Why Does Rehabilitation Not (Always) Work in Osteoarthritis? Does Rehabilitation Need Molecular Biology?
Abstract
:1. Introduction
2. Rehabilitation
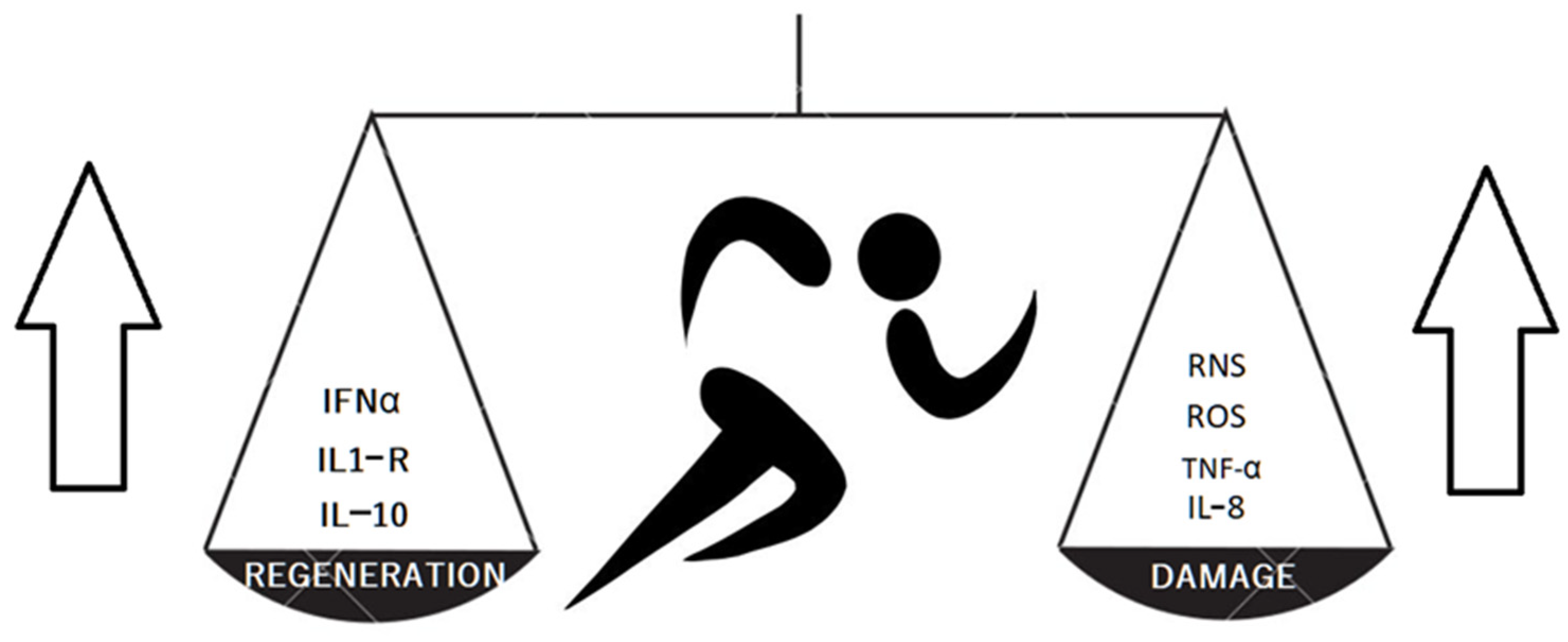
3. Kinesiotherapy-Treatment with Movement
3.1. Non-Coding RNA
3.2. Micro RNA (miRNA)
4. Obesity and Adipose Tissue
Adipokines
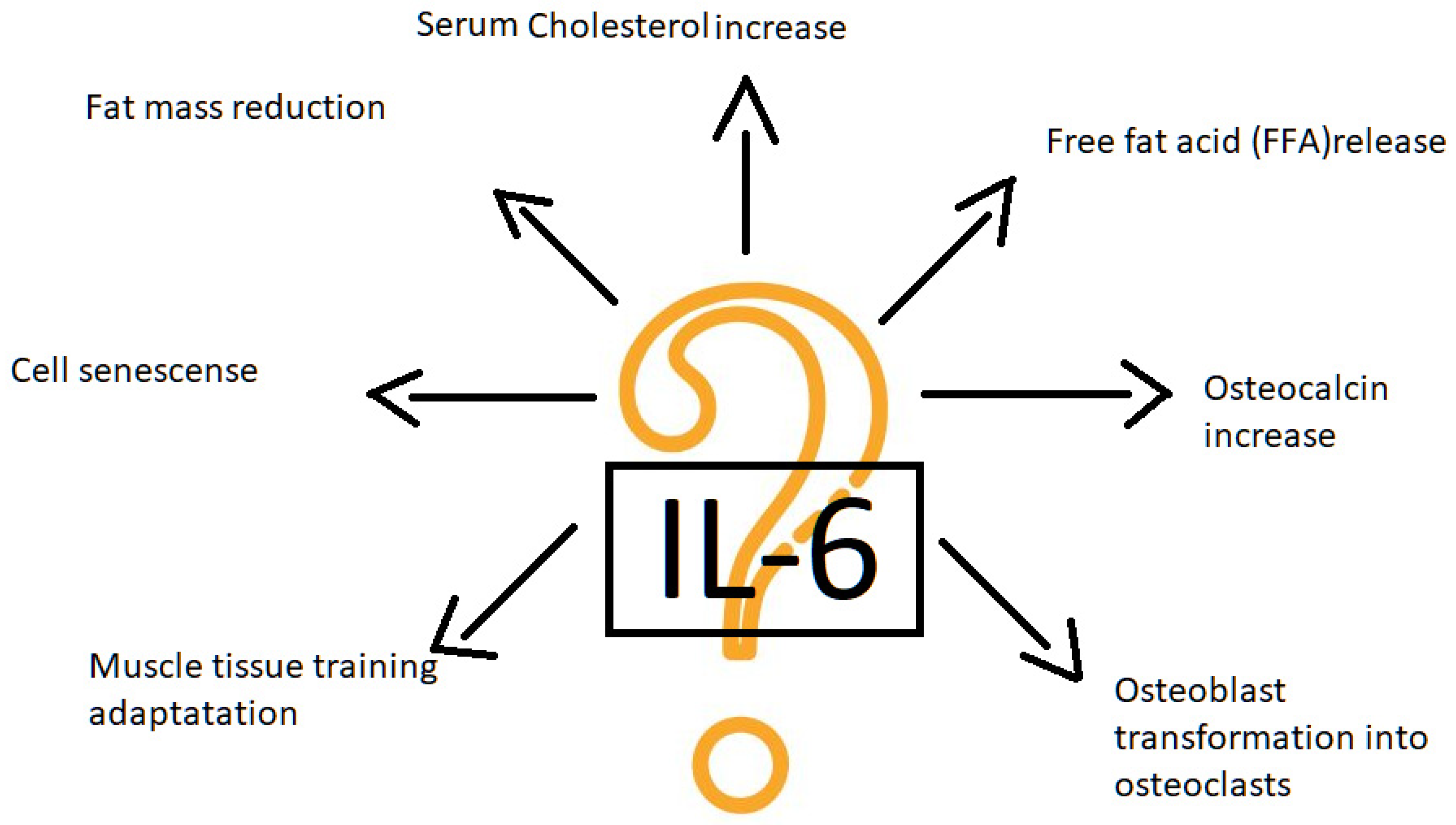
5. Interleukins
6. Physical Therapy
7. Ultrasound Therapy
8. Magnetic Field Therapy
9. Laser Therapy
10. Further Questions and Few Answers
Author Contributions
Funding
Conflicts of Interest
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Gldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Osteoarthritis Society International—OA Definition. Available online: https://oarsi.org/ (accessed on 4 February 2023).
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, S.A.; Gregg, P.J.; Rooney, P. Osteoarthritic cartilage loses its ability to remain avascular. Osteoarthr. Cart. 1999, 7, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Fahy, N.; de Vries-van, M.M.I.; Lehmann, W.; Grotenhuis, N.; Farrel, E.; van der Kraan, P.M.; Murphy, J.M.; Bastiaansen-Jenniskens, Y.M.; van Osch, G.J.V.M. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarization state. Osteoarthr. Cart. 2014, 22, 1167–1175. [Google Scholar] [CrossRef]
- Gómez-Aristizábal, A.; Gadhi, R.; Mahomed, N.N.; Marshall, K.W.; Viswanathan, S. Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: A cohort study. Arthritis Res. Ther. 2019, 21, 26. [Google Scholar] [CrossRef]
- Kemble, S.; Croft, A.P. Critical Role of Synovial Tissue–Resident Macrophage and Fibroblast Subsets in the Persistence of Joint Inflammation. Front. Immunol. 2021, 12, 715894. [Google Scholar] [CrossRef]
- Kawanishi, M.; Kami, K.; Nishimura, Y.; Minami, K.; Senba, E.; Umemoto, Y.; Kinoshita, T.; Tajima, F. Exercise-induced increase in M2 macrophages accelerates wound healing in young mice. Physiol. Rep. 2022, 10, e15447. [Google Scholar] [CrossRef]
- Jang, J.S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef]
- Katsuola, G.; Kreitmaier, P.; Zeggini, E. Insights into the molecular landscape of osteoarthritis in human tissues. Curr. Opin. Rheumatol. 2022, 34, 79–90. [Google Scholar] [CrossRef]
- Cho, Y.; Jeong, S.; Kim, H.; Kang, D.; Lee, J.; Kang, S.-J.; Kim, J.-H. Disease-modifying therapeutic strategies in osteoarthritis: Current status and future directions. Exp. Mol. Med. 2021, 53, 1689–1696. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, X.; Su, S.; Du, S.S.H. Identifying the shared genes and KEGG pathways of Resolvin D1-targeted network and osteoarthritis using bioinformatics. Bioengineered 2022, 13, 9839–9854. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N.Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Rausch Osthoff, A.-K.; Niedermann, K.; Braun, J.; Adams, J.; Brodin, N.; Dagfinrud, H.; Duruoz, T.; Esbensen, B.A.; Günther, K.-P.; Hurkmans, E.; et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018, 77, 1251–1260. [Google Scholar] [CrossRef]
- Dhillon, R.J.-S.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Elagizi, A.; Kachur, S.; Carbone, S.; Lavie, C.J.; Blair, S.N. A Review of Obesity, Physical Activity and Cardiovascular Disease. Curr. Obes. Rep. 2020, 9, 571–581. [Google Scholar] [CrossRef]
- Boutron, I.; Tubach, F.; Giradeau, B.; Ravaud, P. Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. J. Clin. Epidemiol. 2004, 57, 543–550. [Google Scholar] [CrossRef]
- Fregni, F.; Imamura, M.; Chien, H.F.; Lew, H.L.; Boggio, P.; Kaptchuk, T.J.; Riberto, M.; Hsing, W.T.; Battistella, L.R.; Furlan, A. Challenges and Recommendations for Placebo Controls in Randomized Trials in Physical and Rehabilitation Medicine: A Report of the International Placebo Symposium Working Group. Am. J. Phys. Med. Rehabil. 2010, 89, 160–172. [Google Scholar] [CrossRef]
