Supplementary UV-B Radiation Effects on Photosynthetic Characteristics and Important Secondary Metabolites in Eucommia ulmoides Leaves
Abstract
1. Introduction
2. Results
2.1. Leaf Anatomy Analysis
2.2. Effect of sUV-B Radiation on the Growth Indexes of E. ulmoides
2.3. Effects of sUV-B Radiation on Photosynthesis of E. ulmoides
2.4. Changes in the Content of Secondary Metabolites of E. ulmoides
2.5. Correlation Analysis
2.6. Denovo Assembly and Sequence Annotation
2.7. Sequence Annotation and Classification
2.8. Functional Classification of Differentially Expressed Genes
2.9. DEGs Related to Plant Auxin and Phenylpropanoid Biosynthesis
2.10. Validation and Expression Analyses of Key Enzyme Genes
3. Discussion
4. Materials and Methods
4.1. Test Materials and Treatment Conditions
4.2. Measurement of Physiological Indexes of E. ulmoides
4.2.1. Number of Branches, Number of Leaves, Leaf Length, Leaf Width, Leaf Area and Thickness Statistics of E. ulmoides
4.2.2. Leaf Anatomy
4.2.3. Measurement of Dry Weight and Fresh Weight of Leaves
4.2.4. Measurement of Photosynthetic Parameters
4.2.5. Measurement of Photosynthetic Pigments
4.2.6. Measurement of Chlorophyll Fluorescence Parameters
4.3. Determination of Secondary Metabolite Content of E. ulmoides
4.4. cDNA Library Preparation and Illumina Sequencing for Transcriptome Analysis
4.5. Denovo Assembly and Functional Annotation Analysis of Illumina Sequencing
4.6. Identification of Differentially Expressed Genes (DEGs)
4.7. Quantitative Real-Time PCR Validation
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.R.; Wang, J.H.; Li, M.X.; Hao, D.J.; Yang, Y.; Zhang, C.L.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Lentini, G. Plant-derived anticancer agents: Lessons from the pharmacology of geniposide and its aglycone genipin. Biomedicines 2018, 6, 2. [Google Scholar] [CrossRef]
- Trim, A.R.; Hill, R. The preparation and properties of aucubin, asperuloside and some related glycosides. Biochem. J. 1952, 50, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Oyebanji, M.; Dong, X.; Zhang, Q.Q.; Yao, G.H.; Huang, Q. Determination of chlorogenic acid in Eucommia ulmoides Oliver extracts by near-infrared spectroscopy. Spectrosc. Lett. 2021, 54, 571–580. [Google Scholar] [CrossRef]
- Bacelar, E.; Moutinho-Pereira, J.; Ferreira, H.; Correia, C. Enhanced Ultraviolet-B Radiation Affect Growth, Yield and Physiological Processes on Triticale Plants. Procedia Environ. Sci. 2015, 29, 219–220. [Google Scholar] [CrossRef]
- Ahmad, P.; Prasad, M. UV-B Radiation, its effects and defense mechanisms in terrestrial plants. Environ. Adapt. Stress Toler. Plants Era Clim. Chang. 2012, 57–83. [Google Scholar] [CrossRef]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef]
- Mishra, P.; Prasad, S.M. Low dose UV-B radiation induced mild oxidative stress impact on physiological and nutritional competence of Spirulina (Arthrospira) species. Plant Stress 2021, 2, 100039. [Google Scholar] [CrossRef]
- Geraldo Rogério, F.C.; Vinícius, N.G.; Leonardo, Z.; Elias, T.W.; José Eduardo, M.P. UV-B effects on growth, photosynthesis, total antioxidant potential and cell wall components of shade-tolerant and sun-tolerant ecotypes of Paubrasilia echinate. Flora 2020, 271, 151679. [Google Scholar]
- Mohammad, K.; Abdolamir, R.; Shapor, L.; Lack, S. Physiological Indices, Phenological Characteristics and Trait Evaluation of Canola Genotypes Response to Different Planting Dates. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 153–163. [Google Scholar]
