Silencing of the Laccase (lacc2) Gene from Pleurotus ostreatus Causes Important Effects on the Formation of Toxocyst-like Structures and Fruiting Body
Abstract
1. Introduction
2. Results
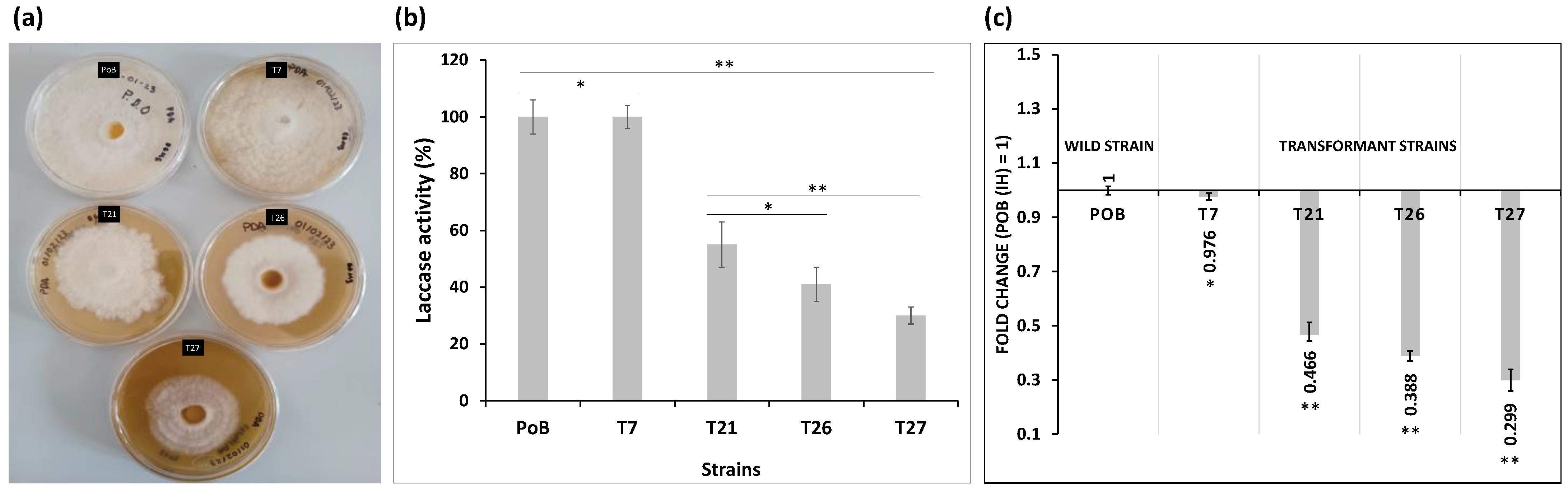
2.1. Evaluation of Laccase (lacc2) Gene Expression
2.2. Expression of the lacc2 Gene in Different Structures of P. ostreatus and Transformant (T7)
2.3. Growth Analysis of P. ostreatus and Transformants in Solid Culture
2.3.1. Effect on Mycelium
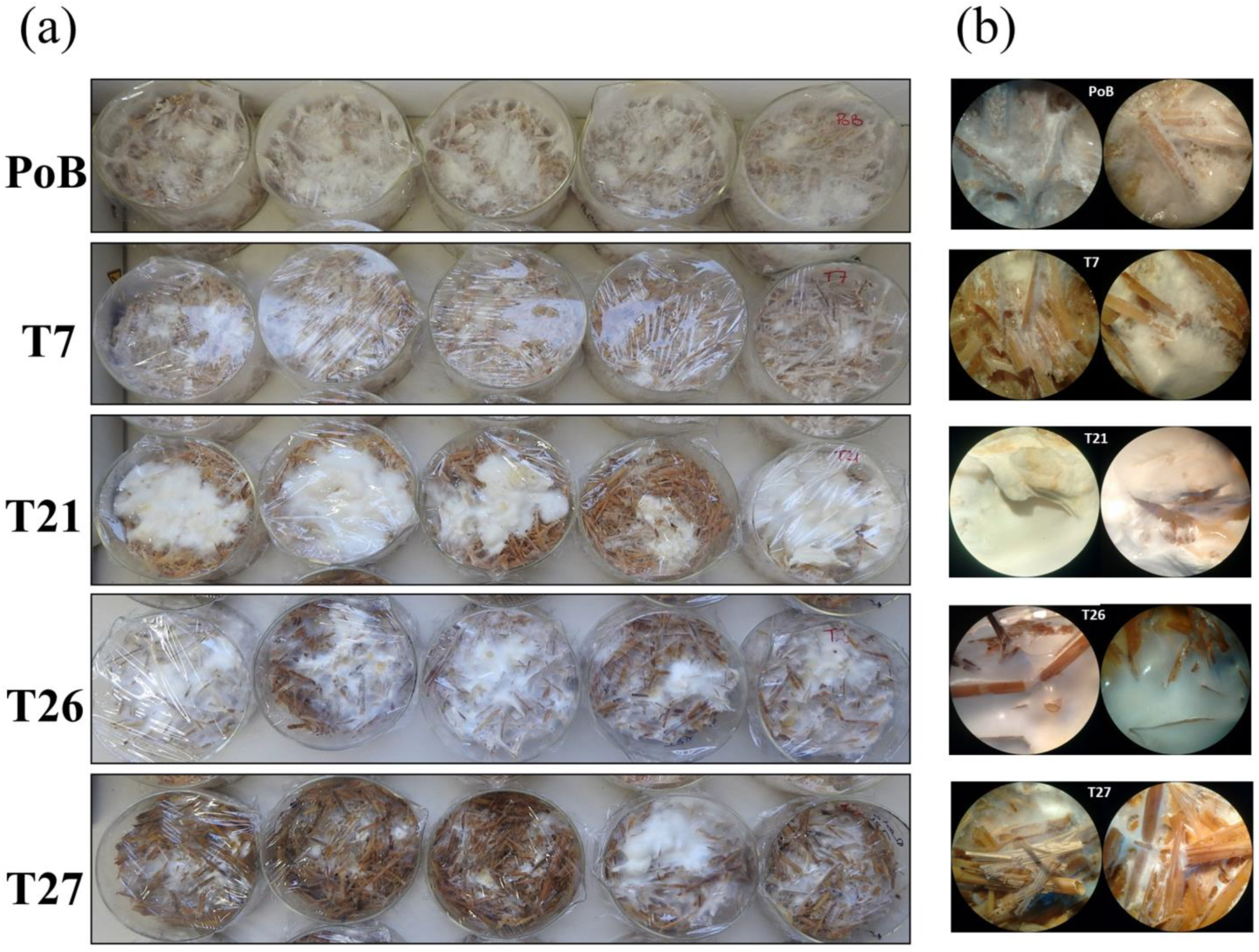
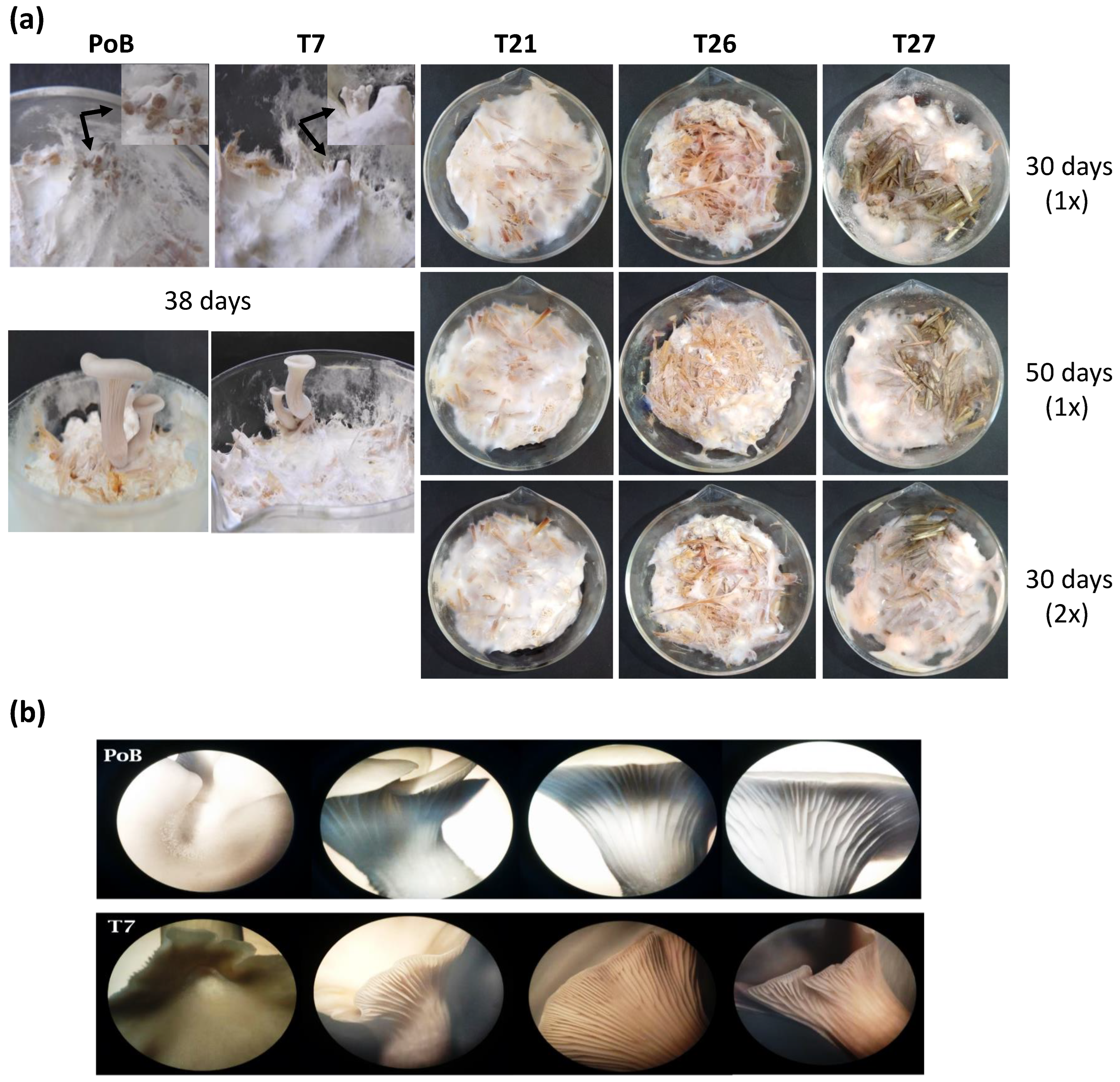
2.3.2. Effect on the Formation of Primordia and Fruiting Bodies
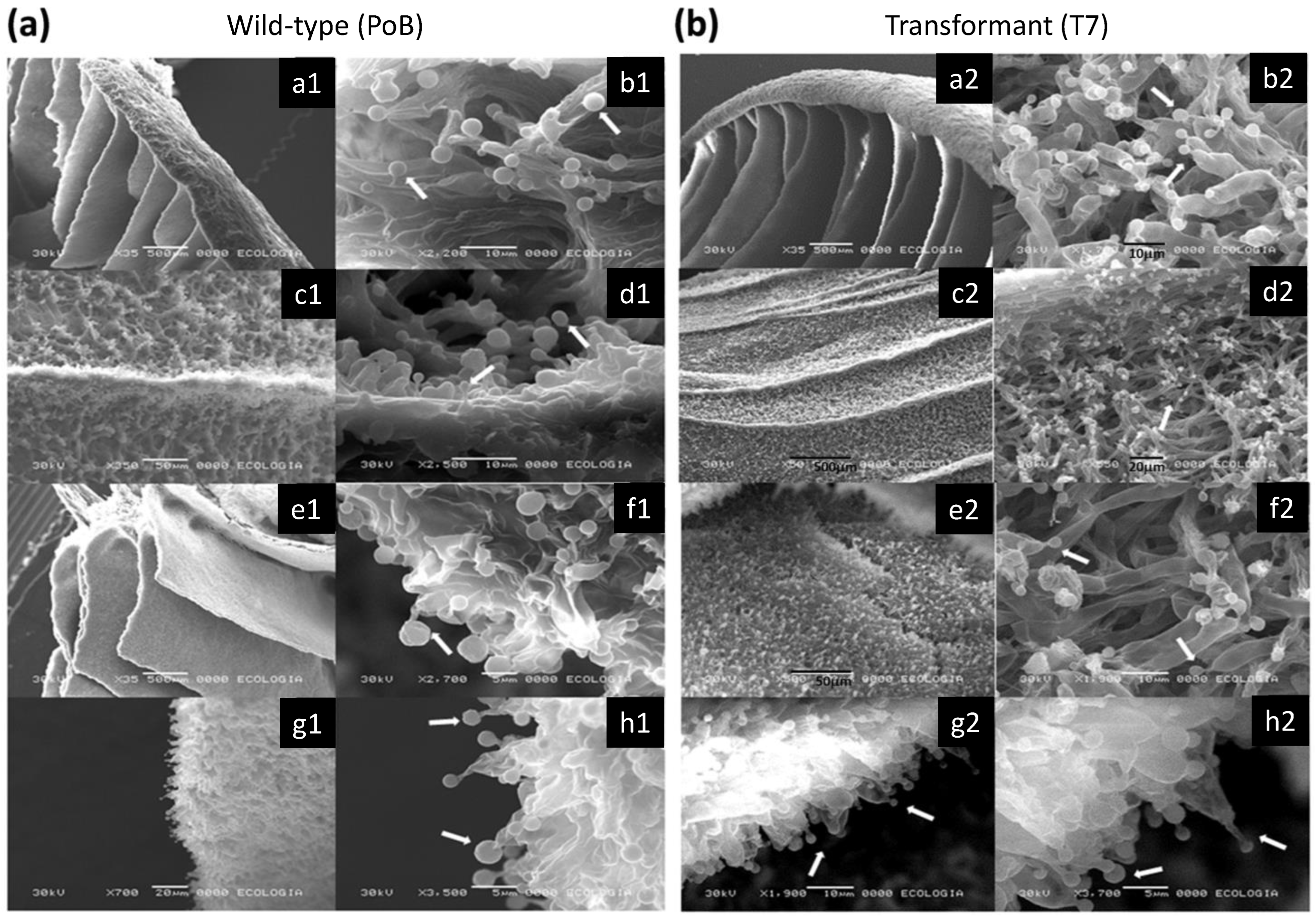
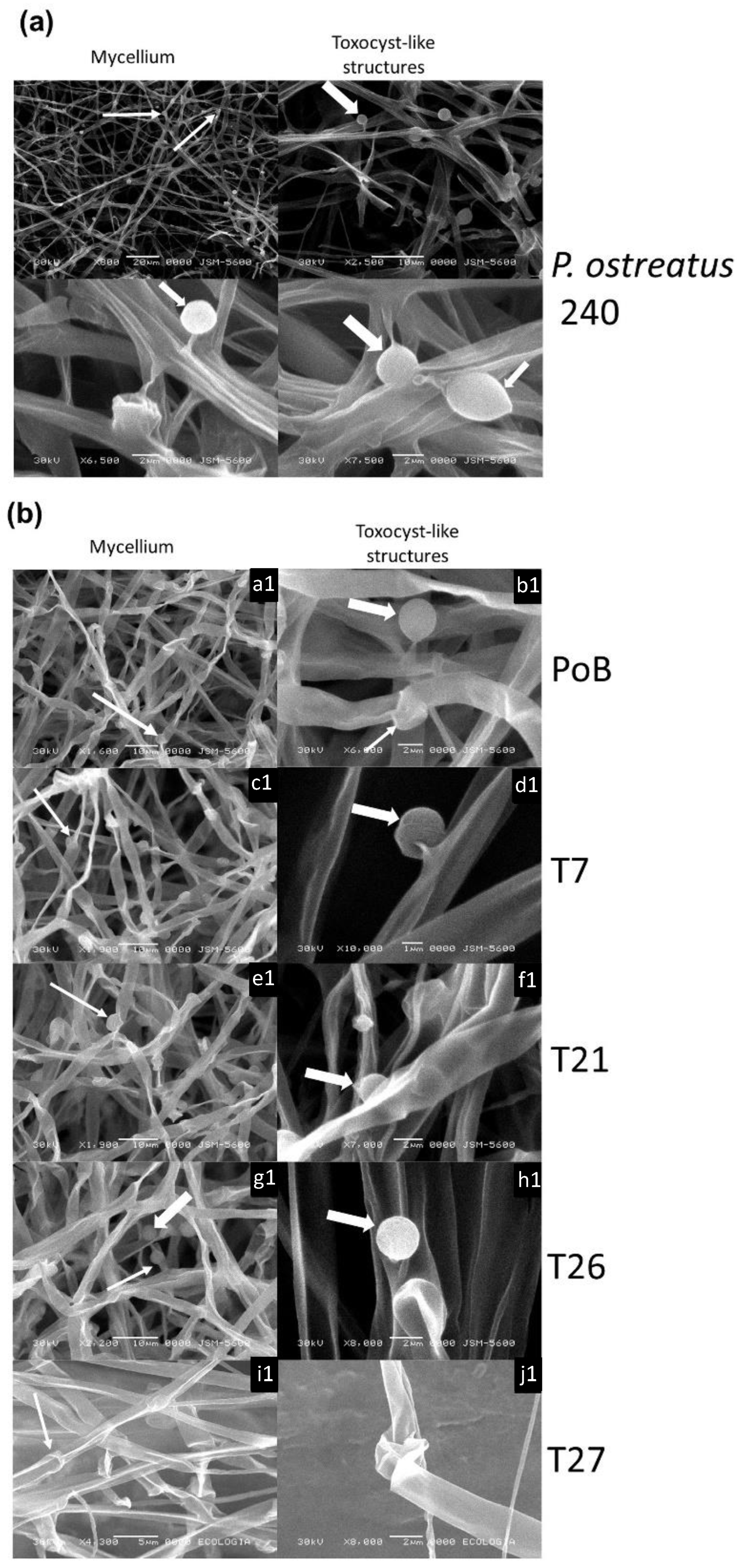
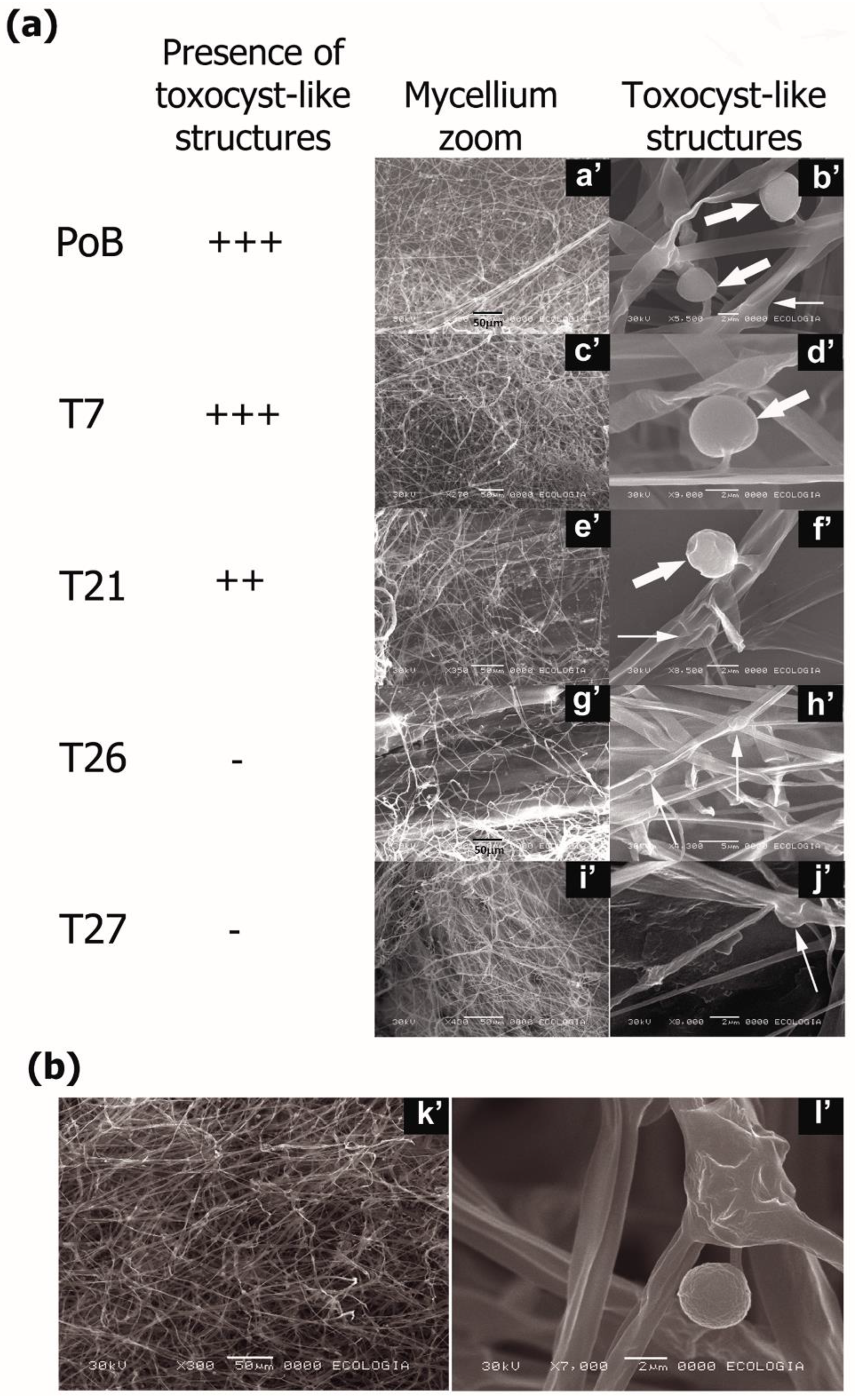
2.4. Effect of Laccase Gene Silencing on Micro- and Ultrastructure of Fruiting Bodies
2.5. Effect of Laccase Gene Silencing on Mycelial Ultrastructure
3. Discussion
4. Materials and Methods
4.1. Fungal Strains
4.2. Culture Conditions
4.3. Biomass Determination of P. ostreatus PoB and Transformant Strains
4.4. Extracellular Laccase Activity Determination
4.5. RNA Extraction
4.6. cDNA Synthesis and PCR
4.7. Macro- and Microscopic Morphology Evaluation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Sharma, A.; Tripathi, A. Biological activities of Pleurotus spp. polysaccharides: A review. J. Food Biochem. 2021, 45, e13748. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2020, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sekan, A.S.; Myronycheva, O.S.; Karlsson, O.; Gryganskyi, A.P.; Blume, Y. Green potential of Pleurotus spp. in biotechnology. PeerJ 2019, 7, e6664. [Google Scholar] [CrossRef] [PubMed]
- Werghemmi, W.; Fayssal, S.A.; Mazouz, H.; Hajjaj, H.; Hajji, L. Olive and green tea leaves extract in Pleurotus ostreatus var. florida culture media: Effect on mycelial linear growth rate, diameter and growth induction index. IOP Conf. Ser. Earth Environ. Sci. 2022, 1090, 012020. [Google Scholar] [CrossRef]