- Villamar, M.F.; Contreras, V.S.; Kuntz, R.E.; Fregni, F. The Reporting of Blinding in Physical Medicine and Rehabilitation Randomized Controlled Trials: A Systematic Review. J. Rehabil. Med. 2013, 45, 6–13. [Google Scholar] [CrossRef]
- Lv, Z.; Yang, Y.X.; Li, J.; Fei, Y.; Guo, H.; Sun, Z.; Lu, J.; Xu, X.; Jiang, Q.; Ikegawa, S.; et al. Molecular Classification of Knee Osteoarthritis Front. Cell Dev. Biol. 2021, 9, 725568. [Google Scholar] [CrossRef]
- Vassao, P.G.; de Souza, A.C.F.; da Silveira Campos, R.M.; Garcia, L.A.; Tucci, H.T.; Renno, A.C.M. Effects of photobiomodulation and a physical exercise program on the expression of inflammatory and cartilage degradation biomarkers and functional capacity in women with knee osteoarthritis: A randomized blinded study. Adv. Rheumatol. 2021, 61. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-R.; Yoo, J.J.; Kim, H.A. Therapeutics in Osteoarthritis Based on an Understanding of Its Molecular Pathogenesis. Int. J. Mol. Sci. 2018, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- American Kinesiotherapy Association. Available online: https://akta.org/about/history (accessed on 4 February 2023).
- Przeździak, B. Historia Rehabilitacji na świecie i w Polsce in Rehabilitacja Medyczna; Kwolek, A., Ed.; Elsevier Urban & Partner: Wrocław, Poland, 2012; pp. 4–9. [Google Scholar]
- Alves, M.D.d.J.; Silva, D.d.S.; Pereira, E.V.M.; Pereira, D.D.; de Sousa Fernandes, M.S.; Santos, D.F.C.; Oliveira, D.P.M.; Vieira-Souza, L.M.; Aidar, F.J.; de Souza, R.F. Changes in Cytokines Concentration Following Long-Distance Running: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 838069. [Google Scholar] [CrossRef] [PubMed]
- Balan, E.; De Groote, E.; Bouillon, M.; Vieconte, N.; Mahieu, M.N.; Aslain, D.; Nielens, H.; Decottignies, A.; Deldicque, L. No effect of the endurance training status on senescence despite reduced inflammation in skeletal muscle of older individuals. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E447–E454. [Google Scholar] [CrossRef]
- Nakamura, Y.; Saitou, M.; Komura, S.; Matsumoto, K.; Ogawa, H.; Miyagawa, T.; Saitou, T.; Imai, Y.; Takayanagi, H.; Akiyama, H. Reduced dynamic loads due to hip dislocation induce acetabular cartilage degeneration by IL-6 and MMP3 via the STAT3/periostin/NF-κB axis. Sci. Rep. 2022, 12, 12207. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.T. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef]
- Jimi, E.; Huang, F.; Nakatomi, C. NF-κB Signaling Regulates Physiological and Pathological Chondrogenesis. Int. J. Mol. Sci. 2019, 20, 6275. [Google Scholar] [CrossRef]
- Kong, H.; Wang, X.-Q.; Zhang, X.-A. Exercise for Osteoarthritis: A Literature Review of Pathology and Mechanism. Front. Aging Neurosci. 2022, 14, 854026. [Google Scholar] [CrossRef]
- Di Meo, S.; Napolitano, G.; Venditti, P. Mediators of Physical Activity Protection against ROS-Linked Skeletal Muscle Damage. Int. J. Mol. Sci. 2019, 20, 3024. [Google Scholar] [CrossRef]
- Chen, C.-W.; Chen, C.-C.; Jian, C.-Y.; Lin, P.-H.; Chou, J.-C.; Teng, H.-S.; Hu, S.; Lieu, F.-K.; Wang, P.S.; Wang, S.-W. Attenuation of exercise effect on inflammatory responses via novel role of TLR4/PI3K/Akt signaling in rat splenocytes. J. Appl. Physiol. 2016, 121, 870–877. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics–challenges and potential solutions. Nat. Rev. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Ebert, M.S.; Sharp, P.A. MicroRNA sponges: Progress and possibilities. RNA 2010, 16, 2043–2050. [Google Scholar] [CrossRef]
- Endisha, H.; Datta, P.; Sharma, A.; Nakamura, S.; Rossomacha, E.; Younan, C.; Ali, S.A.; Tavallee, G.; Lively, S.; Potla, P.; et al. MicroRNA-34a5p Promotes Joint Destruction During Osteoarthritis. Arthritis Rheumatol. 2021, 73, 426–439. [Google Scholar] [CrossRef]
