Characterization of Human Subcutaneous Adipose Tissue and Validation of the Banking Procedure for Autologous Transplantation
Abstract
:1. Introduction
2. Results
2.1. AT Long-Term Cryopreservation
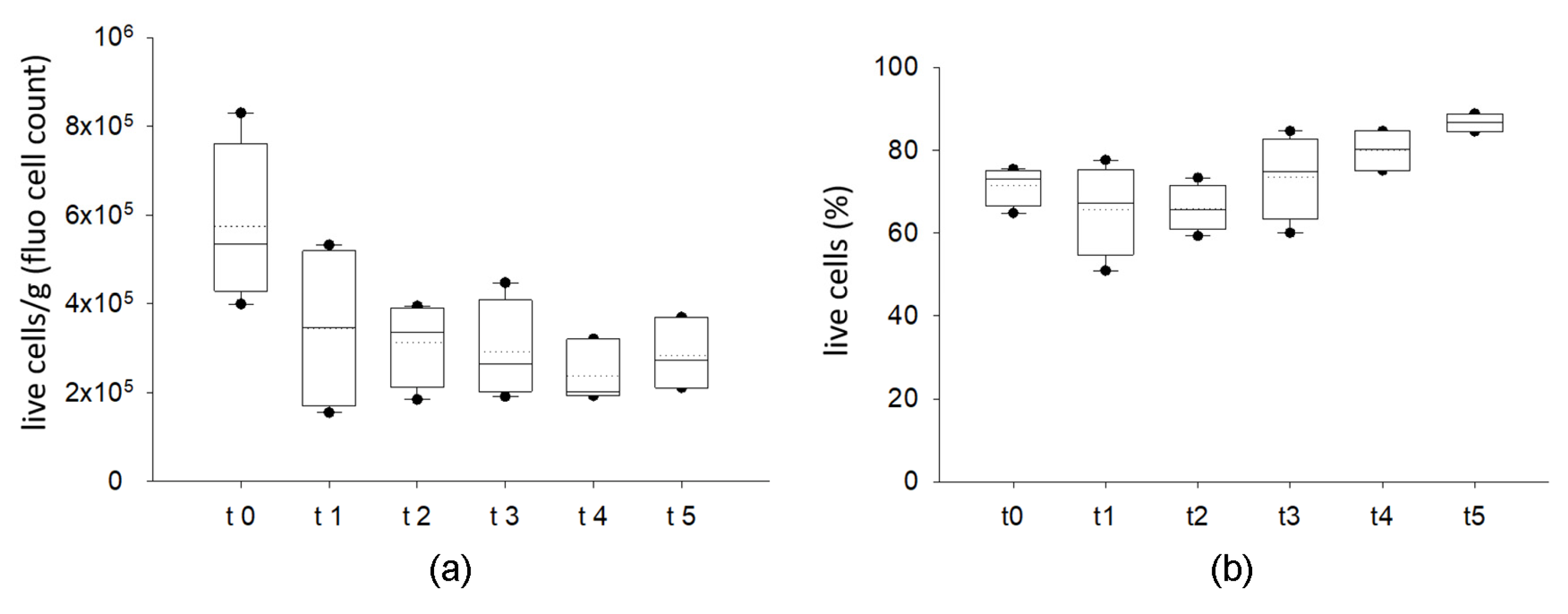
2.1.1. Cryopreserved AT Maintains Viable Stromal Vascular Fraction
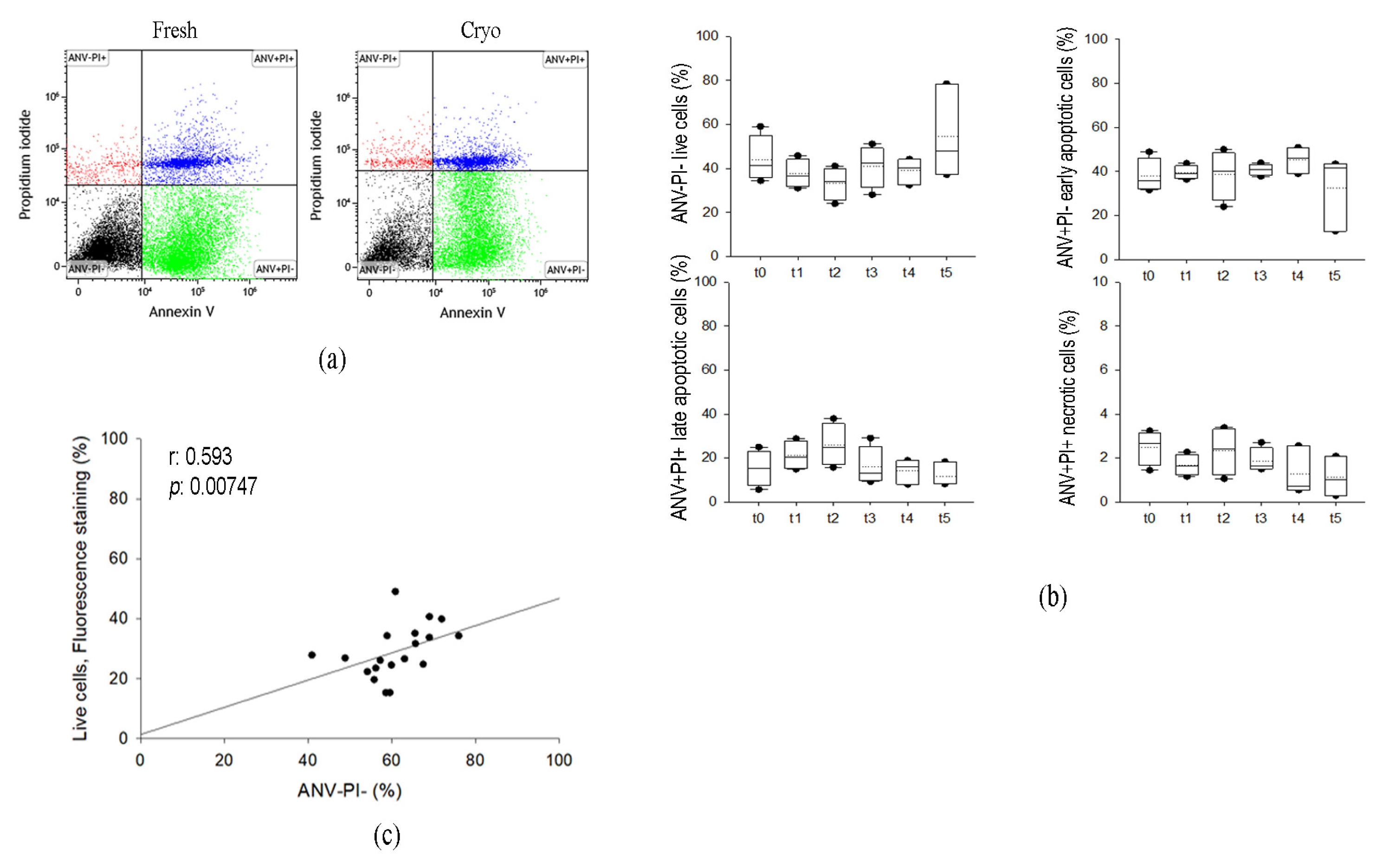
2.1.2. Cryopreservation of AT Does Not Affect Annexin V Nor Propidium Iodide Staining in SVF
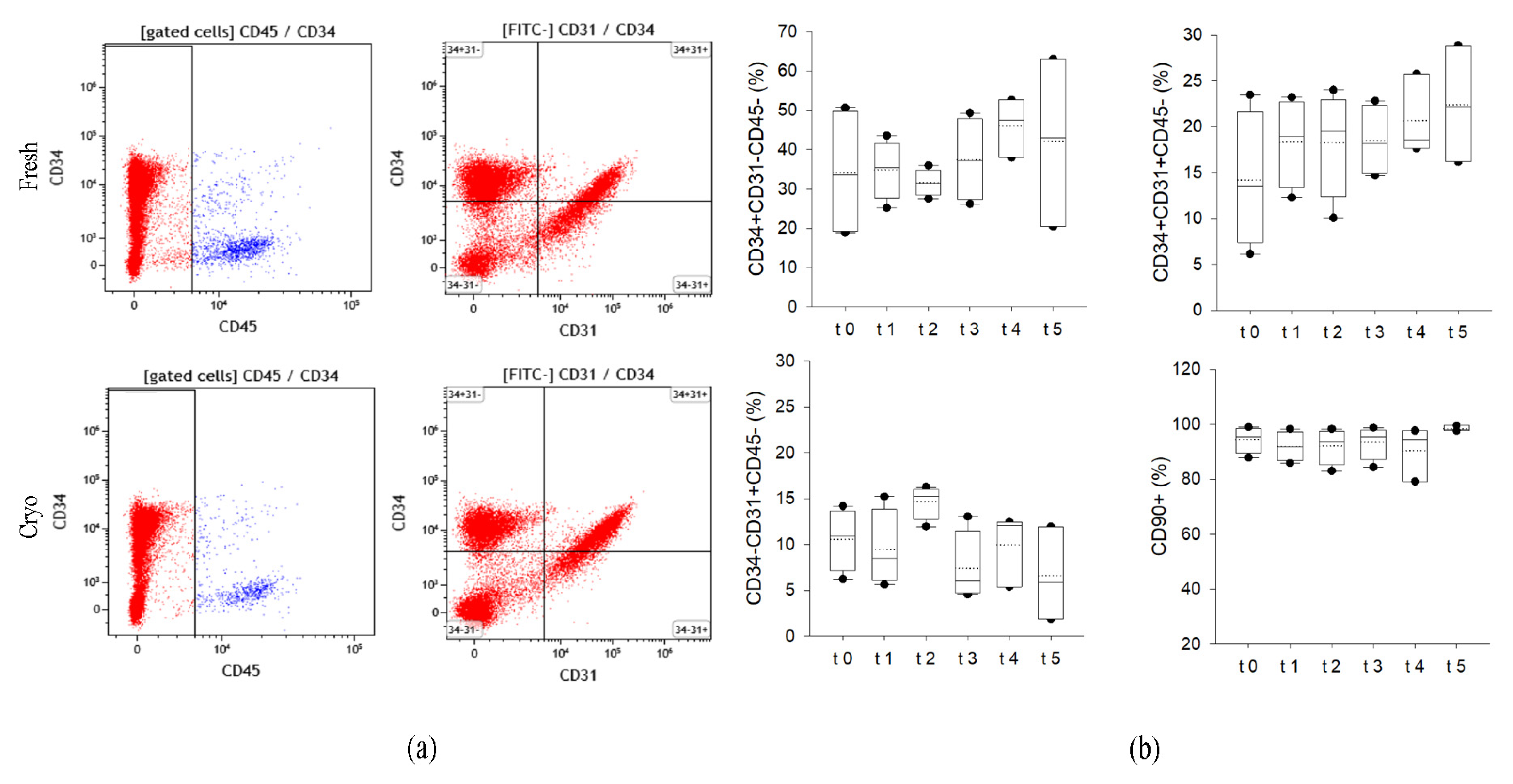
