8-Methoxyflindersine-Induced Apoptosis and Cell Cycle Disorder Involving MAPK Signaling Activation in Human Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results
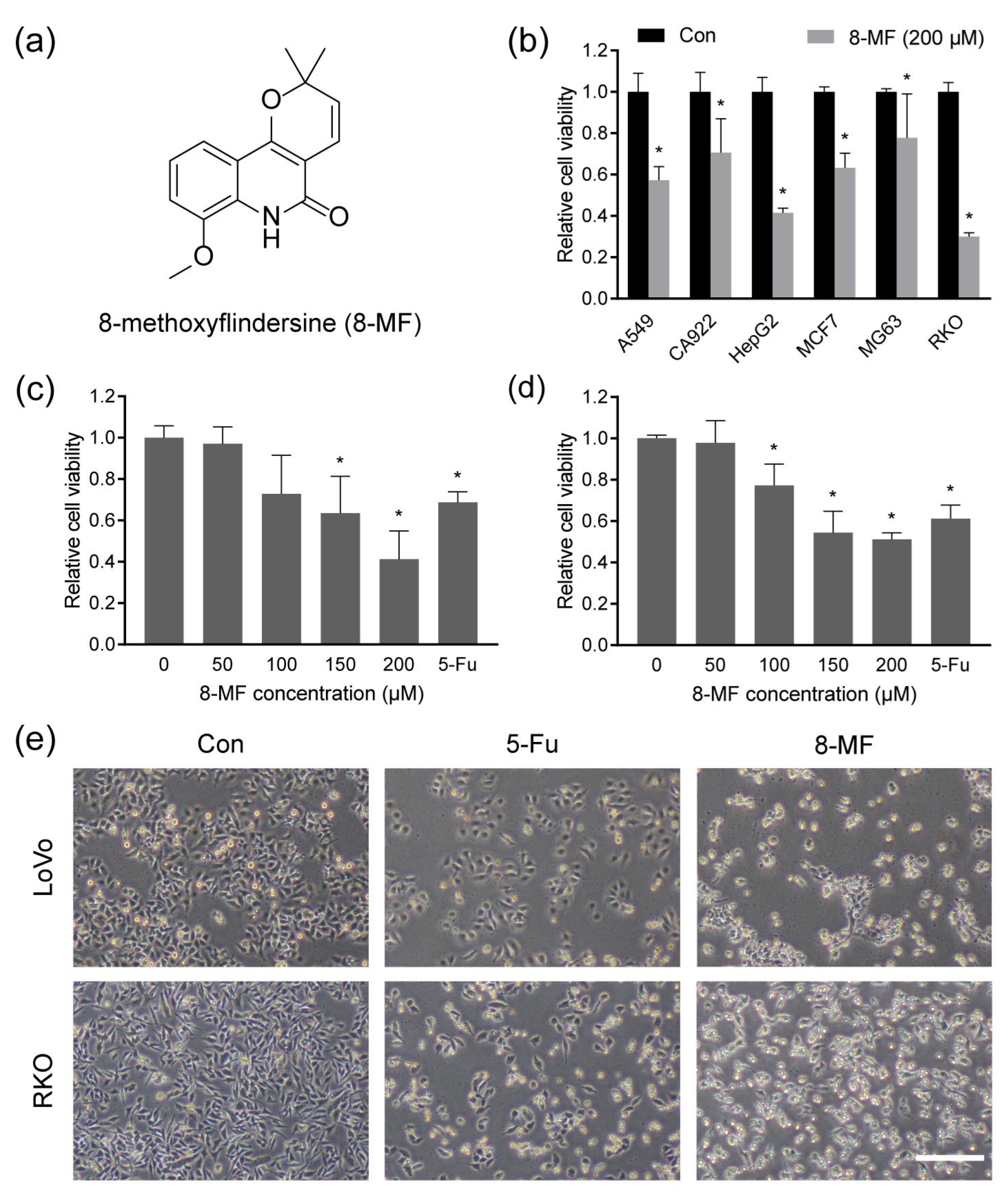
2.1. 8-MF Reduced CRC Cells Viability
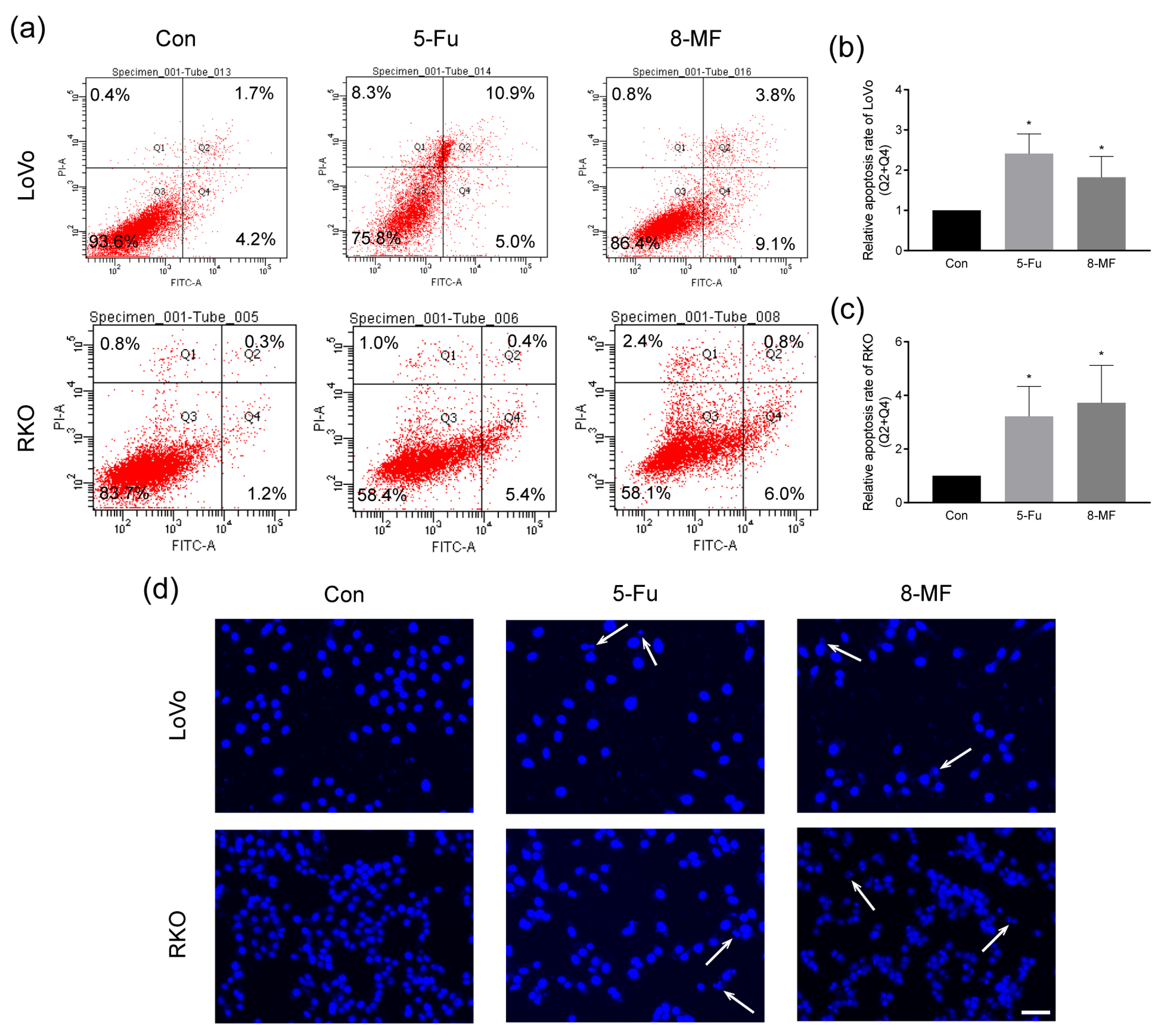
2.2. 8-MF–Induced CRC Cells Apoptosis
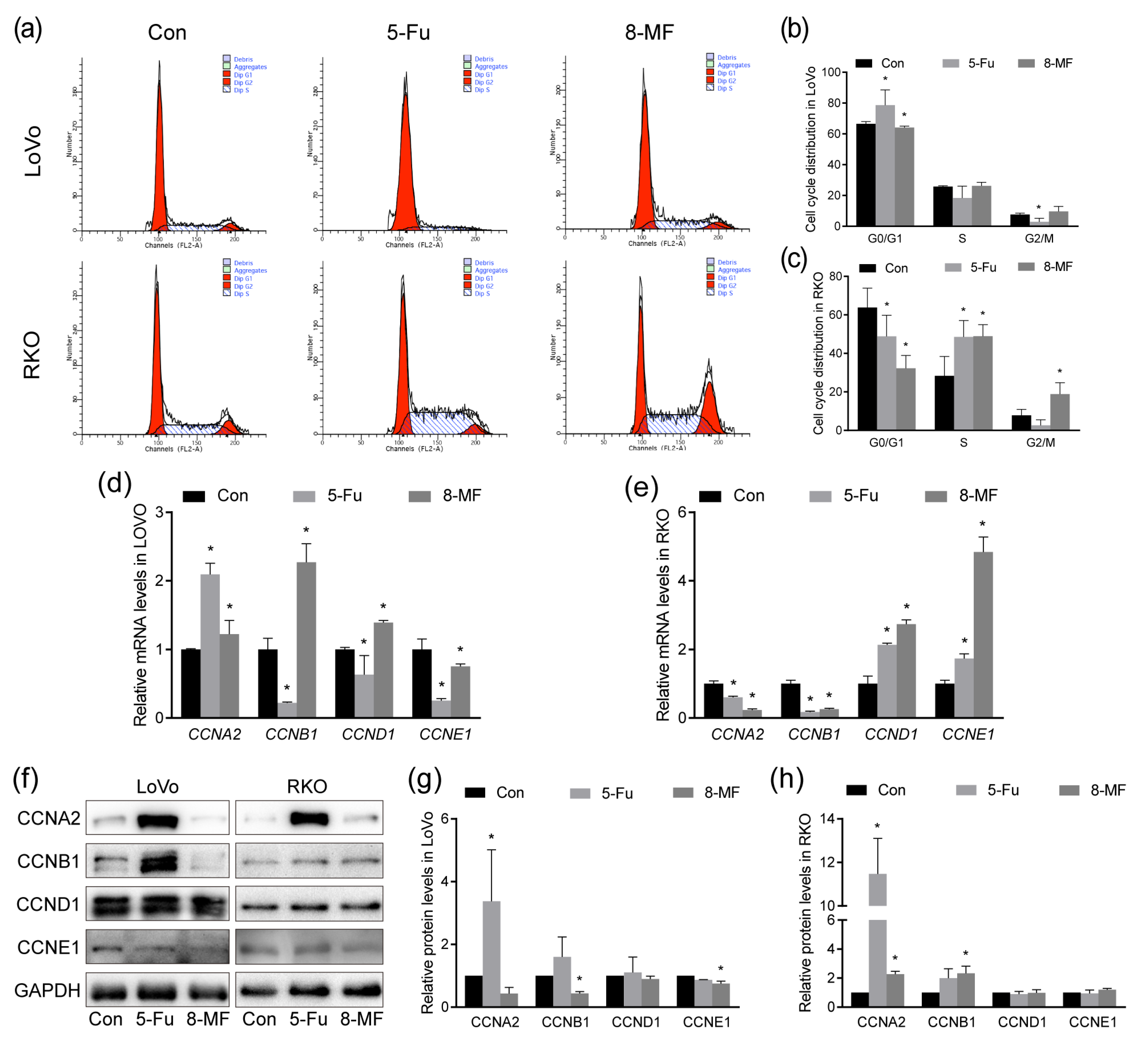
2.3. 8-MF–Induced CRC Cell Cycle Disorder
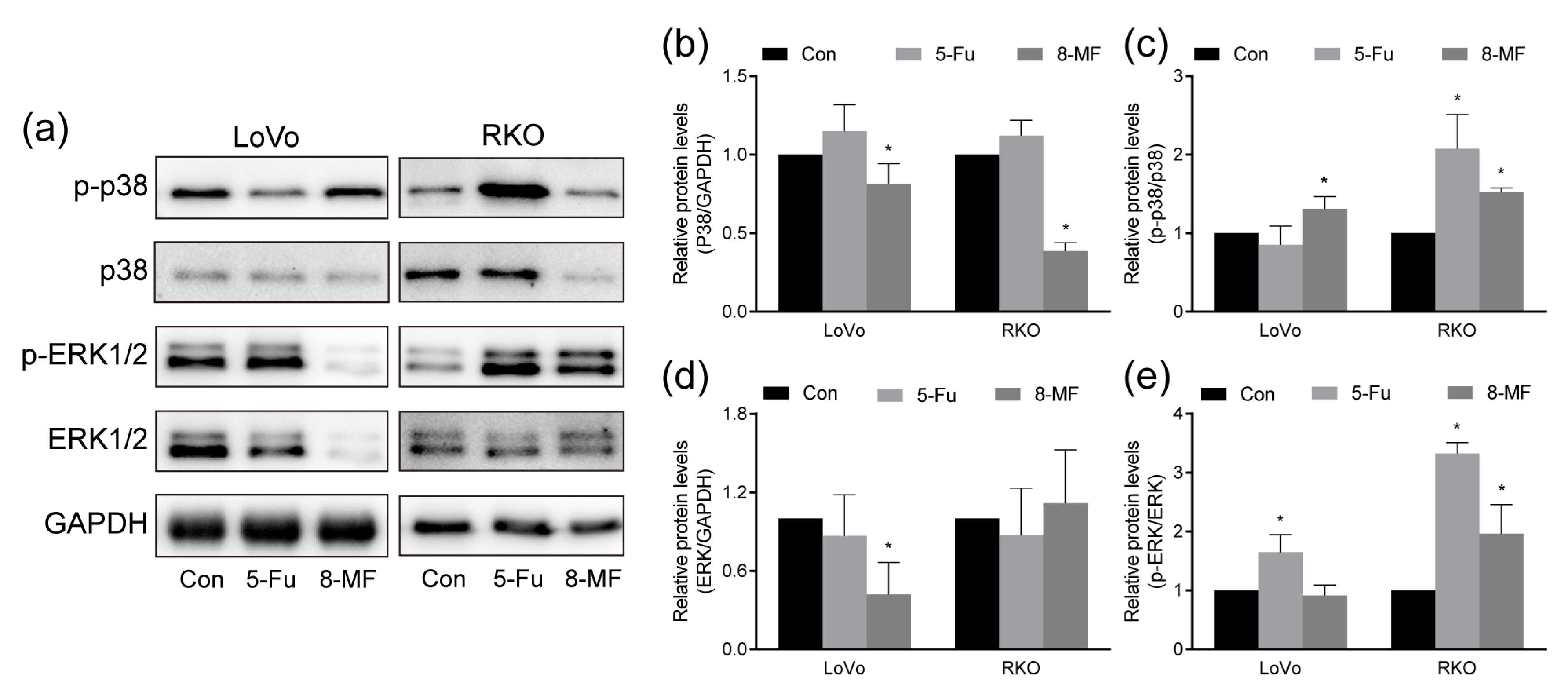
2.4. MAPK Signaling Was Involved in 8-MF-Induced Inhibition of CRC Cells
2.5. Interactions between 8-MF and Proteins
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. DAPI Staining Assay
4.5. Annexin V-FITC/PI Apoptosis Assay
4.6. Cell Cycle Analysis
4.7. Real-Time PCR
4.8. Western Blotting
4.9. Protein–Protein Interaction and Molecular Docking
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.D.; Nagtegaal, I.D. Terminology, molecular features, epidemiology, and management of serrated colorectal neoplasia. Gastroenterology 2019, 157, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Menon, H.; Chulkina, M.; Yee, N.S.; Pinchuk, I.V. Drug-microbiota interaction in colon cancer therapy: Impact of antibiotics. Biomedicines 2021, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Gelibter, A.J.; Caponnetto, S.; Urbano, F.; Emiliani, A.; Scagnoli, S.; Sirgiovanni, G.; Napoli, V.M.; Cortesi, E. Adjuvant chemotherapy in resected colon cancer: When, how and how long? Surg. Oncol. 2019, 30, 100–107. [Google Scholar] [CrossRef]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Zhang, L.; Wang, Y.; Chen, Q.; Chen, Z.; Xu, L.; Liu, T. Chemical constituents from Dictamnus dasycarpus Turcz. Biochem. Syst. Ecol. 2020, 93, 104134. [Google Scholar] [CrossRef]
- Lang, X.; Zhang, X.; Wang, D.; Zhou, W. In vitro and in vivo metabolic activation of obacunone, a bioactive and potentially hepatotoxic constituent of Dictamni Cortex. Planta Med. 2020, 86, 686–695. [Google Scholar] [CrossRef]
- Yang, B.; Lee, H.B.; Kim, S.; Park, Y.C.; Kim, K.; Kim, H. Decoction of Dictamnus dasycarpus Turcz. root bark ameliorates skin lesions and inhibits inflammatory reactions in mice with contact dermatitis. Pharmacogn. Mag. 2017, 13, 483–487. [Google Scholar] [CrossRef]
