The Role of Fucoxanthin in Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
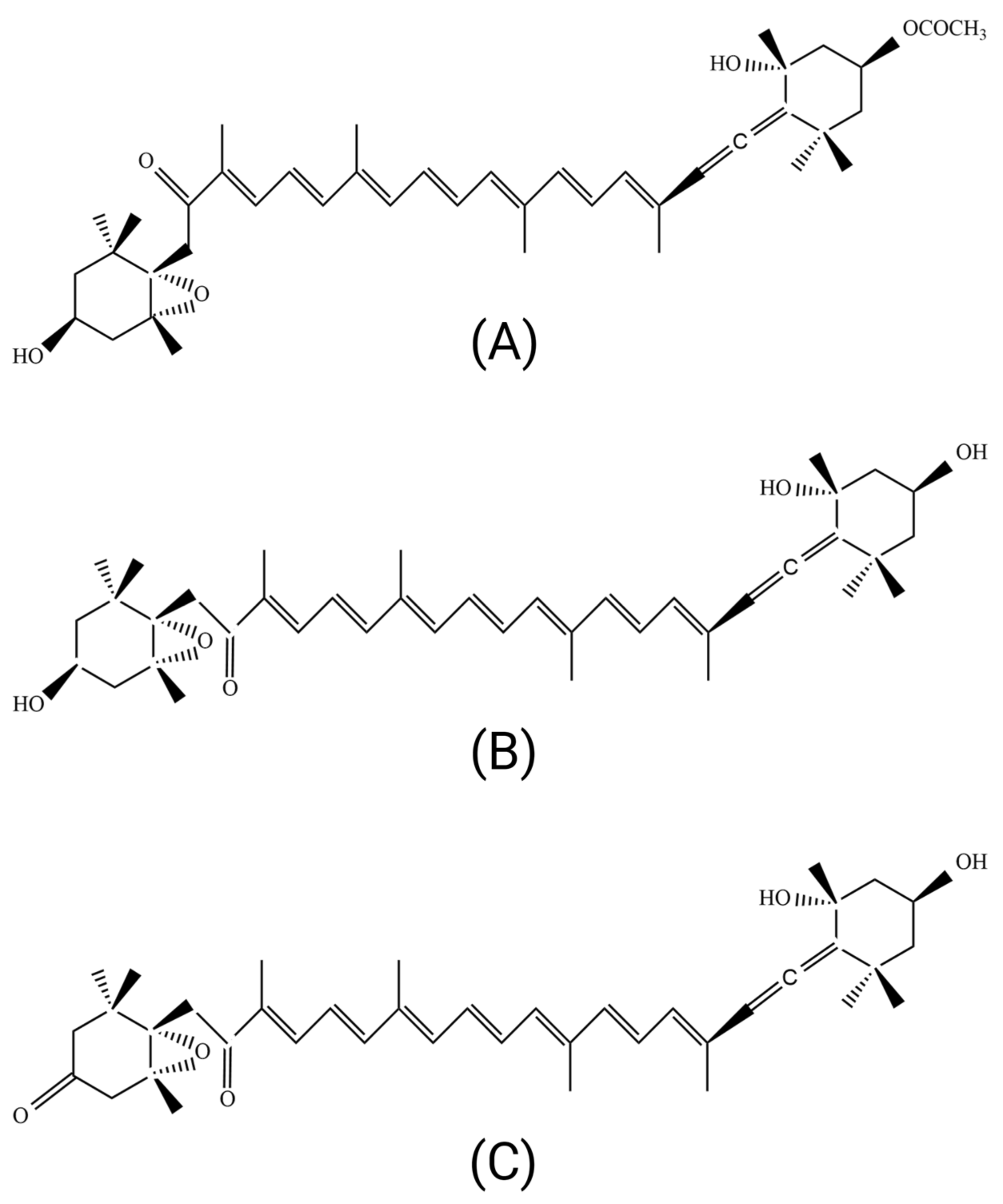