2.1.3. Cryopreserved AT Maintains the Expression of Specific Membrane Markers on SVF Cells
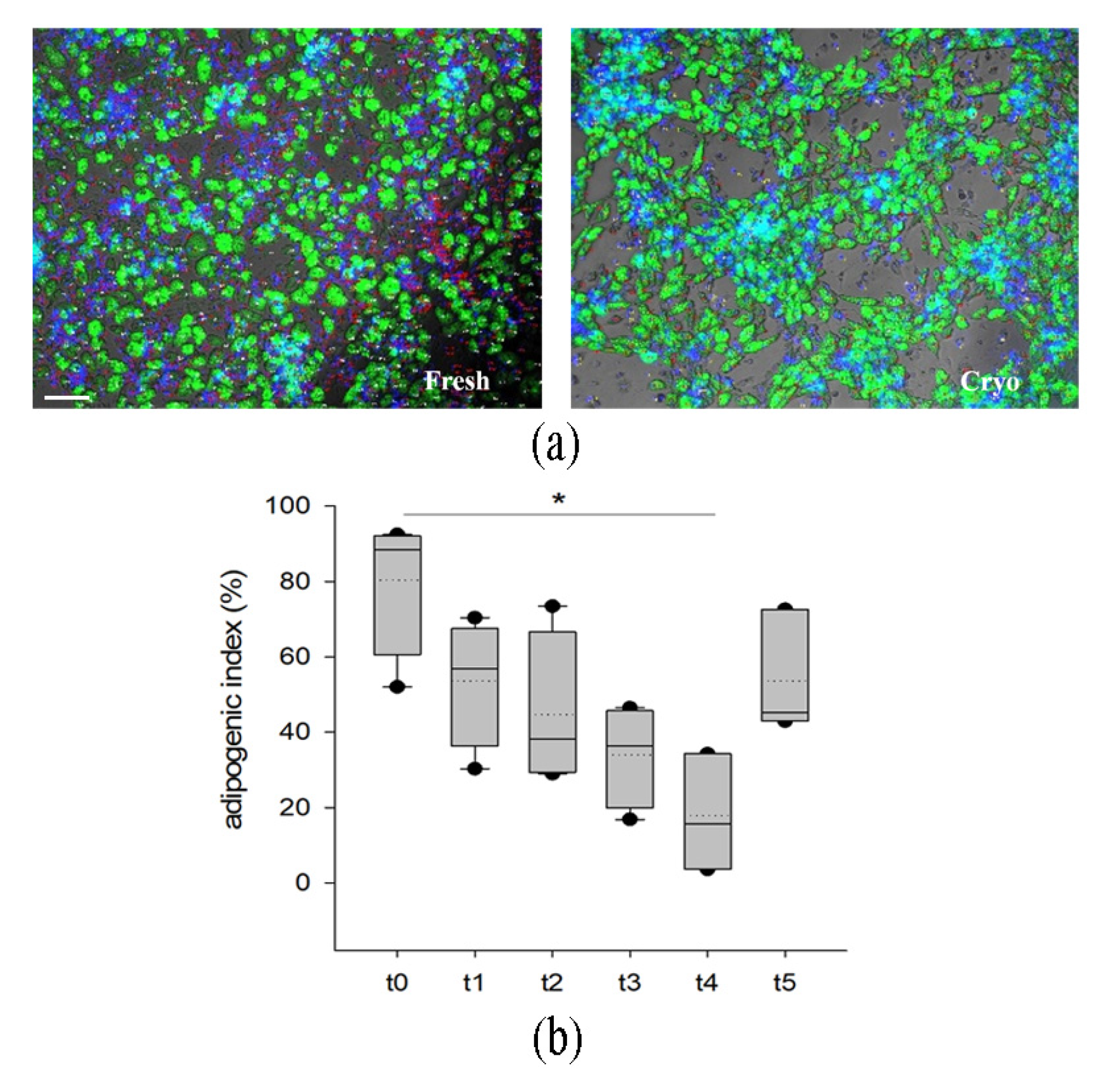
2.1.4. Cryopreserved Stromal Vascular Fraction Retains In Vitro Adipogenic Abilities
2.1.5. Cryopreserved AT Retains Viable AD
2.1.6. Microbiological Tests
2.2. Validation of Quality Controls and Logistic Procedures
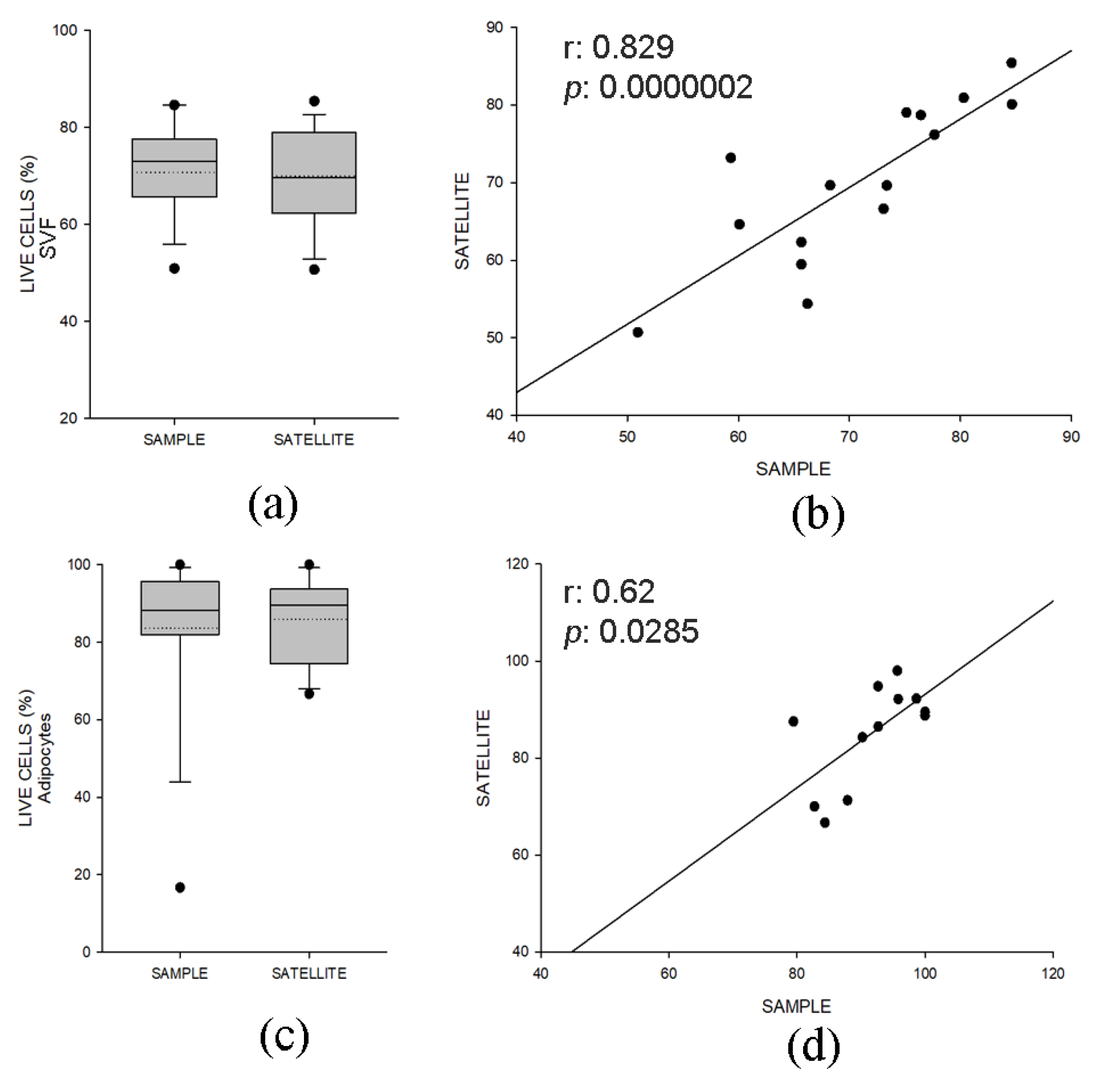
2.2.1. Quality Control Samples Display Similar Viability and Composition of Specimens for Clinical Use