- Bai, Y.; Jin, X.; Jia, X.; Tang, W.; Wang, X.; Zhao, Y. Two new apotirucallane-type isomeric triterpenoids from the root bark of Dictamnus dasycarpus with their anti-proliferative activity. Phytochem. Lett. 2014, 10, 118–122. [Google Scholar] [CrossRef]
- Zhai, W.; Liu, J.; Liu, Q.; Wang, Y.; Yang, D. Rapid identification and global characterization of multiple constituents from the essential oil of Cortex Dictamni based on GC-MS. J. Sep. Sci. 2017, 40, 2671–2681. [Google Scholar] [CrossRef]
- Cabral, R.S.; Allard, P.M.; Marcourt, L.; Young, M.C.; Queiroz, E.F.; Wolfender, J.L. Targeted isolation of indolopyridoquinazoline alkaloids from Conchocarpus fontanesianus based on molecular networks. J. Nat. Prod. 2016, 79, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Alves, I.M.; Abreu, L.S.; Costa, C.O.; Le Hyaric, M.; Guedes, M.L.; Soares, M.B.; Beaerra, D.P.; Velozo, E.S. Pyranochromones from Dictyoloma vandellianum A. Juss and their cytotoxic evaluation. Chem. Biodivers. 2017, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wang, L.; Zhao, L.; Zhang, Q.Y.; Zeng, K.W.; Zhao, M.B.; Jiang, Y.; Tu, P.F.; Guo, X.Y. Anti-inflammatory quinoline alkaloids from the root bark of Dictamnus dasycarpus. Phytochemistry 2020, 172, 112260. [Google Scholar] [CrossRef] [PubMed]