- Elbagory, M.; El-Nahrawy, S.; Omara, A.E.-D.; Eid, E.M.; Bachheti, A.; Kumar, P.; Abou Fayssal, S.; Adelodun, B.; Bachheti, R.K.; Kumar, P.; et al. Sustainable Bioconversion of Wetland Plant Biomass for Pleurotus ostreatus var. florida Cultivation: Studies on Proximate and Biochemical Characterization. Agriculture 2022, 12, 2095. [Google Scholar] [CrossRef]
- Curran, L.M.C.L.K.; Pham, L.T.M.; Sale, K.L.; Simmons, B.A. Review of advances in the development of laccases for the valorization of lignin to enable the production of lignocellulosic biofuels and bioproducts. Biotechnol. Adv. 2022, 54, 107809. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Bilal, M.; Bagheri, A.R.; Vilar, D.S.; Aramesh, N.; Eguiluz, K.I.B.; Ferreira, L.F.R.; Ashraf, S.S.; Iqbal, H.M.N. Oxidoreductases as a versatile biocatalytic tool to tackle pollutants for clean environment—A review. J. Chem. Technol. Biotechnol. 2022, 97, 420–435. [Google Scholar] [CrossRef]
- Vilar, D.D.S.; Bilal, M.; Bharagava, R.N.; Kumar, A.; Nadda, A.K.; Salazar-Banda, G.R.; Eguiluz, K.I.B.; Ferreira, L.F.R. Lignin-modifying enzymes: A green and environmental responsive technology for organic compound degradation. J. Chem. Technol. Biotechnol. 2021, 97, 327–342. [Google Scholar] [CrossRef]
- Montiel-González, A.M.; Marcial, J. Phenoloxidases of Fungi and Bioremediation. In Fungal Bioremediation, Fundamentals and Applications; Tomasini, A., León-Santiesteban, H., Eds.; CRC Press: London, UK; New York, NY, USA, 2019; pp. 62–90. [Google Scholar]
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various types and applications. Biotechnol. Appl. Biochem. 2022, 69, 2658–2672. [Google Scholar] [CrossRef]
