Murine Placental Erythroid Cells Are Mainly Represented by CD45+ Immunosuppressive Erythroid Cells and Secrete CXCL1, CCL2, CCL3 and CCL4 Chemokines
Abstract
1. Introduction
2. Results
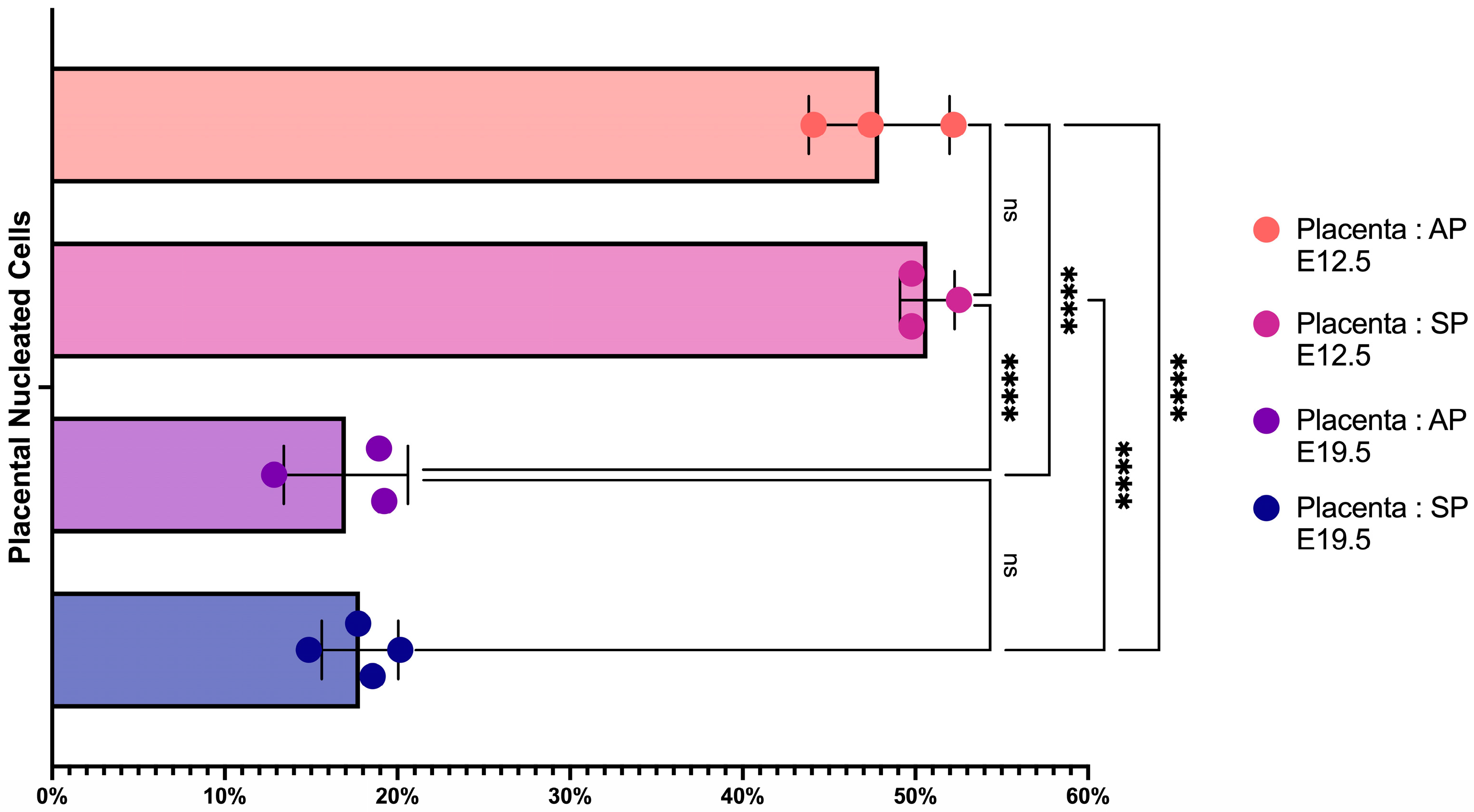
2.1. The Number of Erythroid Cells Decreases in Late-Term Pregnancy
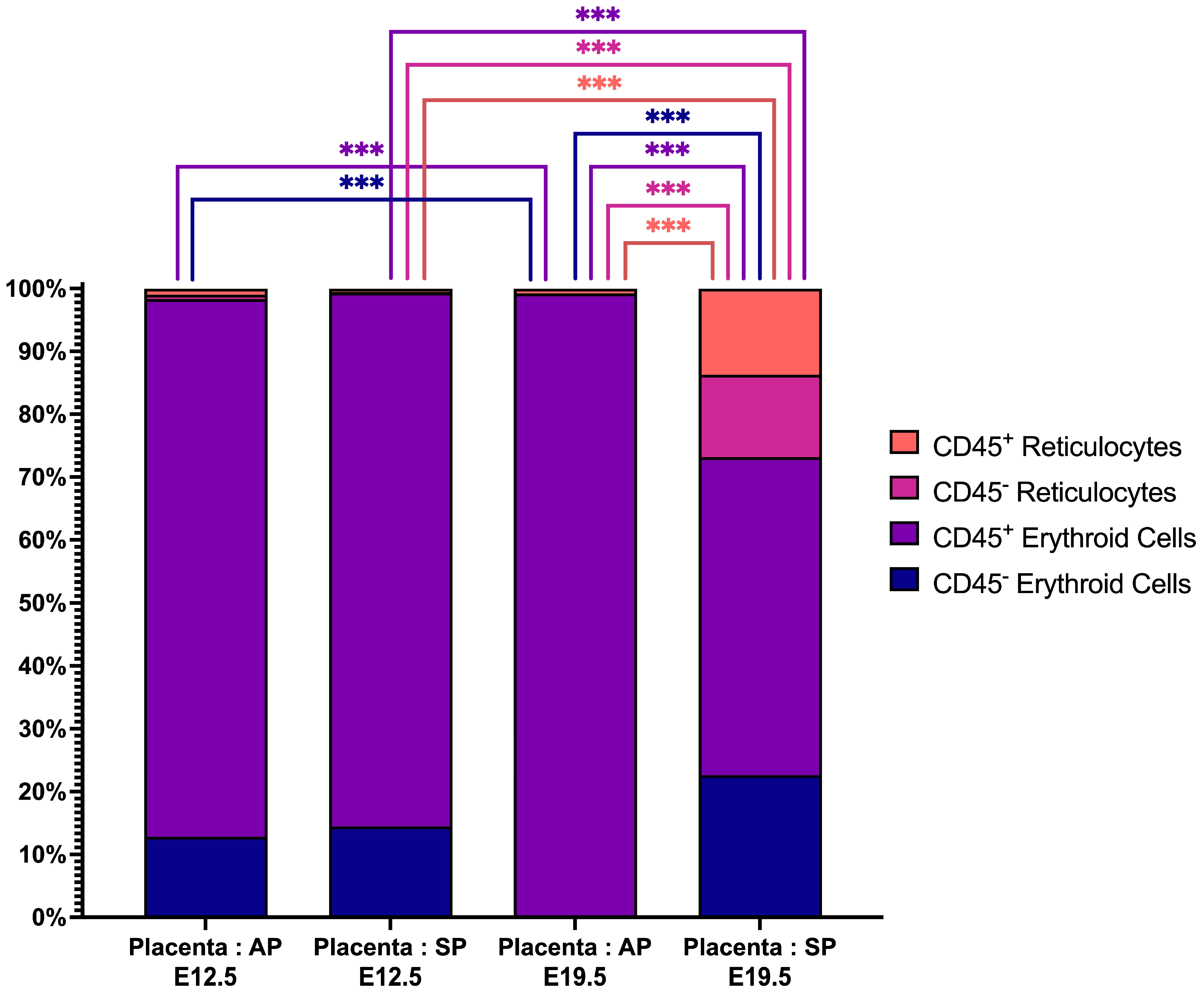
2.2. Placental Erythroid Cells Are Mainly Represented by CD45+ Erythroid Cells
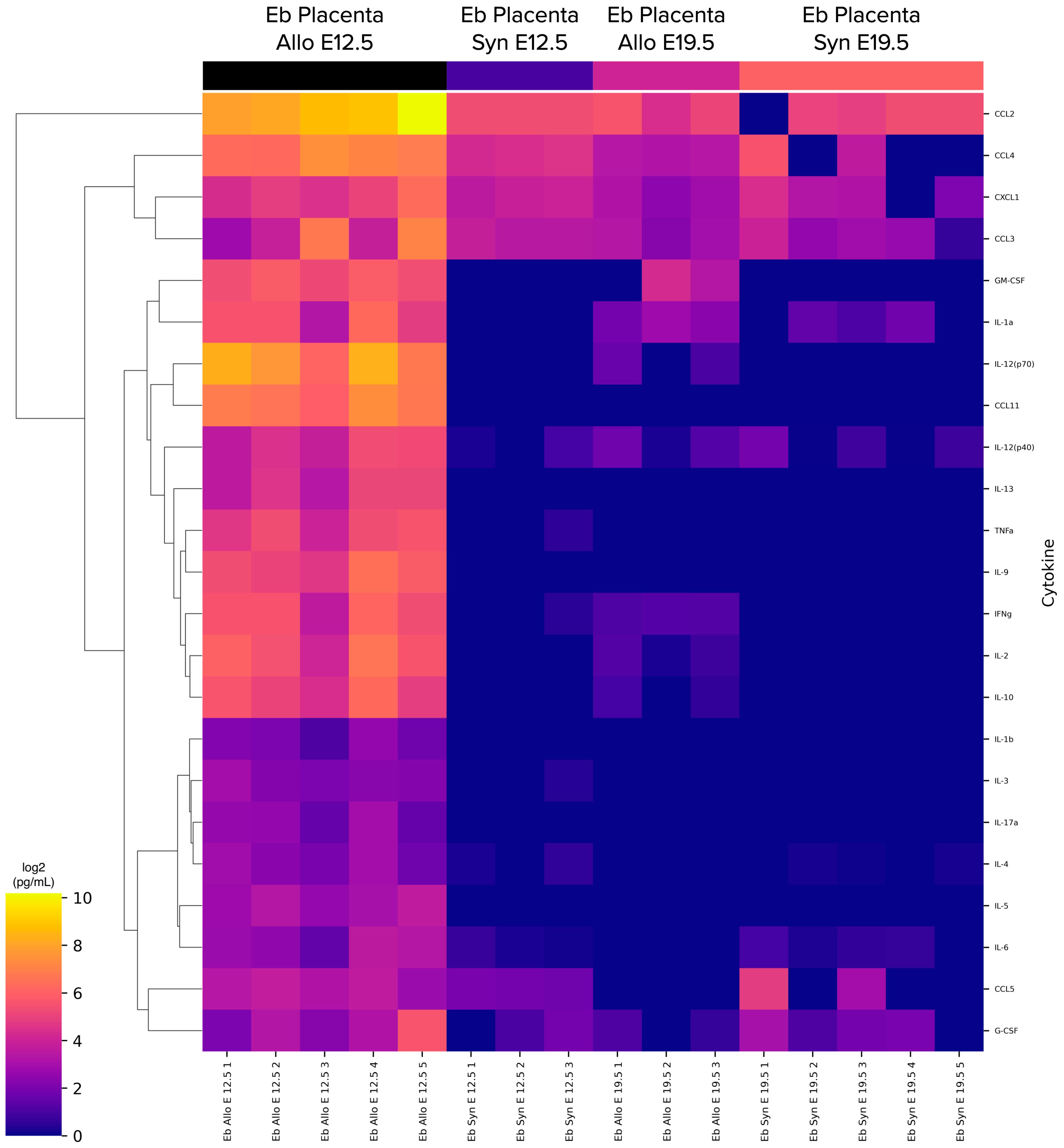
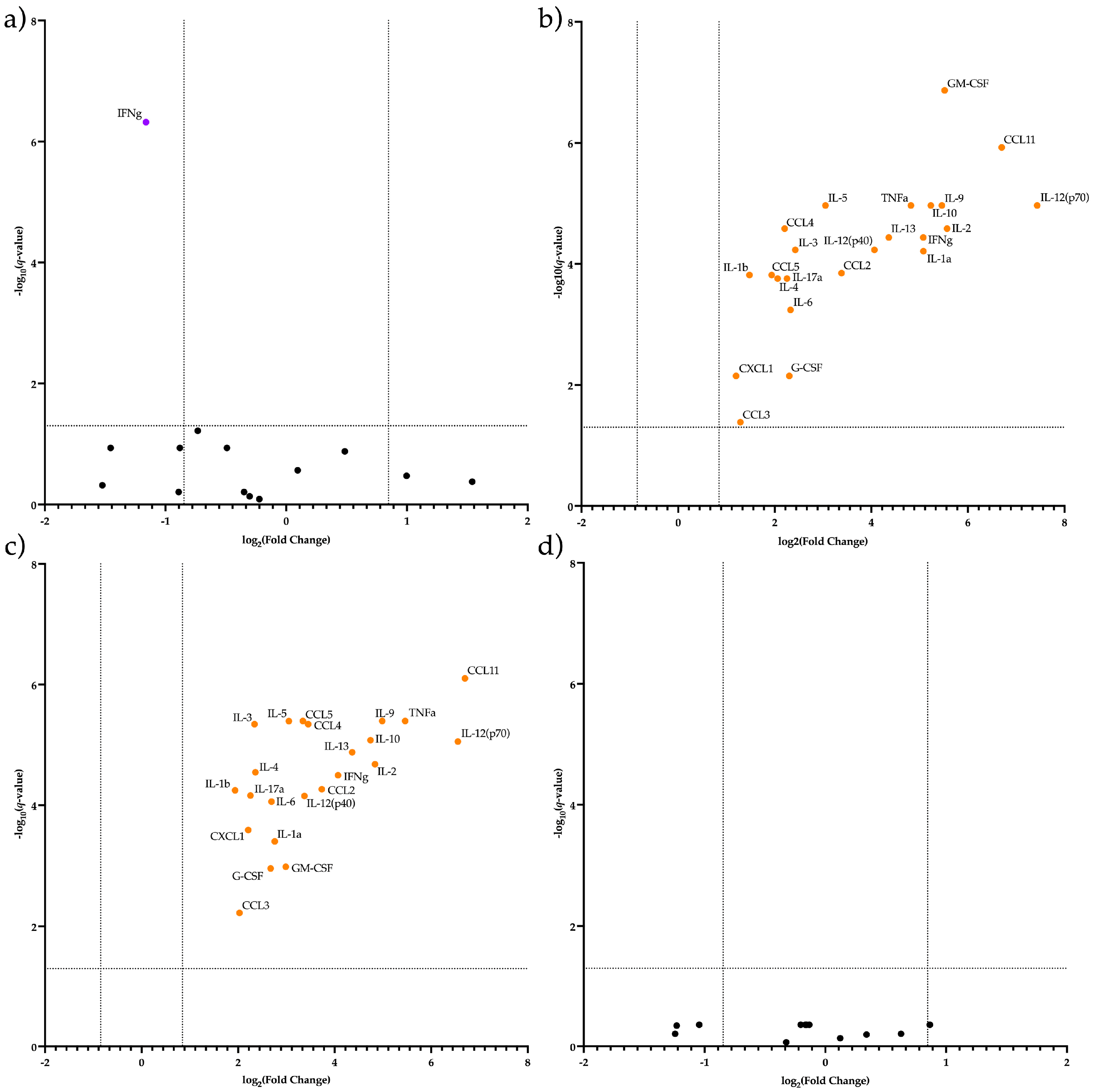
2.3. CECs from the Placenta at E12.5 in AP Have a Different Secretome Compared to Other Studied Terms and Types of Pregnancy
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cell Isolation
4.3. Magnetic Separation
4.4. Viability Staining
4.5. Erythroid Cells’ Flow Cytometry
4.6. Cell Culturing
4.7. Erythroid Cells’ Conditioned Media Harvesting
4.8. Cytokine Quantification in Culture Medium
4.9. Data Analysis: Flow Cytometry Data
4.10. Data Analysis: Cytokine Secretion Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef]
- Wegmann, T.G.; Carlson, G.A. Allogeneic pregnancy as immunoabsorbent. J. Immunol. 1977, 119, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lopez, G.E.; Vazquez, J.; Sun, Y.; Chavarria, M.; Lindner, P.N.; Fredrickson, S.; Karst, N.; Stanic, A.K. Decidual-placental immune landscape during syngeneic murine pregnancy. Front. Immunol. 2018, 9, 2087. [Google Scholar] [CrossRef]