2. Structure and Metabolites of Fucoxanthin
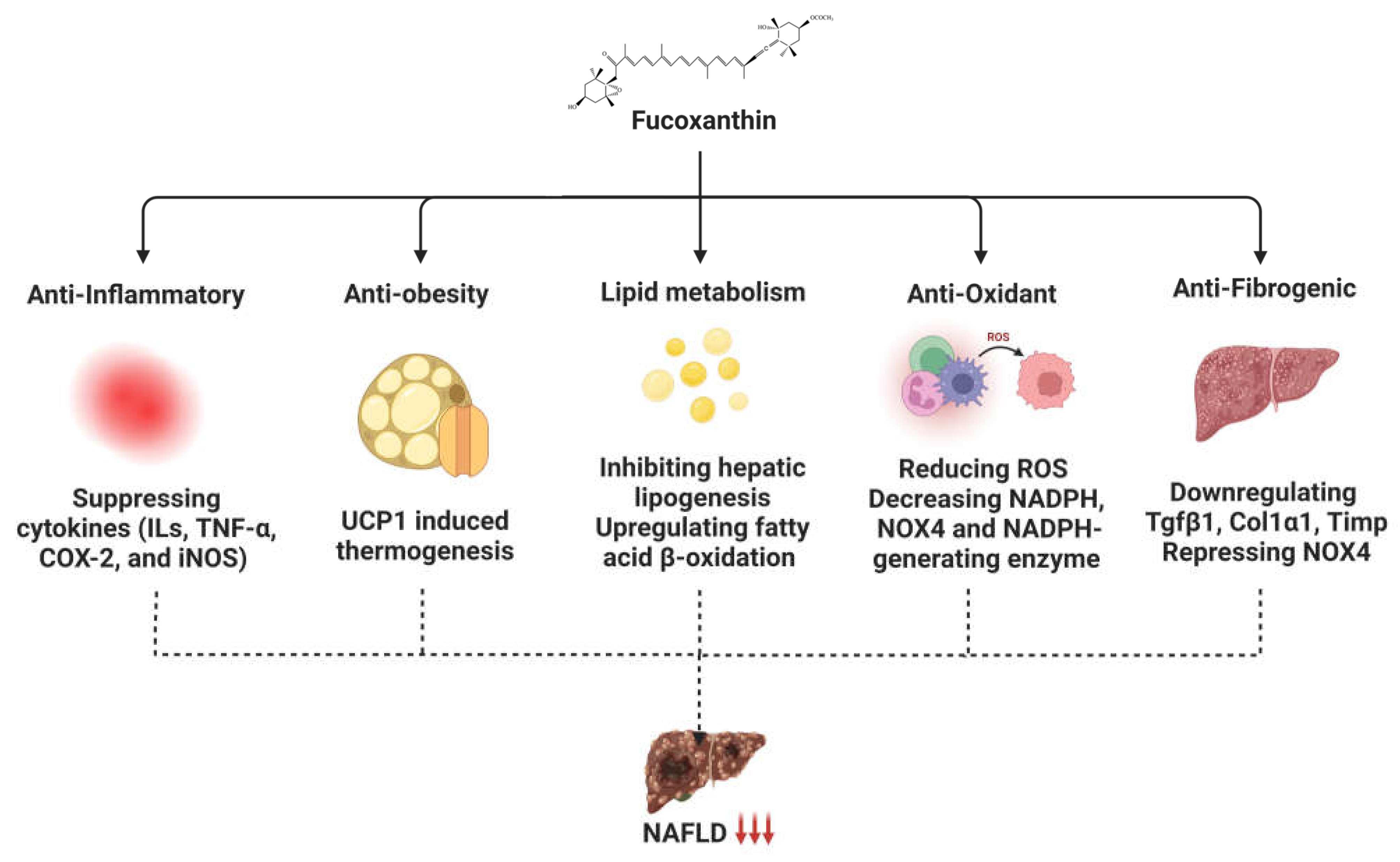
3. Role of Fucoxanthin in Non-Alcoholic Fatty Liver Disease