- Ito, Y.; Matsuzaki, T.; Ayabe, F.; Mokuda, S.; Kurimoto, R.; Matsushima, T.; Tabata, Y.; Inotsume, M.; Tsutsumi, H.; Liu, L.; et al. Both microRNA-455-5p and -3p repress hypoxia-inducible factor-2α expression and coordinately regulate cartilage homeostasis. Nat. Commun. 2021, 12, 4148. [Google Scholar] [CrossRef]
- Lefevebre, V.; Angelozzi, M.; Haseeb, A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019, 61, 39–47. [Google Scholar] [CrossRef]
- Carbonare, L.D.; Mottes, M.; Cheri, S.; Deiana, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Salvagno, G.L.; Lippi, G.; Valenti, M.T. Increased Gene Expression of RUNX2 and SOX9 in Mesenchymal Circulating Progenitors Is Associated with Autophagy during Physical Activity. Oxid. Med. Cell Longev. 2019, 2019, 8456259. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, L.; Zhao, Q.; Wu, Z.; Kong, L. MicroRNA-93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF-κB signaling pathway. Int. J. Mol. Med. 2019, 42, 779–790. [Google Scholar] [CrossRef]
- Guan, Y.-J.; Li, J.; Yang, X.U.; Du, S.; Ding, J.; Gao, Y.; Zhang, Y.; Yang, K.; Chen, Q. Evidence that miR-146a attenuates aging and trauma induced osteoarthritis by inhibiting Notch1, IL-6, and IL-1 mediated catabolism. Aging Cell 2018, 17, e12752. [Google Scholar] [CrossRef]
- Minguzzi, M.; Panichi, V.; D’Adamo, S.; Cetrullo, S.; Cattini, L.; Flamigni, F.; Mariani, E.; Borzì, R.M. Pleiotropic Roles of NOTCH1 Signaling in the Loss of Maturational Arrest of Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2021, 22, 12012. [Google Scholar] [CrossRef]
- Horak, M.; Zlamal, F.; Iliev, R.; Kucera, J.; Cacek, J.; Svobodova, L.; Hlavonova, Z.; Kalina, T.; Slaby, O.; Bienertova-Vasku, J. Exercise- induced circulating microRNA changes in athletes in various training scenarios. PLoS ONE 2018, 13, e0191060. [Google Scholar] [CrossRef] [PubMed]
- Wardle, S.L.; Bailey, M.E.S.; Kilikevicius, A.; Malkova, D.; Wilson, R.H.; Venckunas, T.; Moran, C.N. Plasma MicroRNA Levels Differ between Endurance and Strength Athletes. PLoS ONE 2015, 10, e0122107. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, P.S.; Gilbert, S.J.; Tarn, J.; Charlton, S.; Skelton, A.J.; Barter, M.J.; Duance, V.C.; Young, D.A.; Blain, E.J. Regulation of microRNA-221, -222, -21 and -27 in articular cartilage subjected to abnormal compressive forces. J. Physiol. 2021, 599, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.; Härönen, R.; Mumberg, D.; Bürger, C.; Olsen, B.R.; Budarf, M.L.; Apte, S.S.; Müller, R. Structure of the Human TIMP-3 gene and its cell cycle-regulated promoter. Biochem. J. 1995, 311, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Wilusz, J.E. A360 degree view of circular RNAs; From biogenesis to functions Wiley Interdiscip. Rev. RNA 2018, 9, e1478. [Google Scholar] [CrossRef]
- Salzman, J.; Chen, R.E.; Olsen, M.N.; Wang, P.L.; Brown, P.O. Cell-Type Specific Features of Circular RNA Expression. PLoS Genet. 2013, 9, e1003777. [Google Scholar] [CrossRef]
- He, M.; Jia, Z.; Wen, Y.; Chen, X. Circ_0043947 contributes to interleukin 1β-induced injury in chondrocytes by sponging miR-671-5p to up-regulate RTN3 expression in osteoarthritis pathology. J. Orthop. Surg. Res. 2022, 17, 177. [Google Scholar] [CrossRef]
- Li, G.; Luo, H.; Ding, Z.; Liang, H.; Laio, Z.; Chen, S.; Huang, Y. Silencing of circ_0000205 mitigates interleukin-1β-induced apoptosis and extracellular matrix degradation in chondrocytes via targeting miR-766-3p/ADAMTS5 axis. Innate Immun. 2022, 28, 79–90. [Google Scholar] [CrossRef]
- Collins, K.H.; Lenz, K.L.; Politt, E.N.; Ferguson, D.; Hutson, I.; Springer, L.E.; Oestreich, A.K.; Tang, R.; Choi, Y.-R.; Meyer, G.A.; et al. Adipose tissue is a critical regulator of osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021096118. [Google Scholar] [CrossRef]