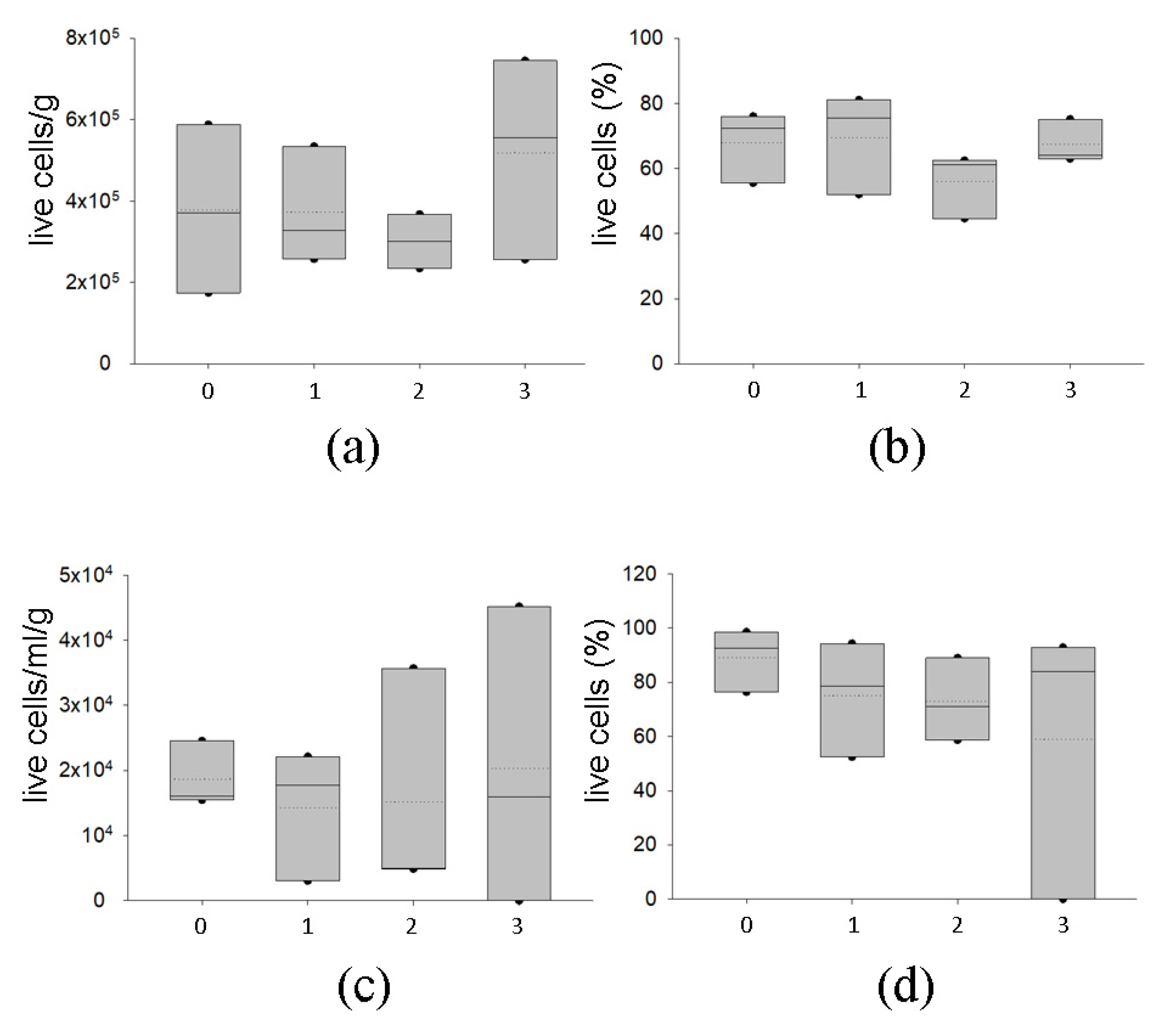
2.2.2. Delivery with Dry Ice of AT at −80 °C Preserves Graft Viability
2.2.3. Temporary Storage of AT at −80 °C Preserves Graft Viability
3. Discussion
4. Materials and Methods
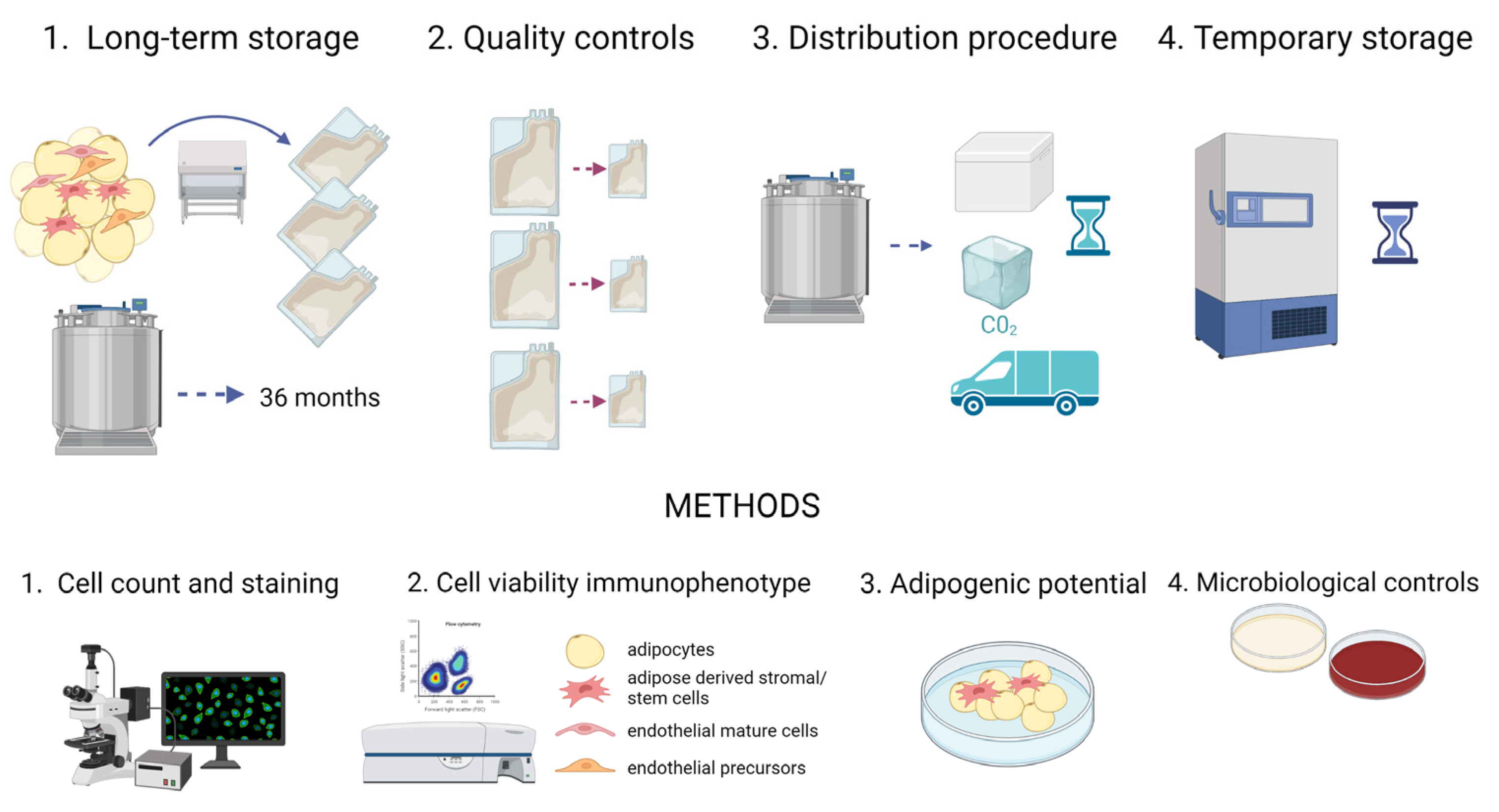
4.1. Study Design
- Long-term storage without affecting tissue viability and tissue safety: AT samples were collected (t0) and stored in vapor phase liquid nitrogen for 1 (t1), 2 (t2), 3 (t3), 14 (t4) and 36 (t5) months; at each time point, the samples were analyzed and compared to the fresh tissue (t0). Moreover, microbiological tests were carried out;
- Quality controls before implantation: AT samples were collected and split in aliquots of 50 and 10 mL, mimicking the aliquots to transplant and paired quality control samples, respectively. The viability and composition of both groups were compared to verify this approach. The storage of AT in aliquots has been proposed to avoid recurrent thawing when multiple filling procedures are necessary in a clinical setting;
- Distribution procedure in dry ice: AT samples were collected and cryopreserved in aliquots. Afterwards, aliquots kept in dry ice for 24 h were compared to aliquots stored in vapor phase liquid nitrogen;
- Temporary storage at −80 °C: After collection, AT samples were cryopreserved in aliquots. Subsequently, some of them were kept at −80 °C for 1, 2 and 3 months and compared to samples stored in vapor phase liquid nitrogen for at least 1 month.
