vivcharenko, V.; Budzyńska, B.; Kulpa-Greszta, M.; Fila, D.; Pązik, R.; Belcarz, A. Poly(levodopa)-Functionalized Polysaccharide Hydrogel Enriched in Fe3O4 Particles for Multiple-Purpose Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 8002. https://doi.org/10.3390/ijms24098002
Michalicha A, Tomaszewska A, Vivcharenko V, Budzyńska B, Kulpa-Greszta M, Fila D, Pązik R, Belcarz A. Poly(levodopa)-Functionalized Polysaccharide Hydrogel Enriched in Fe3O4 Particles for Multiple-Purpose Biomedical Applications. International Journal of Molecular Sciences. 2023; 24(9):8002. https://doi.org/10.3390/ijms24098002
Chicago/Turabian StyleMichalicha, Anna, Anna Tomaszewska, Vladyslav Vivcharenko, Barbara Budzyńska, Magdalena Kulpa-Greszta, Dominika Fila, Robert Pązik, and Anna Belcarz. 2023. "Poly(levodopa)-Functionalized Polysaccharide Hydrogel Enriched in Fe3O4 Particles for Multiple-Purpose Biomedical Applications" International Journal of Molecular Sciences 24, no. 9: 8002. https://doi.org/10.3390/ijms24098002