- Xie, C.; Chen, Q. Adipokines: New Therapeutic Target for Osteoarthritis? Curr. Rheumatol. Rep. 2019, 21, 71. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Liu, S.-C.; Huang, C.C.; Kuo, S.-J.; Tsai, C.-H.; Tang, C.-H. Associations between Adipokines in Arthritic Disease and Implications for Obesity. Int. J. Mol. Sci. 2019, 20, 1505. [Google Scholar] [CrossRef]
- Presle, N.; Pottie, P.; Dumond, H.; Guillaume, C.; Lapicque, F.; Pallu, S.; Mainard, D.; Netter, P.; Terlain, B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthr. Cart. 2006, 14, 690–695. [Google Scholar] [CrossRef]
- Calvet, J.; Orellana, C.; Gratacós, J.; Berenguer-Llergo, A.; Caixàs, A.; Chillarón, J.J.; Pedro-Botet, J.; Garcia-Manrique, M.; Larrosa, M. Synovial fluid adipokines are associated with clinical severity in knee osteoarthritis: A cross-sectional study in female patients with joint effusion. Arthritis Res. Ther. 2016, 18, 207. [Google Scholar] [CrossRef]
- Griffin, T.M.; Huebner, J.L.; Kraus, V.B.; Yan, Z.; Guilak, F. Induction of Osteoarthritis and Metabolic Inflammation by a Very High Fat Diet in Mice: Effects of Short-term Exercise. Arthritis Rheum. 2012, 64, 443–453. [Google Scholar] [CrossRef]
- Tian, D.; Meng, J. Exercise for Prevention and Relief of Cardiovascular Disease: Prognoses, Mechanisms, and Approaches. Oxid. Med. Cell. Longev. 2019, 2019, 3756750. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, J.; Yang, H.; Sun, Y. The role of leptin in osteoarthritis. Medicine 2018, 97, 14. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L.; Wiliams, T.D.; Dobbs, W.C. The Effect of Chronic Exercise Training on Leptin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sport. Med. 2018, 48, 1437–1450. [Google Scholar] [CrossRef]
- Joo, Y.B.; Lee, K.B.; Sul, B.; Lee, H.-S.; Lim, S.H.; Park, Y.-J. Effect of resistance exercise on serum leptin levels in a prospective longitudinal study of women patients with rheumatoid arthritis. Arthritis Res. Ther. 2022, 24, 1–9. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; van der Kraan, P.M.; van der Berg, W.B. TGF-b and osteoarthritis. Osteoarthr. Cart. 2007, 15, 597–604. [Google Scholar] [CrossRef]
- Takahashi, H.; Alves, C.R.R.; Stanford, K.I.; Middelbeek, R.J.W.; Nigro, P.; Ryan, R.E.; Xue, R.; Sakaguchi, M.; Lynes, M.D.; So, K.; et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 2019, 1, 291–303. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.F.; Maharjan, B.R.; Williams, P.F.; Tam, C.S.; McIennan, S.V.; Twigg, S.M. Differential metabolic effects of constant moderate versus high intensity interval training in high-fat fed mice: Possible role of muscle adiponectin. Physiol. Rep. 2018, 6, e13599. [Google Scholar] [CrossRef] [PubMed]
- Orellana, C.; Calvet, J.; Berenguer-Llergo, A.; Albiñana, N.; Narique, M.G.; Lencastre, C.G.; Arévalo, M.; Llop, M.; Caixàs, A.; Gratacós, J. Synovial Adiponectin Was More Associated with Clinical Severity than Synovial Leptin in Women with Knee Osteoarthritis. Cartilage 2021, 13 (Suppl. I), 1675S–1683S. [Google Scholar] [CrossRef] [PubMed]
- Ilia, I.; Nitusca, D.; Marian, C. Adiponectin in Osteoarthritis: Pathophysiology, Relationship with Obesity and Presumptive Diagnostic Biomarker Potential. Diagnostics 2022, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A.; Mier, J.W. Interleukins. Ann. Rev. Med. 1986, 37, 173–178. [Google Scholar] [CrossRef]
- Boraschi, D. What Is IL-1 for? The Functions of Interleukin-1 across Evolution. Front. Immunol. 2022, 13, 872155. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Li, Y.; Wu, H. Glaucocalyxin A Attenuates IL-1β-Induced Inflammatory Response and Cartilage Degradation in Osteoarthritis Chondrocytes via Inhibiting the Activation of NF-κB Signaling Pathway. Dis. Markers 2022, 2022, 6516246. [Google Scholar] [CrossRef]
- Vincent, T.L. IL-1 in osteoarthritis: Time for a critical review of the literature. F1000Research 2019, 8, F1000. [Google Scholar] [CrossRef]