- Kolahi, K.S.; Valent, A.M.; Thornburg, K.L. Cytotrophoblast, not syncytiotrophoblast, dominates glycolysis and oxidative phosphorylation in human term placenta. Sci. Rep. 2017, 7, 42941. [Google Scholar] [CrossRef]
- Van Handel, B.; Prashad, S.L.; Hassanzadeh-Kiabi, N.; Huang, A.; Magnusson, M.; Atanassova, B.; Chen, A.; Hamalainen, E.I.; Mikkola, H.K. The first trimester human placenta is a site for terminal maturation of primitive erythroid cells. Blood J. Am. Soc. Hematol. 2010, 116, 3321–3330. [Google Scholar] [CrossRef]
- Chhabra, A.; Lechner, A.J.; Ueno, M.; Acharya, A.; Van Handel, B.; Wang, Y.; Iruela-Arispe, M.L.; Tallquist, M.D.; Mikkola, H.K. Trophoblasts regulate the placental hematopoietic niche through PDGF-B signaling. Dev. Cell 2012, 22, 651–659. [Google Scholar] [CrossRef]
- Kina, T.; Ikuta, K.; Takayama, E.; Wada, K.; Majumdar, A.S.; Weissman, I.L.; Katsura, Y. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Hematol. 2000, 109, 280–287. [Google Scholar] [CrossRef]
- Delyea, C.; Bozorgmehr, N.; Koleva, P.; Dunsmore, G.; Shahbaz, S.; Huang, V.; Elahi, S. CD71+ Erythroid suppressor cells promote fetomaternal tolerance through arginase-2 and PDL-1. J. Immunol. 2018, 200, 4044–4058. [Google Scholar] [CrossRef]
- Sennikov, S.V.; Eremina, L.V.; Samarin, D.M.; Avdeev, I.V.; Kozlov, V.A. Cytokine gene expression in erythroid cells. Eur. Cytokine Netw. 1996, 7, 771–774. [Google Scholar] [PubMed]