- Loi, M.; Glazunova, O.; Fedorova, T.; Logrieco, A.F.; Mulè, G. Fungal Laccases: The Forefront of Enzymes for Sustainability. J. Fungi 2021, 7, 1048. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Li, G.; Wang, Y.; Nie, F.; Cheng, X.; Abdullah, M.; Lin, Y.; Cai, Y. Systematic Analysis of the Pleurotus ostreatus Laccase Gene (PoLac) Family and Functional Characterization of PoLac2 Involved in the Degradation of Cotton-Straw Lignin. Molecules 2018, 23, 880. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Sun, S.J.; Liu, J.Z.; Hu, K.H.; Zhu, H.X. The level of secreted laccase activity in the edible fungi and their growing cycles are closely related. Curr. Microbiol. 2011, 62, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Castanera, R.; Pérez, G.; Omarini, A.; Alfaro, M.; Pisabarro, A.G.; Faraco, V.; Amore, A.; Ramírez, L. Transcriptional and enzymatic profiling of Pleurotus ostreatus laccase genes in submerged and solid-state fermentation cultures. Appl. Environ. Microbiol. 2012, 78, 4037–4045. [Google Scholar] [CrossRef]
- Pezzella, C.; Lettera, V.; Piscitelli, A.; Giardina, P.; Sannia, G. Transcriptional analysis of Pleurotus ostreatus laccase genes. Appl. Microbiol. Biotechnol. 2013, 97, 705–717. [Google Scholar] [CrossRef]
- Lax, C.; Tahiri, G.; Patiño-Medina, J.A.; Cánovas-Márquez, J.T.; Pérez-Ruiz, J.A.; Osorio-Concepción, M.; Navarro, E.; Calo, S. The Evolutionary Significance of RNAi in the Fungal Kingdom. Int. J. Mol. Sci. 2020, 21, 9348. [Google Scholar] [CrossRef]
- Nakade, K.; Watanabe, H.; Sakamoto, Y.; Sato, T. Gene silencing of the Lentinula edodes lcc1 gene by expression of a homologous inverted repeat sequence. Microbiol. Res. 2011, 166, 484–493. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Nakade, K.; Sato, S.; Yoshimi, A.; Sasaki, K.; Konno, N.; Abe, K. Cell wall structure of secreted laccase-silenced strain in Lentinula edodes. Fungal Biol. 2018, 122, 1192–1200. [Google Scholar] [CrossRef]