- Clements, K.M.; Price, J.S.; Chambers, M.G.; Visco, D.M.; Poole, A.R.; Mason, R.M. Gene Deletion of Either Interleukin-1β, Interleukin-1β–Converting Enzyme, Inducible Nitric Oxide Synthase, or Stromelysin 1 Accelerates the Development of Knee Osteoarthritis in Mice After Surgical Transection of the Medial Collateral Ligament and Partial Medial Meniscectomy. Arthritis Rheum. 2003, 48, 3452–3463. [Google Scholar] [CrossRef]
- Wysocka, A.; Giziński, S.; Lechowski, R. Metaloproteinazy macierzy–ich struktura i znaczenie (pol.). Życie weterynaryjne 2014, 89, 223–227. [Google Scholar]
- Fields, J.K.; Günther, S.; Sundberg, E.J. Structural Basis of IL-1 Family Cytokine Signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Budhiparama, N.C.; Lumban–Gaol, I.; Sudoyo, H.; Magetsari, R.; Wibawa, T. Interleukin-1 genetic polymorphisms in knee osteoarthritis: What do we know? A meta-analysis and systematic review. J. Orthop. Surg. 2022, 30, 23094990221076652. [Google Scholar] [CrossRef]
- Dennis, R.A.; Trappe, T.A.; Simpson, P.; Carroll, C.; Huang, B.E.; Nagarajan, R.; Bearden, E.; Gurley, C.; Duff, G.W.; Evans, W.J.; et al. Interleukin-1 polymorphisms are associated with the inflammatory response in human muscle to acute resistant exercise. J. Physiol. 2004, 560, 617–626. [Google Scholar] [CrossRef]
- Tanaka, T.; Kishimoto, T. Targeting Interleukin-6: All the Way to Treat Autoimmune and Inflammatory Diseases. Int. J. Biol. Sci. 2012, 8, 1227–1236. [Google Scholar] [CrossRef]
- Wiegertjes, R.; van den Loo, F.A.J.; Blaney Davidson, E.N. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology 2020, 59, 2681–2694. [Google Scholar] [CrossRef]
- Latourte, A.; Cherifi, C.; Maillet, J.; Ea, H.-K.; Bouaziz, W.; Funck-Brentano, T.; Cohen-Solal, M.; Hay, E.; Richette, P. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 2016, 76, 1–8. [Google Scholar] [CrossRef]
- Chowdhury, S.; Schulz, L.; Palmisano, B.; Singh, P.; Berger, J.M.; Yadav, V.K.; Mera, P.; Ellingsgaard, H.; Hidalgo, J.; Karsenty, G. Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J. Clin. Investig. 2020, 130, 2888–2902. [Google Scholar] [CrossRef]
- Wedell-Neergaard, A.-S.; Lehrskov, L.L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab. 2019, 29, 844–855. [Google Scholar] [CrossRef]
- Trinh, B.; Peletier, M.; Simonsen, C.; Plomgaard, P.; Karstoft, K.; Pedersen, B.K.; van Hall, G.; Ellingsgaard, H. Blocking endogenous IL-6 impairs mobilization of free fatty acids during rest and exercise in lean and obese men. Cell Rep. Med. 2021, 2, 100396. [Google Scholar] [CrossRef]
- Bennell, K.L.; Buchbinder, R.; Himan, R.S. Physical therapies in the management of osteoarthritis: Current state of the evidence. Curr. Opin. Rheumatol. 2015, 27, 304–311. [Google Scholar] [CrossRef]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718. [Google Scholar] [CrossRef]
- Miller, D.; Smith, N.; Bailey, M.; Czarnota, G.; Hynynen, K.; Makin, I. Overview of Therapeutic Ultrasound Applications and Safety Considerations. J. Ultrasound. Med. 2012, 31, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, V.; Arjmand, B.; Tavirani, M.R.; Razzaghi, M.; Rostami-Nejad, M.; Hamdieh, M. Evaluation of Efficacy of Low-Level Laser Therapy. J. Lasers Med. Sci. 2020, 11, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I.; Paley, C.A.; Jones, G.; Mulvey, M.R.; Wittkopf, P.G. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: A systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open 2022, 12, e051073. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Zhu, Y.; Liao, B.; Tan, Q.; Qi, H.; Zhang, B.; Huang, J.; Du, X.; Bai, D. Low-intensity pulsed ultrasound inhibits VEGFA expression in chondrocytes and protects against cartilage degeneration in experimental osteoarthritis. FEBS Open Bio 2020, 10, 434–443. [Google Scholar] [CrossRef]