- Sennikov, S.V.; Injelevskaya, T.V.; Krysov, S.V.; Silkov, A.N.; Kovinev, I.B.; Dyachkova, N.J.; Zenkov, A.N.; Loseva, M.I.; Kozlov, V.A. Production of hemo-and immunoregulatory cytokines by erythroblast antigen+ and glycophorin A+ cells from human bone marrow. BMC Cell Biol. 2004, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sennikov, S.V.; Krysov, S.V.; Injelevskaya, T.V.; Silkov, A.N.; Kozlov, V.A. Production of cytokines by immature erythroid cells derived from human embryonic liver. Eur. Cytokine Netw. 2001, 12, 274–279. [Google Scholar] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Arenas-Hernandez, M.; Ahn, H.; Panaitescu, B.; Vadillo-Ortega, F.; Sanchez-Torres, C.; Salisbury, K.S.; Hassan, S.S. In vivo T-cell activation by a monoclonal αCD3ε antibody induces preterm labor and birth. Am. J. Reprod. Immunol. 2016, 76, 386–390. [Google Scholar] [CrossRef]
- Dunsmore, G.; Koleva, P.; Ghobakhloo, N.; Sutton, R.; Ambrosio, L.; Meng, X.; Hotte, N.; Nguyen, V.; Madsen, K.L.; Dieleman, L.A.; et al. Lower abundance and impaired function of CD71+ erythroid cells in inflammatory bowel disease patients during pregnancy. J. Crohn’s Colitis 2019, 13, 230–244. [Google Scholar] [CrossRef]
- Miller, D.; Romero, R.; Unkel, R.; Xu, Y.; Vadillo-Ortega, F.; Hassan, S.S.; Gomez-Lopez, N. CD71+ erythroid cells from neonates born to women with preterm labor regulate cytokine and cellular responses. J. Leukoc. Biol. 2018, 103, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Ki, C.S.; Han, K.H. Isolation of nucleated red blood cells in maternal blood for Non-invasive prenatal