- Monika, R.; Kusum, A.; Ayyandar, A.; Juha, M.A.; Rajiv, P. Assessment of leaf morphological, physiological, chemical and stoichiometry functional traits for understanding the functioning of Himalayan temperate forest ecosystem. Sci. Rep. 2021, 11, 23807. [Google Scholar]
- Sakalauskaite, J.; Viskelis, P.; Duchovskis, P.; Dambrauskiene, E.; Sakalauskiene, S.; Samuoliene, G.; Brazaityte, A. Supplementary UV-B irradiation effects on basil (Ocimum basilicum L.) growth and phytochemical properties. Food Agric. Environ. 2012, 10, 342–346. [Google Scholar]
- Singh, S.; Sarkar, A.; Agrawal, S.B.; Agrawal, M. Impact of ambient and supplemental ultraviolet-B stress on kidney bean plants: An insight into oxidative stress management. Protoplasma 2014, 251, 1395–1405. [Google Scholar] [CrossRef]
- Dotto, M.; Casati, P. Developmental reprogramming by UV-B radiation in plants. Plant Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef]
- Gonçalves, B.; Correia, C.M.; Silva, A.P.; Bacelar, E.A.; Santos, A.; Moutinho-Pereira, J.M. Leaf structure and function of sweet cherry tree (Prunus avium L.) cultivars with open and dense canopies. Sci. Hortic. 2008, 116, 381–387. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, V.; Singh, V.; Varadwaj, P. Extrapolation of significant genes and transcriptional regulatory networks involved in Zea mays in response in UV-B stress. Genes Genom. 2018, 40, 973–990. [Google Scholar] [CrossRef]
- Arróniz-Crespo, M.; Gwynn-Jones, D.; Callaghan, T.V.; Núñez-Olivera, E.; Martínez-Abaigar, J.; Horton, P.; Phoenix, G.K. Impacts of long-term enhanced UV-B radiation on bryophytes in two sub-Arctic heathland sites of contrasting water availability. Ann. Bot. 2011, 108, 557–565. [Google Scholar] [CrossRef]
- Sakalauskaitė, J.; Viskelis, P.; Dambrauskienė, E.; Sakalauskienė, S.; Samuolienė, G.; Brazaitytė, A.; Duchovskis, P.; Urbonavičienė, D. The effects of different UV-B radiation intensities on morphological and biochemical characteristics in Ocimum basilicum L. J. Sci. Food agric. 2012, 93, 1266–1271. [Google Scholar] [CrossRef]
- Pandey, J.; Chaplot, K. Effects of enhanced UV-B radiation on physiological and biochemical characteristics of wheat. Res. Crops 2007, 8, 401–405. [Google Scholar]
- Fujimura, S.; Shi, P.; Iwama, K.; Zhang, X.Z.; Gopal, J.; Jitsuyama, Y. Effect of altitude on the response of net photosynthetic rate to carbon dioxide increase by spring wheat. Plant Prod. Sci. 2010, 13, 141–149. [Google Scholar] [CrossRef]
- Diogo, D.P.; Hélio, N.M.; Amélia, T.H.; Luís Mauro, G.R.; Janette, P.F.; Germano, F.N. The alkaloid brachycerine contributes to protection against acute UV-B damage in Psychotria. Ind. Crops Prod. 2020, 147, 112216. [Google Scholar]
- Takshak, S.; Agrawal, S.B. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol. B Biol. 2019, 193, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fang, M.F.; Yue, M.; Chai, Y.F.; Wang, H.; Li, Y.F. Advances in influence of UV-Bradiation on medicinal plant secondary metabolism. China J. Chin. Mater. Med. 2012, 37, 2248–2251. [Google Scholar]
- Chakraborty, K.; Paulraj, R. Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 2010, 122, 31–41. [Google Scholar] [CrossRef]
- Unal, D.; Tuney, I.; Sukatar, A. The role of external polyamines on photosynthetic responses, lipid peroxidation, protein and chlorophyll a content under the UV-A (352 nm) stress in Physcia semipinnata. J. Photochem. Photobiol. B Biol. 2008, 90, 64–68. [Google Scholar] [CrossRef]