4.2. Human Sample Fat Harvesting
4.3. Decontamination, Cryopreservation, Storage and Thawing Procedures
4.4. Characterization of AT
4.4.1. Cell Isolation (SVF-AD)
4.4.2. Cell Count and Cell Viability Quantification
4.4.3. Annexin V Staining and Analysis
4.4.4. Immunophenotyping and Flow Cytometry
4.4.5. Adipogenic Differentiation
4.5. Microbiological Testing
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milan, G.; Conci, S.; Sanna, M.; Favaretto, F.; Bettini, S.; Vettor, R. ASCs and their role in obesity and metabolic diseases. Trends Endocrinol. Metab. 2021, 32, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Scheller, E.L.; MacDougald, O.A. Adipose tissue stem cells meet preadipocyte commitment: Going back to the future. J. Lipid Res. 2012, 53, 227–246. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Zvonic, S.; Garrett, S.; Mitchell, J.B.; Floyd, Z.E.; Hammill, L.; Kloster, A.; Di Halvorsen, Y.; Ting, J.P.; Storms, R.W.; et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 2006, 24, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Koh, Y.J.; Moon, H.R.; Ryoo, H.G.; Cho, C.H.; Kim, I.; Koh, G.Y. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood 2010, 115, 957–964. [Google Scholar] [CrossRef]
- Kim, K.-S.; Seeley, R.J.; Sandoval, D.A. Signalling from the periphery to the brain that regulates energy homeostasis. Nat. Rev. Neurosci. 2018, 19, 185–196. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Pontecorvi, P.; Anastasiadou, E.; Napoli, C.; Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020, 8, 236. [Google Scholar] [CrossRef]
- Cousin, B.; André, M.; Arnaud, E.; Pénicaud, L.; Casteilla, L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem. Biophys. Res. Commun. 2003, 301, 1016–1022. [Google Scholar] [CrossRef]
- Yoshimura, K.; Shigeura, T.; Matsumoto, D.; Sato, T.; Takaki, Y.; Aiba-Kojima, E. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 2006, 208, 64–76. [Google Scholar] [CrossRef]
- Suh, A.; Pham, A.; Cress, M.J.; Pincelli, T.; TerKonda, S.P.; Bruce, A.J.; Zubair, A.C.; Wolfram, J.; Shapiro, S.A. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res. Rev. 2019, 54, 100933. [Google Scholar] [CrossRef]
- Onur Erol, O.; Agaoglu, G.; Jawad, M.A. Combined Non-Ablative Laser and Microfat Grafting for Burn Scar Treatment. Aesthet. Surg. J. 2019, 39, NP55–NP67. [Google Scholar] [CrossRef]
- Copeland, R.; Martin, J. Chronic prosthesis-related residual limb ulcer treated with autologous micro-fragmented adipose tissue. Regen. Ther. 2021, 18, 21–23. [Google Scholar] [CrossRef]
- Lonardi, R.; Leone, N.; Gennai, S.; Trevisi Borsari, G.; Covic, T.; Silingardi, R. Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: A randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res. Ther. 2019, 10, 223. [Google Scholar] [CrossRef]
- Bennett, K.G.; Qi, J.; Kim, H.M.; Hamill, J.B.; Wilkins, E.G.; Mehrara, B.J.; Kozlow, J.H. Association of Fat Grafting With Patient-Reported Outcomes in Postmastectomy Breast Reconstruction. JAMA Surg. 2017, 152, 944–950. [Google Scholar] [CrossRef]
- Strong, A.L.; Rubin, J.P.; Kozlow, J.H.; Cederna, P.S. Fat Grafting for the Treatment of Scleroderma. Plast. Reconstr. Surg. 2019, 144, 1498–1507. [Google Scholar] [CrossRef]
- Billings, E.; May, J.W. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast. Reconstr. Surg. 1989, 83, 368–381. [Google Scholar] [CrossRef]
- Coleman, S.R. Structural fat grafting. Aesthet. Surg. J. 1998, 18, 386–388. [Google Scholar] [CrossRef]
- Coleman, S.R. Long-term survival of fat transplants: Controlled demonstrations. Aesthet. Plast. Surg. 1995, 19, 421–425. [Google Scholar] [CrossRef]
- Hivernaud, V.; Lefourn, B.; Guicheux, J.; Weiss, P.; Festy, F.; Girard, A.C.; Roche, R. Autologous Fat Grafting in the Breast: Critical Points and Technique Improvements. Aesthet. Plast. Surg. 2015, 39, 547–561. [Google Scholar] [CrossRef]
- Crowley, C.A.; Smith, W.P.W.; Seah, K.T.M.; Lim, S.-K.; Khan, W.S. Cryopreservation of Human Adipose Tissues and Adipose-Derived Stem Cells with DMSO and/or Trehalose: A Systematic Review. Cells 2021, 10, 1837. [Google Scholar] [CrossRef]
- Erol, O.O.; Agaoglu, G. Facial Rejuvenation With Staged Injections of Cryopreserved Fat and Tissue Cocktail: Clinical Outcomes in the Past 10 Years. Aesthet. Surg. J. 2013, 33, 639–653. [Google Scholar] [CrossRef]
- Butterwick, K.J.; Bevin, A.A.; Iyer, S. Fat transplantation using fresh versus frozen fat: A side-by-side two-hand comparison pilot study. Dermatol. Surg. 2006, 32, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Lidagoster, M.I.; Cinelli, P.B.; Leveé, E.M.; Sian, C.S. Comparison of autologous fat transfer in fresh, refrigerated, and frozen specimens: An animal model. Ann. Plast. Surg. 2000, 44, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Bakhach, J. The cryopreservation of composite tissues: Principles and recent advancement on cryopreservation of different type of tissues. Organogenesis 2009, 5, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Alotto, D.; Belisario, D.C.; Casarin, S.; Fumagalli, M.; Cambieri, I.; Piana, R.; Stella, M.; Ferracini, R.; Castagnoli, C. Adipose Derived-Mesenchymal Stem Cells Viability and Differentiating Features for Orthopaedic Reparative Applications: Banking of Adipose Tissue. Stem Cells Int. 2016, 2016, 4968724. [Google Scholar] [CrossRef]
- Shaik, S.; Wu, X.; Gimble, J.M.; Devireddy, R. Non-toxic freezing media to retain the stem cell reserves in adipose tissues. Cryobiology. 2020, 96, 137–144. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, E.; Kim, K.J.; Jun, Y.J.; Rhie, J.W. Cryopreservation of lipoaspirates: In vitro measurement of the viability of adipose-derived stem cell and lipid peroxidation. Int Wound J. 2020, 5, 1282–1290. [Google Scholar] [CrossRef]
- Badowski, M.S.; Muise, A.; Harris, D.T. Long-Term Biobanking of Intact Tissue from Lipoaspirate. J. Clin. Med. 2019, 8, 327. [Google Scholar] [CrossRef]
- Ohashi, M. Cryopreserved fat: Our clinical experience and applications. Plast. Aesthetic Res. 2020, 7, 26. [Google Scholar] [CrossRef]
- Ohashi, M.; Chiba, A.; Nakai, H.; Fukuda, E.; Higuchi, T. Serial Injections of Cryopreserved Fat at −196 °C for Tissue Rejuvenation, Scar Treatment, and Volume Augmentation. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1742. [Google Scholar] [CrossRef]
- Hanson, S.E.; Kapur, S.K.; Hwang, R.F.; Dryden, M.S. Autologous fat grafting in breast reconstruction: Implications for follow-up and surveillance. Gland Surg. 2021, 1, 487–493. [Google Scholar] [CrossRef]
- Guide to the Quality and Safety of Tissues and Cells for Human Application—European Directorate for the Quality of Medicines & HealthCare—Liferay DXP. European Directorate for the Quality of Medicines & HealthCare. Available online: https://www.edqm.eu/en/ (accessed on 29 July 2022).