diagnosis. Biomed. Microdevices 2015, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.I.; Seledtsova, G.V.; Samarin, D.M.; Taraban, V.Y.; Sennikov, S.V.; Kozlov, V.A. Characterization of erythroid cell-derived natural suppressor activity. Immunobiology 1998, 198, 361–374. [Google Scholar] [CrossRef]
- Cui, L.; Takada, H.; Takimoto, T.; Fujiyoshi, J.; Ishimura, M.; Hara, T. Immunoregulatory function of neonatal nucleated red blood cells in humans. Immunobiology 2016, 221, 853–861. [Google Scholar] [CrossRef]
- Dulay, A.T.; Buhimschi, I.A.; Zhao, G.; Luo, G.; Abdel-Razeq, S.; Cackovic, M.; Rosenberg, V.A.; Pettker, C.M.; Thung, S.F.; Bahtiyar, M.O.; et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborn. Gynecology 2008, 198, 421–426. [Google Scholar] [CrossRef]
- Norton, M.T.; Fortner, K.A.; Bizargity, P.; Bonney, E.A. Pregnancy alters the proliferation and apoptosis of mouse splenic erythroid lineage cells and leukocytes. Biol. Reprod. 2009, 81, 457–464. [Google Scholar] [CrossRef]
- Oguro, H.; McDonald, J.G.; Zhao, Z.; Umetani, M.; Shaul, P.W.; Morrison, S.J. 27-Hydroxycholesterol induces hematopoietic stem cell mobilization and extramedullary hematopoiesis during pregnancy. J. Clin. Investig. 2017, 127, 3392–3401. [Google Scholar] [CrossRef]
- Tsuda, S.; Nakashima, A.; Shima, T.; Saito, S. New paradigm in the role of regulatory T cells during pregnancy. Front. Immunol. 2019, 10, 573. [Google Scholar] [CrossRef]