- Schmitz-Hoerner, R.; Weissenböck, G. Contribution of phenolic compounds to the UV-B screening capacity of developing barley primary leaves in relation to DNA damage and repair under elevated UV-B levels. Phytochemistry 2003, 64, 243–255. [Google Scholar] [CrossRef]
- Dolzhenko, Y.; Bertea, C.M.; Occhipinti, A.; Bossi, S.; Maffei, M.E. UV-B modulates the interplay between terpenoids and flavonoids in peppermint. J. Photochem. Photobiol. B Biol. 2010, 100, 67–75. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta (BBA)—Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Wargent, J.J.; Gegas, V.C.; Jenkins, G.I. UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation. New Phytol. 2019, 183, 315–326. [Google Scholar] [CrossRef]
- Apoorva; Deepanshi, J.; Shashi, P.R.; Agrawal, S.B. Untangling the UV-B radiation-induced transcriptional network regulating plant morphogenesis and secondary metabolite production. Environ. Exp. Bot. 2021, 192, 104655. [Google Scholar] [CrossRef]
- Ido, S.; Levy, M.; Naomi, O. Hormones in tomato leaf development. Dev. Biol. 2016, 419, 132–142. [Google Scholar]
- Tripathi, S.C.; Sayre, K.D.; Kaul, J.N.; Narang, R.S. Growth and morphology of spring wheat (Triticum aestivum L.) culms and their association with lodging: Effects of genotypes, N levels and ethephon. Field Crop. Res. 2003, 84, 271–290. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Luo, L.; Zheng, L.Q. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar]
- Kutchan, T.M. Predictive metabolic engineering in plants: Still full of surprises. Trends Biotechnol. 2005, 23, 381–383. [Google Scholar] [CrossRef]
- Muro-Villanueva, F.; Mao, X.Y.; Chapple, C. Linking phenylpropanoid metabolism, lignin deposition, and plant growth inhibition. Curr. Opin. Biotechnol. 2019, 56, 202–208. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Transcriptional networks for lignin biosynthesis: More complex than we thought. Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef]
- Li, Q.J.; Xue, Y.X. Total leaf area estimation based on the total grid area measured using mobile laser scanning. Comput. Electron. Agric. 2023, 204, 107503. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Chen, Y.E.; Su, Y.Q.; Zhang, C.M.; Ma, J.; Mao, H.T.; Yang, Z.H.; Yuan, M.; Zhang, Z.W.; Yuan, S.; Zhang, H.Y. Comparison of Photosynthetic Characteristics and Antioxidant Systems in Different Wheat Strains. J. Plant Growth Regul. 2018, 37, 347–359. [Google Scholar] [CrossRef]
- Zhang, Q.C. Study on the extraction technology of chemical active components from Eucommia ulmoides leaves. Liaoning Chem. Ind. 2022, 51, 1379–1381. [Google Scholar]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fang, L.; Zheng, H. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2016, 34, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Guan, Z.J.; Sun, M. Evolutionary association of stomatal traits with leaf vein density in Paphiopedilum, Orchidaceae. PLoS ONE 2012, 7, e40080. [Google Scholar] [CrossRef]
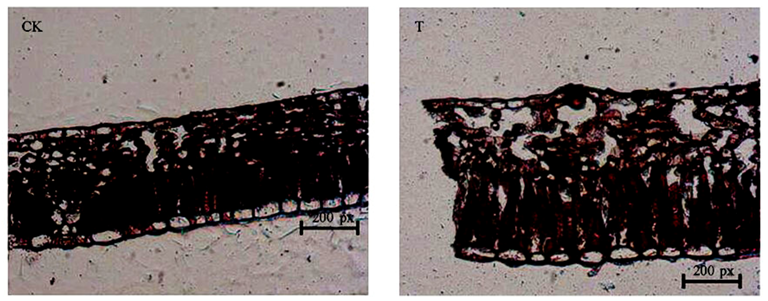






| Samples | Epidermal Cell (px) | Palisade Tissue (px) |
|---|---|---|
| CK | 80.00 ± 4 b | 145.45 ± 7.27 b |
| T | 166.67 ± 8.33 a | 364.64 ± 18.23 a |
| Treatment | Number of Leaves (Piece) | Leaf Width (cm) | Leaf Length (cm) | Leaf Area (cm2) | Leaf Thickness (mm) | Branch Number (Branch) | Leaf Fresh Weight (g) | Leaf Dry Weight (g) | Relative Water Content (%) |