- Piccolo, N.S.; Piccolo, M.S.; de Paula Piccolo, N.; de Paula Piccolo, P.; de Paula Piccolo, N.; Daher, R.P.; Lobo, R.P.; Daher, S.P.; Sarto Piccolo, M.T. Fat Grafting for Treatment of Facial Burns and Burn Scars. Clin. Plast. Surg. 2020, 47, 119–130. [Google Scholar] [CrossRef]
- Fang, H.A.; Soto, E.; Pigg, R.; Smith, M.; Boyd, C.J.; Ananthasekar, S.; Fix, R.J.; Kilic, A.; Denney, B.; Patcha, P.; et al. The Safety of Fat Grafting: An Institutional Retrospective Review. Ann. Plast. Surg. 2022, 88, S473–S477. [Google Scholar] [CrossRef]
- Massiah, G.; De Palma, G.; Negri, A.; Mele, F.; Loisi, D.; Paradiso, A.V.; Ressa, C.M. Cryopreservation of adipose tissue with and without cryoprotective agent addition for breast lipofilling: A cytological and histological study. Cryobiology 2021, 103, 141–146. [Google Scholar] [CrossRef]
- Zanata, F.; Bowles, A.; Frazier, T.; Curley, J.L.; Bunnell, B.A.; Wu, X.; Wade, J.; Devireddy, R.; Gimble, J.M.; Ferreira, L.M. Effect of Cryopreservation on Human Adipose Tissue and Isolated Stromal Vascular Fraction Cells: In Vitro and In Vivo Analyses. Plast. Reconstr. Surg. 2018, 141, 232e–243e. [Google Scholar] [CrossRef]
- Shaik, S.; Wu, X.; Gimble, J.; Devireddy, R. Effects of Decade Long Freezing Storage on Adipose Derived Stem Cells Functionality. Sci. Rep. 2018, 8, 8162. [Google Scholar] [CrossRef]
- Jossen, V.; Muoio, F.; Panella, S.; Harder, Y.; Tallone, T.; Eibl, R. An Approach towards a GMP Compliant In-Vitro Expansion of Human Adipose Stem Cells for Autologous Therapies. Bioengineering 2020, 7, 77. [Google Scholar] [CrossRef]
- Panella, S.; Muoio, F.; Jossen, V.; Harder, Y.; Eibl-Schindler, R.; Tallone, T. Chemically Defined Xeno- and Se-rum-Free Cell Culture Medium to Grow Human Adipose Stem Cells. Cells 2021, 10, 466. [Google Scholar] [CrossRef]
- Conti, G.; Jurga, M.; Benati, D.; Bernardi, P.; Mosconi, E.; Rigotti, G.; Buvé, M.; Van Wemmel, K.; Sbarbati, A. Cryopreserved Subcutaneous Adipose Tissue for Fat Graft. Aesthet. Plast. Surg. 2015, 39, 800–817. [Google Scholar] [CrossRef]
- Schroeter, J.; Wilkemeyer, I.; Schiller, R.A.; Pruss, A. Validation of the Microbiological Testing of Tissue Preparations Using the BACTECTM Blood Culture System. Transfus. Med. Hemother. 2012, 39, 387–390. [Google Scholar] [CrossRef]
- Cui, X.D.; Gao, D.Y.; Fink, B.F.; Vasconez, H.C.; Pu, L.L.Q. Cryopreservation of human adipose tissues. Cryobiology 2007, 55, 269–278. [Google Scholar] [CrossRef]
- Zhang, P.Q.; Tan, P.C.; Gao, Y.M.; Zhang, X.J.; Xie, Y.; Zheng, D.N.; Zhou, S.B.; Li, Q.F. The effect of glycerol as a cryoprotective agent in the cryopreservation of adipose tissue. Stem Cell Res. Ther. 2022, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Erdim, M.; Tezel, E.; Numanoglu, A.; Sav, A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: An experimental study. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Wolter, T.P.; von Heimburg, D.; Stoffels, I.; Groeger, A.; Pallua, N. Cryopreservation of mature human adipocytes: In vitro measurement of viability. Ann. Plast. Surg. 2005, 55, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Oh, J.; Choi, T.; Kim, J.; Han, K.; Ha, S.; Lee, K. Viability of fat cells over time after syringe suction lipectomy: The effects of cryopreservation. Ann. Plast. Surg. 2010, 65, 354–360. [Google Scholar] [CrossRef]
- Moscatello, D.K.; Dougherty, M.; Narins, R.S.; Lawrence, N. Cryopreservation of human fat for soft tissue augmentation: Viability requires use of cryoprotectant and controlled freezing and storage. Dermatol. Surg. 2005, 31, 1506–1510. [Google Scholar] [CrossRef]
- Guo, B.; Sawkulycz, X.; Heidari, N.; Rogers, R.; Liu, D.; Slevin, M. Characterisation of Novel Angiogenic and Potent Anti-Inflammatory Effects of Micro-Fragmented Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 3271. [Google Scholar] [CrossRef]
- Tennant, J.R. Evaluation of the trypan blue technique for determination of cell viability. Transplantation 1964, 2, 685–694. [Google Scholar] [CrossRef]
- Miklíková, M.; Jarkovská, D.; Čedíková, M.; Švíglerová, J.; Kuncová, J.; Nalos, L.; Kubíková, T.; Liška, V.; Holubová, M.; Lysák, D.; et al. Beneficial effects of mesenchymal stem cells on adult porcine cardiomyocytes in non-contact co-culture. Physiol. Res. 2018, 67, S619–S631. [Google Scholar] [CrossRef]
- Guimarães-Camboa, N.; Evans, S.M. Are Perivascular Adipocyte Progenitors Mural Cells or Adventitial Fibroblasts? Cell Stem Cell 2017, 20, 587–589. [Google Scholar] [CrossRef]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef]
- Laschke, M.W.; Karschnia, P.; Scheuer, C.; Heß, A.; Metzger, W.; Menger, M.D. Effects of cryopreservation on adipose tissue-derived microvascular fragments. J. Tissue Eng. Regen. Med. 2018, 12, 1020–1030. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar]