- Krop, J.; Heidt, S.; Claas, F.H.J.; Eikmans, M. Regulatory T cells in pregnancy: It is not all about FoxP3. Front. Immunol. 2020, 11, 1182. [Google Scholar] [CrossRef]
- Niedbala, W.; Wei, X.-Q.; Cai, B.; Hueber, A.J.; Leung, B.P.; McInnes, I.B.; Liew, F.Y. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur. J. Immunol. 2007, 37, 3021–3029. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiao, Y.D.; Li, X.; Xu, J.L.; Ye, Q.J.; Jiang, N.; Zhang, H.; Wu, X.Y. Intratumoral CD45+CD71+ erythroid cells induce immune tolerance and predict tumor recurrence in hepatocellular carcinoma. Cancer Lett. 2021, 499, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Chau, S.E.; Murthi, P.; Wong, M.H.; Whitley, G.S.; Brennecke, S.P.; Keogh, R.J. Control of extravillous trophoblast function by the eotaxins CCL11, CCL24 and CCL26. Hum. Reprod. 2013, 28, 1497–1507. [Google Scholar] [CrossRef]
- Dybedal, I.; Larsen, S.; Jacobsen, S.E. IL-12 directly enhances in vitro murine erythropoiesis in combination with IL-4 and stem cell factor. J. Immunol. 1995, 154, 4950–4955. [Google Scholar] [CrossRef] [PubMed]
- de Wolf, J.T.; Beentjes, J.A.M.; Esselink, M.T.; Smit, J.W.; Halie, M.R.; Vellenga, E. Interleukin-4 suppresses the interleukin-3 dependent erythroid colony formation from normal human bone marrow cells. Br. J. Haematol. 1990, 74, 246–250. [Google Scholar] [CrossRef]