- Suresh, T.; Dhanabal, T.; Nandha Kumar, R.; Mohan, P.S. Synthesis of pyranoquinoline alkaloids via (4+2) cycloaddition reaction. Heterocycl. Commun. 2005, 11, 79–84. [Google Scholar] [CrossRef]
- Hohlemwerger, S.V.A.; Tavares, W.; de Carvalho, A.M.; Cézar Vieira, P.; da Silva Velozo, E. 2-Quinolone alkaloids from the rare species of Rutaceae Andreadoxa flava Kallunki. Biochem. Syst. Ecol. 2004, 32, 627–629. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X. Long noncoding RNAs: Functions and mechanisms in colon cancer. Mol. Cancer 2020, 19, 167. [Google Scholar] [CrossRef]
- Fu, X.; Duanmu, J.; Li, T.; Jiang, Q. A 7-lncRNA signature associated with the prognosis of colon adenocarcinoma. PeerJ 2020, 8, e8877. [Google Scholar] [CrossRef]
- El-Mesery, M.; Seher, A.; El-Shafey, M.; El-Dosoky, M.; Badria, F.A. Repurposing of quinoline alkaloids identifies their ability to enhance doxorubicin-induced sub-G0/G1 phase cell cycle arrest and apoptosis in cervical and hepatocellular carcinoma cells. Biotechnol. Appl. Bioc. 2021, 68, 832–840. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, C.M.; Park, S.H.; Nam, M.J. Esculetin induces cell cycle arrest and apoptosis in human colon cancer LoVo cells. Environ. Toxicol. 2019, 34, 1129–1136. [Google Scholar] [CrossRef]
- Lee, J.; Lee, I.; Han, B.; Park, J.O.; Jang, J.; Park, C.; Kang, W.K. Effect of simvastatin on cetuximab resistance in human colorectal cancer with KRAS mutations. J. Natl. Cancer Inst. 2011, 103, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Roock, W.D.; Vriendt, V.D.; Normanno, N.; Ciardiello, F.; Tejpar, S. KRAS, BRAF, PIK3CA, and PTEN mutations: Implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011, 12, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]


| Compound | Molecular Formula | Target Name | PDB ID | Minimum Binding Energy (kcal/mol) |
|---|---|---|---|---|
| 8-methoxyflindersine (8-MF) | C15H15NO3 | p38 | 4MYG | −7.13 |
| ERK | 2Y9Q | −7.35 |
| Gene | Primer Sequence |
|---|---|
| GAPDH | forward:5′-GCACCGTCAAGGCTGAGAAC-3′ reverse:5′-TGGTGAAGACGCCAGTGGA-3′ |
| CCNA2 | forward:5′-CCTGGACCCAGAAAACCATT-3′ reverse:5′-AACACTCACTGGCTTTTCATCT-3′ |
| CCNB1 | forward:5′-ACCTGTGTCAGGCTTTCTCT-3′ reverse:5′-TTGGTCTGACTGCTTGCTCTT-3′ |