- Jin, W.; Li, J.; Feng, H.; You, S.; Zhang, L.; Norvienyeku, J.; Hu, K.; Sun, S.; Wang, Z. Importance of a Laccase Gene (Lcc1) in the Development of Ganoderma tsugae. Int. J. Mol. Sci. 2018, 19, 471. [Google Scholar] [CrossRef]
- Ma, S.X.; Cao, K.K.; Liu, N.; Meng, C.; Cao, Z.Y.; Dai, D.Q.; Jia, H.; Zang, J.P.; Li, Z.Y.; Hao, Z.M.; et al. The StLAC2 gene is required for cell wall integrity, DHN-melanin synthesis and the pathogenicity of Setosphaeria turcica. Fungal Biol. 2017, 121, 589–601. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Q.; Jia, H.; Zhao, B.; Zhu, Z.; Cao, Z.; Dong, J. Characterization of laccase gene StLAC6 and its involvement in the pathogenicity and peroxisome function in Setosphaeria turcica. J. Integr. Agric. 2022, 21, 2019–2030. [Google Scholar] [CrossRef]
- Armas-Tizapantzi, A.; Marcial, J.; Fernández, F.J.; Estrada Torres, A.; Pérez-Godínez, E.A.; Montiel-González, A.M. Laccase gene silencing negatively effects growth and development in Pleurotus ostreatus. IJBT 2019, 18, 42–51. [Google Scholar]
- Heydari, R.; Pourjam, E.; Goltapeh, E.M. Antagonistic effect of some species of Pleurotus on the root-knot nematode, Meloidogyne javanica in vitro. Plant Pathol. J. 2006, 5, 173–177. [Google Scholar] [CrossRef]
- Truong, B.N.; Okazaki, K.; Fukiharu, T.; Takeuchi, Y.; Futai, K.; Le, X.T.; Suzuki, A. Characterization of the nematocidal toxocyst in Pleurotus subgen. Coremiopleurotus. Mycoscience 2007, 48, 222–230. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, Y.Y.; Chang, Y.C.; Pon, W.L.; Lee, S.P.; Wali, N.; Nakazawa, T.; Honda, Y.; Shie, J.J.; Hsueh, Y.P. A carnivorous mushroom paralyzes and kills nematodes via a volatile ketone. Sci. Adv. 2023, 9, eade4809. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Shahane, S.; Shivam; Majumdar, R.; Mishra, U. Mode of Action, Properties, Production, and Application of Laccase: A Review. Recent Pat. Biotechnol. 2019, 13, 19–32. [Google Scholar] [CrossRef]