- Sonoda, Y.; Okuda, T.; Yokota, S.; Maekawa, T.; Shizumi, Y.; Nishigaki, H.; Misawa, S.; Fujii, H.; Abe, T. Actions of human interleukin-4/B-cell stimulatory factor-1 on proliferation and differentiation of enriched hematopoietic progenitor cells in culture. Blood 1997, 96, 781–789. [Google Scholar]
- Wang, Y.; Gao, A.; Zhao, H.; Lu, P.; Cheng, H.; Dong, F.; Gong, Y.; Ma, S.; Zheng, Y.; Zhang, H.; et al. Leukemia cell infiltration causes defective erythropoiesis partially through MIP-1α/CCL3. Leukemia 2016, 30, 1897–1908. [Google Scholar] [CrossRef]
- Shynlova, O.; Nedd-Roderique, T.; Li, Y.; Dorogin, A.; Lye, S.J. Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. J. Cell. Mol. Med. 2013, 17, 90–102. [Google Scholar] [CrossRef]
- Melsen, J.E.; van Ostaijen-Ten Dam, M.M.; Lankester, A.C.; Schilham, M.W.; van den Akker, E.B. A comprehensive workflow for applying single-cell clustering and pseudotime analysis to flow cytometry data. J. Immunol. 2020, 205, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Höllt, T.; Pezzotti, N.; van Unen, V.; Koning, F.; Eisemann, E.; Lelieveldt, B.; Vilanova, A. Cytosplore: Interactive immune cell phenotyping for large single-cell datasets. Comput. Graph. Forum 2016, 35, 171–180. [Google Scholar] [CrossRef]
- Bedre, R. Bioinformatics data analysis and visualization toolkit (2.0.9). Zenodo 2022. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, X.; Peltz, G. GSEApy: A comprehensive package for performing gene set enrichment analysis in Python. Bioinformatics 2023, 39, btac757. [Google Scholar] [CrossRef]


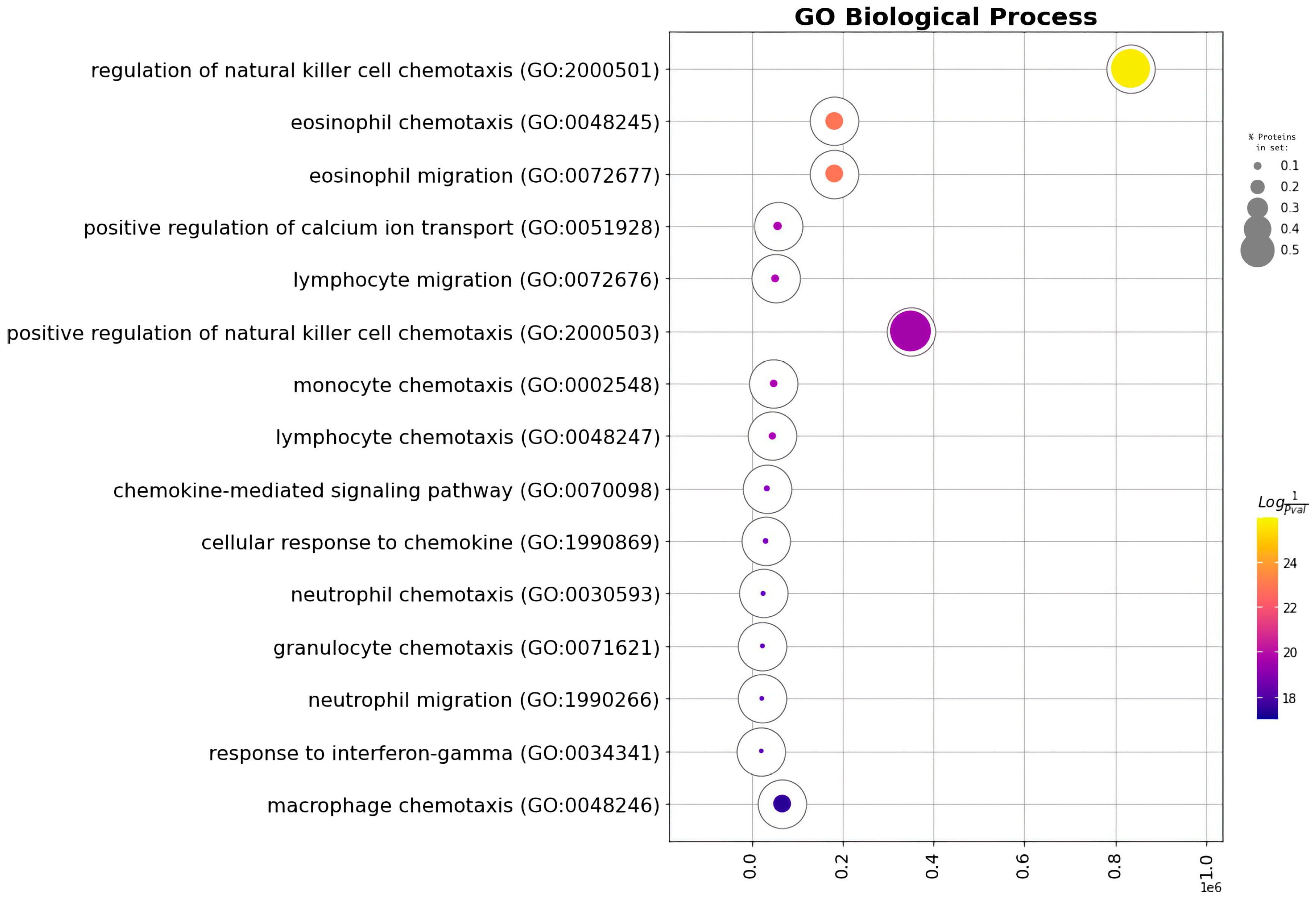