| CCND1 | forward:5′-GCTGCGAAGTGGAAACCATC-3′ reverse:5′-CCTCCTTCTGCACACATTTGAA-3′ |
| CCNE1 | forward:5′-AAGGAGCGGGACACCATGA-3′ reverse:5′-ACGGTCACGTTTGCCTTCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Fu, Y.; Liu, Y.; Wu, Y.; Chen, J.; Zhang, L.; Wang, R.; Chen, Z.; Liu, T. 8-Methoxyflindersine-Induced Apoptosis and Cell Cycle Disorder Involving MAPK Signaling Activation in Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2023, 24, 8039. https://doi.org/10.3390/ijms24098039
Zhang D, Fu Y, Liu Y, Wu Y, Chen J, Zhang L, Wang R, Chen Z, Liu T. 8-Methoxyflindersine-Induced Apoptosis and Cell Cycle Disorder Involving MAPK Signaling Activation in Human Colorectal Cancer Cells. International Journal of Molecular Sciences. 2023; 24(9):8039. https://doi.org/10.3390/ijms24098039
Chicago/Turabian StyleZhang, Dianbao, Yunmei Fu, Ying Liu, Yifan Wu, Jiayu Chen, Luting Zhang, Rui Wang, Zaixing Chen, and Tao Liu. 2023. "8-Methoxyflindersine-Induced Apoptosis and Cell Cycle Disorder Involving MAPK Signaling Activation in Human Colorectal Cancer Cells" International Journal of Molecular Sciences 24, no. 9: 8039. https://doi.org/10.3390/ijms24098039