- Viswanathan, S.; Ciccocioppo, R.; Galipeau, J.; Krampera, M.; Le Blanc, K.; Martin, I.; Moniz, K.; Nolta, J.; Phinney, D.G.; Shi, Y.; et al. Consensus International Council for Commonality in Blood Banking Automation–International Society for Cell & Gene Therapy statement on standard nomenclature abbreviations for the tissue of origin of mesenchymal stromal cells. Cytotherapy 2021, 23, 1060–1063. [Google Scholar]
- Sharma, S.; Muthu, S.; Jeyaraman, M.; Ranjan, R.; Jha, S.K. Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications. World J. Stem Cells 2021, 13, 1360–1381. [Google Scholar] [CrossRef]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R.; et al. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef]
- Lynes, M.D.; Tseng, Y.H. Deciphering adipose tissue heterogeneity. Ann. N. Y. Acad. Sci. 2018, 1411, 5–20. [Google Scholar] [CrossRef]
- Raajendiran, A.; Krisp, C.; Souza, D.P.; Ooi, G.; Burton, P.R.; Taylor, R.A.; Molloy, M.P.; Watt, M.J. Proteome analysis of human adipocytes identifies depot-specific heterogeneity at metabolic control points. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E1068–E1084. [Google Scholar] [CrossRef]
- Bowles, A.C.; Wise, R.M.; Gerstein, B.Y.; Thomas, R.C.; Ogelman, R.; Febbo, I.; Bunnell, B.A. Immunomodulatory Effects of Adipose Stromal Vascular Fraction Cells Promote Alternative Activation Macrophages to Repair Tissue Damage. Stem Cells 2017, 35, 2198–2207. [Google Scholar] [CrossRef]
- Serafini, A.; Riello, E.; Trojan, D.; Cogliati, E.; Palù, G.; Manganelli, R.; Paolin, A. Evaluation of new antibiotic cocktails against contaminating bacteria found in allograft tissues. Cell Tissue Bank. 2016, 17, 619–628. [Google Scholar] [CrossRef]
- Paolin, A.; Spagnol, L.; Battistella, G.; Trojan, D. Evaluation of allograft decontamination with two different antibiotic cocktails at the Treviso Tissue Bank Foundation. PLoS ONE 2018, 13, e0201792. [Google Scholar] [CrossRef]
- Montagner, G.; Trojan, D.; Cogliati, E.; Manea, F.; Vantini, A.; Paolin, A. Stability analysis of the antibiotic cocktail used by Treviso Tissue Bank Foundation for tissues decontamination. Cell Tissue Bank. 2018, 19, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.; Franzin, C.; Pozzobon, M.; Favaretto, F.; Rossi, C.A.; Calcagno, A. Adipogenic potential of skeletal muscle satellite cells. Clin. Lipidol. 2009, 4, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Belligoli, A.; Compagnin, C.; Sanna, M.; Favaretto, F.; Fabris, R.; Busetto, L.; Foletto, M.; Dal Prà, C.; Serra, R.; Prevedello, L.; et al. Characterization of subcutaneous and omental adipose tissue in patients with obesity and with different degrees of glucose impairment. Sci. Rep. 2019, 9, 11333. [Google Scholar]
- Zimmerlin, L.; Donnenberg, V.S.; Rubin, J.P.; Donnenberg, A.D. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A 2013, 83, 134–140. [Google Scholar] [CrossRef]


| FITC | PE | PerCP.Cy5.5 | |
|---|---|---|---|
| Unstained | - | - | - |
| Negative | IgG1 | IgG1 | IgG1 |
| Sample 1 | CD45 | CD31 | CD34 |
| Sample 2 | CD45 CD31 | CD90 | CD34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favaretto, F.; Compagnin, C.; Cogliati, E.; Montagner, G.; Dell’Antonia, F.; Berna, G.; Vettor, R.; Milan, G.; Trojan, D. Characterization of Human Subcutaneous Adipose Tissue and Validation of the Banking Procedure for Autologous Transplantation. Int. J. Mol. Sci. 2023, 24, 8190. https://doi.org/10.3390/ijms24098190
Favaretto F, Compagnin C, Cogliati E, Montagner G, Dell’Antonia F, Berna G, Vettor R, Milan G, Trojan D. Characterization of Human Subcutaneous Adipose Tissue and Validation of the Banking Procedure for Autologous Transplantation. International Journal of Molecular Sciences. 2023; 24(9):8190. https://doi.org/10.3390/ijms24098190
Chicago/Turabian StyleFavaretto, Francesca, Chiara Compagnin, Elisa Cogliati, Giulia Montagner, Francesco Dell’Antonia, Giorgio Berna, Roberto Vettor, Gabriella Milan, and Diletta Trojan. 2023. "Characterization of Human Subcutaneous Adipose Tissue and Validation of the Banking Procedure for Autologous Transplantation" International Journal of Molecular Sciences 24, no. 9: 8190. https://doi.org/10.3390/ijms24098190
APA StyleFavaretto, F., Compagnin, C., Cogliati, E., Montagner, G., Dell’Antonia, F., Berna, G., Vettor, R., Milan, G., & Trojan, D. (2023). Characterization of Human Subcutaneous Adipose Tissue and Validation of the Banking Procedure for Autologous Transplantation. International Journal of Molecular Sciences, 24(9), 8190. https://doi.org/10.3390/ijms24098190