| GO Term | Overlap | Q-Value | Score | Proteins |
|---|---|---|---|---|
| Chemokine-mediated signaling pathway | 4/56 | 0.000000536 | 1,884,465 | CCL4, CCL3, CCL2, CXCL1 |
| Cellular response to chemokine | 4/60 | 0.00000536 | 1,861,481 | CCL4, CCL3, CCL2, CXCL1 |
| Regulation of natural killer cell chemotaxis | 3/7 | 0.00000536 | 344,521.8 | CCL4, CCL3, CCL2 |
| Neutrophil chemotaxis | 4/70 | 0.00000536 | 1,810,210 | CCL4, CCL3, CCL2, CXCL1 |
| Granulocyte chemotaxis | 4/73 | 0.00000536 | 1,796,267 | CCL4, CCL3, CCL2, CXCL1 |
| Neutrophil migration | 4/77 | 0.000000555 | 1,778,550 | CCL4, CCL3, CCL2, CXCL1 |
| Eosinophil chemotaxis | 3/16 | 0.00000344 | 93,174.6 | CCL4, CCL3, CCL2 |
| Eosinophil migration | 3/16 | 0.00000344 | 93,174.6 | CCL4, CCL3, CCL2 |
| Inflammatory response | 4/230 | 0.0000311 | 1,414,560 | CCL4, CCL3, CCL2, CXCL1 |
| Positive regulation of calcium ion transport | 3/37 | 0.0000382 | 30,956.93 | CCL4, CCL3, CCL2 |
| Lymphocyte migration | 3/40 | 0.0000441 | 28,054.03 | CCL4, CCL3, CCL2 |
| Monocyte chemotaxis | 3/42 | 0.0000441 | 26,382.39 | CCL4, CCL3, CCL2 |
| Lymphocyte chemotaxis | 3/44 | 0.0000005 | 24,884.33 | CCL4, CCL3, CCL2 |
| Response to interferon-gamma | 3/80 | 0.000288 | 11,810.81 | CCL4, CCL3, CCL2 |
| Positive regulation of natural killer cell chemotaxis | 2/5 | 0.000314 | 100,096.6 | CCL4, CCL3 |