- Luo, Q.; Ji, S.; Li, Z.; Huang, T.; Fan, S.; Xi, Q. Effects of ultrasound therapy on the synovial fluid proteome in a rabbit surgery-induced model of knee osteoarthritis. Biomed. Eng. Online 2019, 18, 18. [Google Scholar] [CrossRef]
- Xia, P.; Wang, Q.; Song, J.; Wang, X.; Wang, X.; Lin, Q.; Cheng, K.; Chen, A.; Li, X. Low-Intensity Pulsed Ultrasound Enhances the Efficacy of Bone Marrow–Derived MSCs in Osteoarthritis Cartilage Repair by Regulating Autophagy-Mediated Exosome Release. Cartilage 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Draper, D.O.; Klyve, D.; Ortiz, R.; Best, T.M. Effect of low-intensity long-duration ultrasound on the symptomatic relief of knee osteoarthritis: A randomized, placebo controlled double-blind study. J. Orthop. Surg. Res. 2018, 13, 257. [Google Scholar] [CrossRef]
- Zadeh-Haghighi, H.; Simon, C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J. R. Soc. Interface 2020, 19, 20220325. [Google Scholar] [CrossRef]
- Yang, X.; Hongchen, H.; Wenwen, Y.; Perry, T.A.; He, C. Effects of Pulsed Electromagnetic Field Therapy on Pain, Stiffness, Physical Function, and Quality of Life in Patients with Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Phys. Ther. 2020, 100, 1118–1131. [Google Scholar] [CrossRef]
- Parate, D.; Kadir, N.D.; Celik, C.; Lee, E.H.; Hui, J.P.; Franco-Obregón, A.; Yang, Z. Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Curr. Stem. Cell Res. Ther. 2020, 11, 46. [Google Scholar] [CrossRef]
- Hamid, H.A.; Sarmadi, V.H.; Prasad, V.; Ramasay, R.; Miskon, A. Electromagnetic field exposure as a plausible approach to enhance the proliferation and differentiation of mesenchymal stem cells in clinically relevant scenarios. J. Zhejiang Univ. Sci. B 2022, 23, 42–57. [Google Scholar] [CrossRef]
- Lei, H.; Pan, L.; Wu, R.; Lv, Y. Innate Immune Regulation Under Magnetic Fields with Possible Mechanisms and Therapeutic Applications. Front. Immunol. 2020, 11, 582772. [Google Scholar] [CrossRef]
- Tong, J.; Chen, Z.; Sun, G.; Zhou, J.; Zeng, Y.; Zhong, P.; Deng, C.; Chen, X.; Liu, L.; Wang, S.; et al. The Efficacy of Pulsed Electromagnetic Fields on Pain, Stiffness, and Physical Function in Osteoarthritis: A Systematic Review and Meta-Analysis. Pain Res. Manag. 2022, 2022, 9939891. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Nambi, G. Does low level laser therapy has effects on inflammatory biomarkers IL-1β, IL-6, TNF-α, and MMP-13 in osteoarthritis of rat models—A systemic review and meta-analysis. Lasers Med. Sci. 2020, 36, 475–484. [Google Scholar] [CrossRef]
- Saygun, I.; Nizam, N.; Ural, A.U.; Serdar, M.A.; Avcu, F.; Tözüm, T.F. Low-Level Laser Irradiation Affects the Release of Basic Fibroblast Growth Factor (bFGF), Insulin-Like Growth Factor-I (IGF-I), and Receptor of IGF-I (IGFBP3) from Osteoblasts Photomed. Laser Surg. 2012, 30, 149–154. [Google Scholar] [CrossRef]
- Reyegani, S.M.; Raeissadat, S.A.; Heidari, S.; Moradi-Joo, M. Safety and Effectiveness of Low-Level Laser Therapy in Patients with Knee Osteoarthritis: A Systematic Review and Meta-analysis. J. Lasers Med. Sci. 2017, 8 (Suppl. 1), S12–S19. [Google Scholar] [CrossRef]
- Akaltun, M.S.; Altindag, O.; Turan, N.; Gursoy, S.; Gur, A. Efficacy of high intensity laser therapy in knee osteoarthritis: A double-blind controlled randomized study. Clin. Rheumatol. 2020, 40, 1989–1995. [Google Scholar] [CrossRef]
- Peat, F.J.; Colbath, A.C.; Bentsen, L.M.; Goodrich, L.R.; King, M.R. In Vitro Effects of High-Intensity Laser Photobiomodulation on Equine Bone Marrow-Derived Mesenchymal Stem Cell Viability and Cytokine Expression. Photomed. Laser Surg. 2017, 36, 83–91. [Google Scholar] [CrossRef]
- Arienti, C.; Armijo-Olivo, S.; Minozzi, S.; Tjosvold, L.; Lazzarini, S.G.; Patrini, M.; Negrini, S. Methodological Issues in Rehabilitation Research: A Scoping Review. Arch. Phys. Med. Rehabil. 2021, 102, 1614–1622. [Google Scholar] [CrossRef]