|---|---|---|---|---|---|---|---|---|---|
| CK | 139.00 ± 6.95 a | 6.62 ± 0.33 a | 12.59 ± 0.63 b | 61 ± 3.05 b | 0.23 ± 0.012 b | 11 ± 1.00 b | 163.77 ± 8.19 b | 57.39 ± 2.87 b | 0.64 ± 0.008 a |
| T | 132.00 ± 6.6 a | 9.24 ± 0.46 a | 17.32 ± 0.87 a | 121 ± 6.05 a | 0.31 ± 0.016 a | 32 ± 1.15 a | 247.12 ± 12.36 a | 92.84 ± 4.64 a | 0.62 ± 0.006 a |
| Treatment | Chlorophyll a (Chl a) | Chlorophyll b (Chl b) | Carotenoid (Car) | Chlorophyll (Chl) | Chlorophyll Ratio (Chl a/b) |
|---|---|---|---|---|---|
| CK | 0.89 ± 0.055 a | 0.47 ± 0.025 a | 0.082 ± 0.006 a | 1.36 ± 0.079 a | 1.87 ± 0.028 a |
| T | 0.91 ± 0.023 a | 0.49 ± 0.013 a | 0.069 ± 0.007 b | 1.405 ± 0.036 a | 1.85 ± 0.036 b |
| Treatment | Net Photosynthetic Rate (Pn) | Stomatal Conductance (Cond) | Intercellular CO2 Concentration (Ci) | Transpiration Rate (Tr) | Maximum Light and Efficiency (Fv/Fm) | Actual Photosynthetic Rate(Y(II)) |
|---|---|---|---|---|---|---|
| CK | 13.34 ± 0.63 a | 0.27 ± 0.033 a | 281.8 ± 12.14 a | 4.53 ± 0.32 a | 0.71 ± 0.073 a | 0.47 ± 0.04 b |
| T | 14.03 ± 1.46 a | 0.25 ± 0.037 a | 273.89 ± 21.6 a | 6.13 ± 0.19 a | 0.69 ± 0.043 a | 0.52 ± 0.057 a |
| Treatment/Secondary Metabolites | Aucubin (ug/g) | Chlorogenic Acid (ug/g) | Genipin (ug/g) | Geniposide (ug/g) | Geniposidic Acid (ug/g) |
|---|---|---|---|---|---|
| CK | 68.85 ± 0.004 a | 47.46 ± 0.008 b | 0.31 ± 0.005 a | 39.84 ± 0.213 a | 121.84 ± 0.008 a |
| T | 4.6857 ± 0.005 b | 83.0679 ± 2.98 a | 0.35 ± 0.004 a | 9.15 ± 0.002 b | 4.85 ± 0.045 b |
| All (≥200 bp) | ≥500 bp | ≥1000 bp | N50 | GC (%) | Total Length | Max Length | Min Length | Average Length | |
|---|---|---|---|---|---|---|---|---|---|
| Transcript | 62,287 | 40,111 | 25,753 | 1762 | 42 | 70,449,627 | 14,647 | 224 | 1131 |
| Unigene | 42,333 | 23,627 | 14,132 | 1582 | 42.22 | 41,056,860 | 14,647 | 224 | 969 |
| Unigene | SwissProt | TrEMBL | NR | Pfam | KOG | GO | KO |
|---|---|---|---|---|---|---|---|
| 42,333 | 14,988 | 24,272 | 24,500 | 19,708 | 19,523 | 19,282 | 6790 |
| 100% | 35.40% | 57.30% | 57.90% | 46.60% | 46.10% | 45.50% | 16.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Li, D.; Tang, Z.; Wei, H.; Zhang, Y.; Yang, J.; Zhao, C.; Liu, Y.; Wang, W. Supplementary UV-B Radiation Effects on Photosynthetic Characteristics and Important Secondary Metabolites in Eucommia ulmoides Leaves. Int. J. Mol. Sci. 2023, 24, 8168. https://doi.org/10.3390/ijms24098168
Xiao S, Li D, Tang Z, Wei H, Zhang Y, Yang J, Zhao C, Liu Y, Wang W. Supplementary UV-B Radiation Effects on Photosynthetic Characteristics and Important Secondary Metabolites in Eucommia ulmoides Leaves. International Journal of Molecular Sciences. 2023; 24(9):8168. https://doi.org/10.3390/ijms24098168
Chicago/Turabian StyleXiao, Siqiu, Dewen Li, Zhonghua Tang, Hongling Wei, Ying Zhang, Jing Yang, Chunjian Zhao, Ying Liu, and Wei Wang. 2023. "Supplementary UV-B Radiation Effects on Photosynthetic Characteristics and Important Secondary Metabolites in Eucommia ulmoides Leaves" International Journal of Molecular Sciences 24, no. 9: 8168. https://doi.org/10.3390/ijms24098168
APA StyleXiao, S., Li, D., Tang, Z., Wei, H., Zhang, Y., Yang, J., Zhao, C., Liu, Y., & Wang, W. (2023). Supplementary UV-B Radiation Effects on Photosynthetic Characteristics and Important Secondary Metabolites in Eucommia ulmoides Leaves. International Journal of Molecular Sciences, 24(9), 8168. https://doi.org/10.3390/ijms24098168