- González-González, P.; Gómez-Manzo, S.; Tomasini, A.; Pérez, J.L.M.Y.; Nieto, E.G.; Anaya-Hernández, A.; Ortiz Ortiz, E.; Rodríguez, R.A.C.; Marcial-Quino, J.; Montiel-González, A.M. Laccase Production from Agrocybe pediades: Purification and Functional Characterization of a Consistent Laccase Isoenzyme in Liquid Culture. Microorganisms 2023, 11, 568. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; de Los Santos, M.H.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Factories 2019, 18, 200. [Google Scholar] [CrossRef]
- Goudopoulou, A.; Krimitzas, A.; Typas, M.A. Differential gene expression of ligninolytic enzymes in Pleurotus ostreatus grown on olive oil mill wastewater. Appl. Microbiol. Biotechnol. 2010, 88, 541–551. [Google Scholar] [CrossRef]
- Piscitelli, A.; Giardina, P.; Lettera, V.; Pezzella, C.; Sannia, G.; Faraco, V. Induction and transcriptional regulation of laccases in fungi. Curr. Genom. 2011, 12, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Martínez-Morales, F.; Trejo-Hernández, M.R. Fungal laccases: Induction and production. Rev. Mex. Ing. Química 2013, 12, 473–488. [Google Scholar]
- Yang, J.; Wang, G.; Ng, T.B.; Lin, J.; Ye, X. Laccase Production and Differential Transcription of Laccase Genes in Cerrena sp. in Response to Metal Ions, Aromatic Compounds, and Nutrients. Front. Microbiol. 2016, 6, 1558. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Vázquez, A.; Tomasini, A.; Armas-Tizapantzi, A.; Marcial-Quino, J.; Montiel-González, A.M. Extracellular proteases and laccases produced by Pleurotus ostreatus PoB: The effects of proteases on laccase activity. Int. Microbiol. 