- Xu, H.; Kang, J.-H.; Choi, S.-E.; Park, D.-J.; Kweon, S.-S.; Lee, Y.-H.; Kim, H.-Y.; Lee, J.-K.; Shin, M.-H.; Lee, S.-S. Increased adiponectin levels are associated with higher radiographic scores in the knee joint, but not in the hand joint. Sci. Rep. 2021, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Habibi, A.; Karia, R.; Ward, S.; Schwarzkopf, R.; Rozell, J.C.; Slover, J. Patient Reported Outcomes Following Intraarticular Hyaluronic Acid for Knee Osteoarthritis. J. Arthroplast. 2023. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; Stagno, D.; Minetto, M.A.; Cisari, C.; Baricich, A.; INvernizzi, M. Long-term effects of intra-articular oxygen-ozone therapy versus hyaluronic acid in older people affected by knee osteoarthritis: A randomized single-blind extension study. J. Back Musculoskelet. Rehabil. 2020, 33, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qi, X.; Zhang, Z. Intra-articular oxygen-ozone versus hyaluronic acid in knee osteoarthritis: A meta-analysis of randomized controlled trials. Int. J. Surg. 2018, 58, 3–10. [Google Scholar] [CrossRef]
- Nelson, F.R.; Zvirbulis, R.A.; Zonca, B.; Li, K.W.; Turner, S.M.; Pasierb, M.; Wilton, P.; Martinez Puig, D.; Wu, W. The effects of an oral preparation containing hyaluronic acid (Oralvisc®) on obese knee osteoarthritis patients determined by pain, function, bradykinin, leptin, inflammatory cytokines, and heavy water analyses. Rheumatol. Int. 2014, 35, 43–52. [Google Scholar] [CrossRef]
- Lam, T.Y.T.; Cheung, M.F.K.; Munro, Y.L.; Lim, K.M.; Shung, D.; Sung, J.J.Y. Randomized Controlled Trials of Artificial Intelligence in Clinical Practice: Systematic Review. J. Med. Internet Res. 2022, 24, e37188. [Google Scholar] [CrossRef]
- Weiss, R.; Karimijafarbigloo, S.; Roggenbuck, D.; Rödiger, S. Applications of Neural Networks in Biomedical Data Analysis. Biomedicines 2022, 10, 1469. [Google Scholar] [CrossRef]
- Ali, S.A.; Pastrello, C.; Kaur, N.; Peffers, M.J.; Ormseth, M.J.; Jurisica, I. A Network Biology Approach to Understanding the Tissue-Specific Roles of Non-Coding RNAs in Arthritis. Front. Endocrinol. 2021, 12, 744747. [Google Scholar] [CrossRef]
- Erdemir, A.; Hunter, P.J.; Holzapfel, G.A.; Loew, L.M.; Middleton, J.; Jacobs, C.R.; Nithiarasu, P.; Löhner, R.; Wei, G. Perspectives on Sharing Models and Related Resources in Computational Biomechanics Research. J. Biomech. Eng. 2018, 140, 2. [Google Scholar] [CrossRef]
- Liao, Y.; Vakanski, A.; Xian, M.; Paul, D.; Baker, R. A Review of Computational Approaches for Evaluation of Rehabilitation Exercises. Comput. Biol. Med. 2020, 119, 103687. [Google Scholar] [CrossRef]

| Article Type | Human/Animal | Sample Size (Characteristic) | Main Results/Conclusion | Ref. |
|---|---|---|---|---|
| Research article | Human | 6 advanced OA patients | M1 polarized synovial macrophages inhibits mesenchymal stem cells into chondrocytes transformation | [5] |
| Research article | Human | 86 advanced knee OA patients | Synovial macrophage/monocyte M1 polarization correlates with OA symptoms specially stiffness, function and quality of life. Macrophage polarization modulation may be effective in novel treatment strategies | [6] |
| Review, data synthesis | Human | Suggestion of extracting 4 subtypes of knee osteoarthritis (cartilage degradation driven subtype, bone-remodeling driven subtype, pain driven subtype and inflammation driven subtype). Proposed subtypes present different molecular OA progression background and may demand different treatment strategies | [21] | |
| Randomized blinded study | Human | 42 with confirmed knee OA | Exercise program increases a patient’s functional status. Adding IR photobiomodulation to exercise results in increasing serum IL-10 concentration | [22] |
| Review/meta-analysis | Human | 76 research articles | Long distance running increases serum IL-6, IL-1, IL-8, IL-10 and TNFα concentration and reduce IL-2 concentration. Observed effect is stronger in longer distances | [26] |