| Response to interleukin-1 | 3/86 | 0.000314 | 10,795.79 | CCL4, CCL3, CCL2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarov, K.; Perik-Zavodskii, R.; Perik-Zavodskaia, O.; Alrhmoun, S.; Volynets, M.; Shevchenko, J.; Sennikov, S. Murine Placental Erythroid Cells Are Mainly Represented by CD45+ Immunosuppressive Erythroid Cells and Secrete CXCL1, CCL2, CCL3 and CCL4 Chemokines. Int. J. Mol. Sci. 2023, 24, 8130. https://doi.org/10.3390/ijms24098130
Nazarov K, Perik-Zavodskii R, Perik-Zavodskaia O, Alrhmoun S, Volynets M, Shevchenko J, Sennikov S. Murine Placental Erythroid Cells Are Mainly Represented by CD45+ Immunosuppressive Erythroid Cells and Secrete CXCL1, CCL2, CCL3 and CCL4 Chemokines. International Journal of Molecular Sciences. 2023; 24(9):8130. https://doi.org/10.3390/ijms24098130
Chicago/Turabian StyleNazarov, Kirill, Roman Perik-Zavodskii, Olga Perik-Zavodskaia, Saleh Alrhmoun, Marina Volynets, Julia Shevchenko, and Sergey Sennikov. 2023. "Murine Placental Erythroid Cells Are Mainly Represented by CD45+ Immunosuppressive Erythroid Cells and Secrete CXCL1, CCL2, CCL3 and CCL4 Chemokines" International Journal of Molecular Sciences 24, no. 9: 8130. https://doi.org/10.3390/ijms24098130
APA StyleNazarov, K., Perik-Zavodskii, R., Perik-Zavodskaia, O., Alrhmoun, S., Volynets, M., Shevchenko, J., & Sennikov, S. (2023). Murine Placental Erythroid Cells Are Mainly Represented by CD45+ Immunosuppressive Erythroid Cells and Secrete CXCL1, CCL2, CCL3 and CCL4 Chemokines. International Journal of Molecular Sciences, 24(9), 8130. https://doi.org/10.3390/ijms24098130