2022, 25, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Leatham, G.F.; Stahmann, M.A. Studies on the laccase of Lentinus edodes: Specificity, localization and association with the development of fruiting bodies. Microbiology 1981, 125, 147–157. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Chen, H.; Chen, M.; Ren, A.; Huang, J.; Wang, H.; Zhao, M.; Feng, Z. Cloning and functional analysis of a laccase gene during fruiting body formation in Hypsizygus marmoreus. Microbiol. Res. 2015, 179, 54–63. [Google Scholar] [CrossRef]
- Das, N.; Sengupta, S.; Mukherjee, M. Importance of laccase in vegetative growth of Pleurotus florida. Appl. Environ. Microbiol. 1997, 63, 4120–4122. [Google Scholar] [CrossRef]
- Cesur, A.; Yamamoto, R.; Asada, Y.; Watanabe, A. Relationship between fruiting body development and extracellular laccase production in the edible mushroom Flammulina velutipes. Biochem. Biophys. Rep. 2022, 29, 101204. [Google Scholar] [CrossRef]
- Durán-Sequeda, D.; Suspes, D.; Maestre, E.; Alfaro, M.; Perez, G.; Ramírez, L.; Pisabarro, A.G.; Sierra, R. Effect of Nutritional Factors and Copper on the Regulation of Laccase Enzyme Production in Pleurotus ostreatus. J. Fungi 2021, 8, 7. [Google Scholar] [CrossRef]
- Janusz, G.; Kucharzyk, K.H.; Pawlik, A.; Staszczak, M.; Paszczynski, A.J. Fungal laccase, manganese peroxidase and lignin peroxidase: Gene expression and regulation. Enzym. Microb. Technol. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Alfaro, M.; Castanera, R.; Lavín, J.L.; Grigoriev, I.V.; Oguiza, J.A.; Ramírez, L.; Pisabarro, A.G. Comparative and transcriptional analysis of the predicted secretome in the lignocellulose-degrading basidiomycete fungus Pleurotus ostreatus. Environ. Microbiol. 2016, 18, 4710–4726. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.K.; Das, N.; Sengupta, S.; Mukherjee, M. Accumulation of a natural substrate of laccase in gills of Pleurotus florida during sporulation. Curr. Microbiol. 2000, 41, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Armas-Tizapantzi, A.; Mata, G.; Hernández-Cuevas, L.V.; Montiel-González, A.M. Estructuras tipo toxocistos en Pleurotus ostreatus y P. pulmonarius. Sci. Fungorum 2019, 49, e1250. [Google Scholar] [CrossRef]
- Kwok, O.C.H.; Plattner, R.; Weisleder, D.; Wicklow, D.T. A nematicidal toxin from Pleurotus ostreatus NRRL 3526. J. Chem. Ecol. 1992, 18, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Sainos, E.; Díaz-Godínez, G.; Loera, O.; Montiel-González, A.M.; Sánchez, C. Growth of Pleurotus ostreatus on wheat straw and wheat-grain-based media: Biochemical aspects and preparation of mushroom inoculum. Appl. Microbiol. Biotechnol. 2006, 72, 812–815. [Google Scholar] [CrossRef]
- Scotti, C.T.; Vergoignan, C.; Feron, G.; Durand, A. Glucosamine measurement as indirect method for biomass estimation of Cunninghamella elegans grown in solid state cultivation conditions. Biochem. Eng. J. 2001, 7, 1–5. [Google Scholar] [CrossRef]
- Marcial, J.; Barrios-González, J.; Tomasini, A. Effect of medium composition on pentachlorophenol removal by Amylomyces rouxii in solid-state culture. Process Biochem. 2006, 41, 496–500. [Google Scholar] [CrossRef]

| Strain | Days to Fruiting | Stipe Diameter (mm) | Stipe Height (mm) | Pileus Width (mm) | Pileus Length (mm) | Fresh Weight (g) |
|---|---|---|---|---|---|---|
| PoB | 36.6 ± 0.89 a | 9.022 ± 1.11 a | 75.82 ± 6.16 a | 27.58 ± 3.56 a | 26.33 ±6.01 a | 4.4146 ± 1.26 a |
| T7 | 39 ± 4.6 a | 8.134 ± 0.91 a | 64.3 ± 9.79 a | 26.69 ± 3.34 a | 24.8 ± 4.38 a | 3.4921 ± 0.88 a |