| Research article | Rat | 15 | Reduced dynamic acetabular cartilage load activates Il-6 and MMP3 via the STAT3/periostin/NF-κB axis. Mechanical load is essential for proper cartilage function | [28] |
| Research article | Human/mouse | 37 patients qualified to knee replacement, 34 healthy patients, 120 mice | miR-34a-5p expression is high in plasma and synovium of humans and mice with advanced knee OA. The miR-34a-5p overexpression may be the target for future therapeutic interventions and needs further investigations | [37] |
| Research article | Human/mouse | 21 humans, 23mice | MicroRNA-455-5p and-3p repress hypoxia-inducible factor-2α expression (HIF-2α) and prevent cartilage from HIF-2α procatabolic activity | [38] |
| Research article | Mouse | 36 male mice | MicroRNA-93 protects from apoptosis and inflammation outbreak by TLR4/NF-κB signaling pathway deactivation | [41] |
| Research | Human | 30 young males | Different intense strength training forms performed for 8 weeks change microRNA (miR-16, miR-21, miR-93 and miR-222) plasma concentration | [44] |
| Research | Human | 43 | Circular RNA circ_0000205 silencing reduces IL-1β apoptotic activity and cartilage matrix degradation by ADAMTS5 deactivation and miR-766-3p activation | [51] |
| Research | Human | 35 all qualified to knee arthroplasty | Adipokines are produced in joints and their joint activity has no correlation with serum activity. Leptin has higher level in women | [55] |
| Research | Human | 150 Women with advanced KOA with joint effusion | The synovial fluid level of resistin is directly and visfatin level is inversely associated with clinical severity of knee OA | [56] |
| Review + meta-analysis | Human | 72 RCTs | Chronic training causes decrease in leptin and the effect is associated with fat mass loss | [60] |
| Cross-sectional study | Human | 115 women | Synovial adiponectin showed a statistically stronger association with OA clinical progression comparing with synovial leptin | [65] |
| Review | Human | Il-1 fails as a target in OA therapy | [70] | |
| Research article | Human | 24 sedentary men | Inflammatory response strength to resistant exercise depends on Il-1 single nucleotide polymorphism. Potentially polymorphism may be used to training optimalization | [75] |
| Double-blinded trial | Human | 53 abdominal obese | Il-6 blockade (tocilizumab application) results in no effect of endurance training on fat mass loss. IL-6 plays important metabolic role | [80] |
| Research | Mouse | 14 | Low intensity ultrasound decrease expression of vascular endothelium growth factor A (VEGFA) via p38 MAPK suppression | [87] |
| Review + meta-analysis | Rat | 188 | Low level laser therapy reduces IL-1β, TNF-α, MMP-13. LLLT mechanism has a molecular background | [98] |
| Research | Human | 2402 | Adiponectin synovial level is high in advanced knee OA but not in hand joint. The molecular OA pathology differs due to OA localization | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdziechowski, A.; Gluba-Sagr, A.; Rysz, J.; Woldańska-Okońska, M. Why Does Rehabilitation Not (Always) Work in Osteoarthritis? Does Rehabilitation Need Molecular Biology? Int. J. Mol. Sci. 2023, 24, 8109. https://doi.org/10.3390/ijms24098109
Zdziechowski A, Gluba-Sagr A, Rysz J, Woldańska-Okońska M. Why Does Rehabilitation Not (Always) Work in Osteoarthritis? Does Rehabilitation Need Molecular Biology? International Journal of Molecular Sciences. 2023; 24(9):8109. https://doi.org/10.3390/ijms24098109
Chicago/Turabian StyleZdziechowski, Adam, Anna Gluba-Sagr, Jacek Rysz, and Marta Woldańska-Okońska. 2023. "Why Does Rehabilitation Not (Always) Work in Osteoarthritis? Does Rehabilitation Need Molecular Biology?" International Journal of Molecular Sciences 24, no. 9: 8109. https://doi.org/10.3390/ijms24098109