| p = 0.294 | p = 0.206 | p = 0.057 | p = 0.692 | p = 0.660 | p = 0.219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armas-Tizapantzi, A.; Martínez y Pérez, J.L.; Fernández, F.J.; Mata, G.; Hernández-Cuevas, L.V.; Ortiz Ortiz, E.; García Nieto, E.; Tomasini, A.; Sierra-Palacios, E.; Marcial-Quino, J.; et al. Silencing of the Laccase (lacc2) Gene from Pleurotus ostreatus Causes Important Effects on the Formation of Toxocyst-like Structures and Fruiting Body. Int. J. Mol. Sci. 2023, 24, 8143. https://doi.org/10.3390/ijms24098143
Armas-Tizapantzi A, Martínez y Pérez JL, Fernández FJ, Mata G, Hernández-Cuevas LV, Ortiz Ortiz E, García Nieto E, Tomasini A, Sierra-Palacios E, Marcial-Quino J, et al. Silencing of the Laccase (lacc2) Gene from Pleurotus ostreatus Causes Important Effects on the Formation of Toxocyst-like Structures and Fruiting Body. International Journal of Molecular Sciences. 2023; 24(9):8143. https://doi.org/10.3390/ijms24098143
Chicago/Turabian StyleArmas-Tizapantzi, Anahí, José Luis Martínez y Pérez, Francisco José Fernández, Gerardo Mata, Laura V. Hernández-Cuevas, Elvia Ortiz Ortiz, Edelmira García Nieto, Araceli Tomasini, Edgar Sierra-Palacios, Jaime Marcial-Quino, and et al. 2023. "Silencing of the Laccase (lacc2) Gene from Pleurotus ostreatus Causes Important Effects on the Formation of Toxocyst-like Structures and Fruiting Body" International Journal of Molecular Sciences 24, no. 9: 8143. https://doi.org/10.3390/ijms24098143
APA StyleArmas-Tizapantzi, A., Martínez y Pérez, J. L., Fernández, F. J., Mata, G., Hernández-Cuevas, L. V., Ortiz Ortiz, E., García Nieto, E., Tomasini, A., Sierra-Palacios, E., Marcial-Quino, J., & Montiel-González, A. M. (2023). Silencing of the Laccase (lacc2) Gene from Pleurotus ostreatus Causes Important Effects on the Formation of Toxocyst-like Structures and Fruiting Body. International Journal of Molecular Sciences, 24(9), 8143. https://doi.org/10.3390/ijms24098143