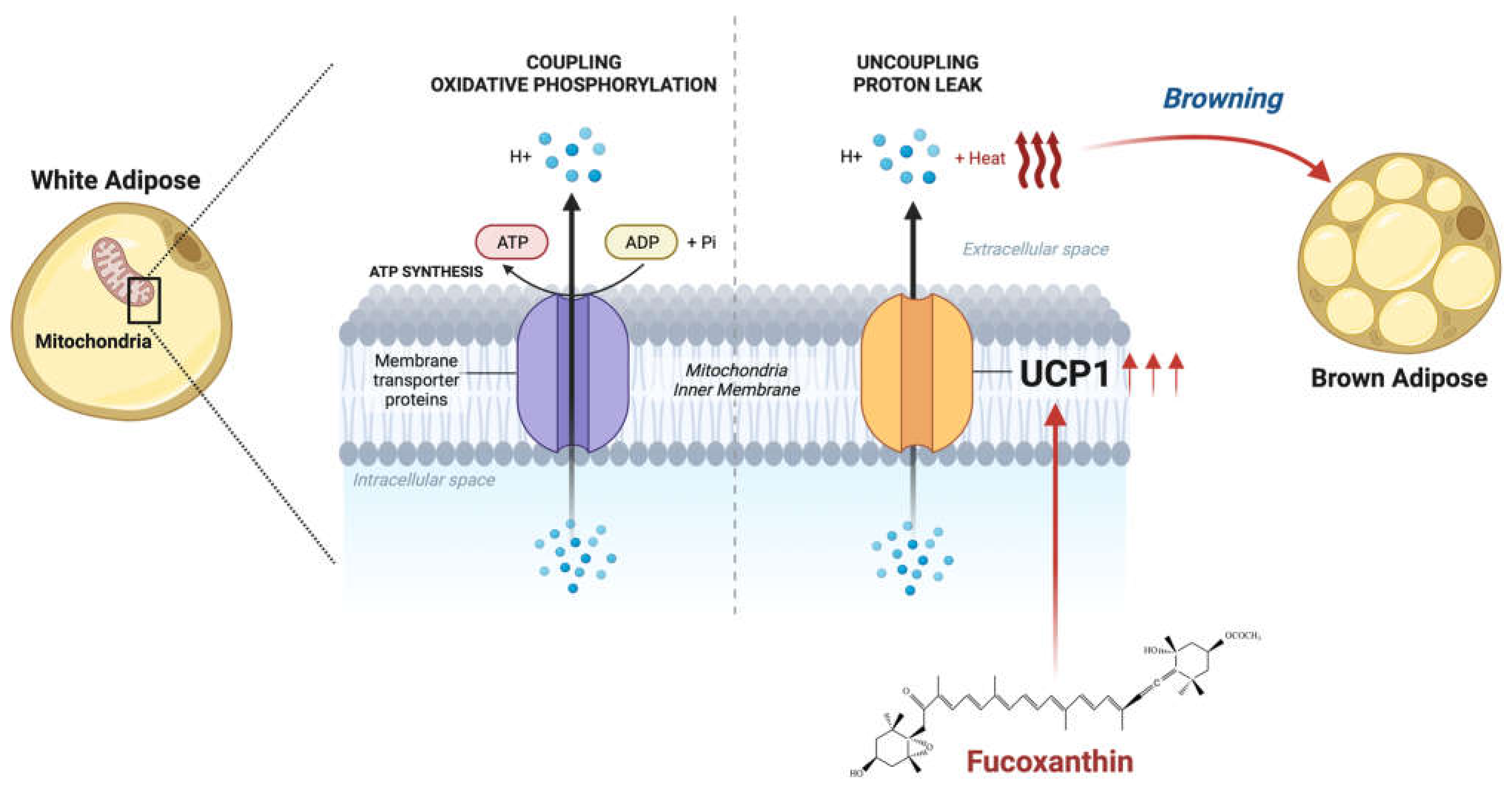
3.1. Fucoxanthin Affects Mitochondrial Homeostasis through Thermogenic Activity
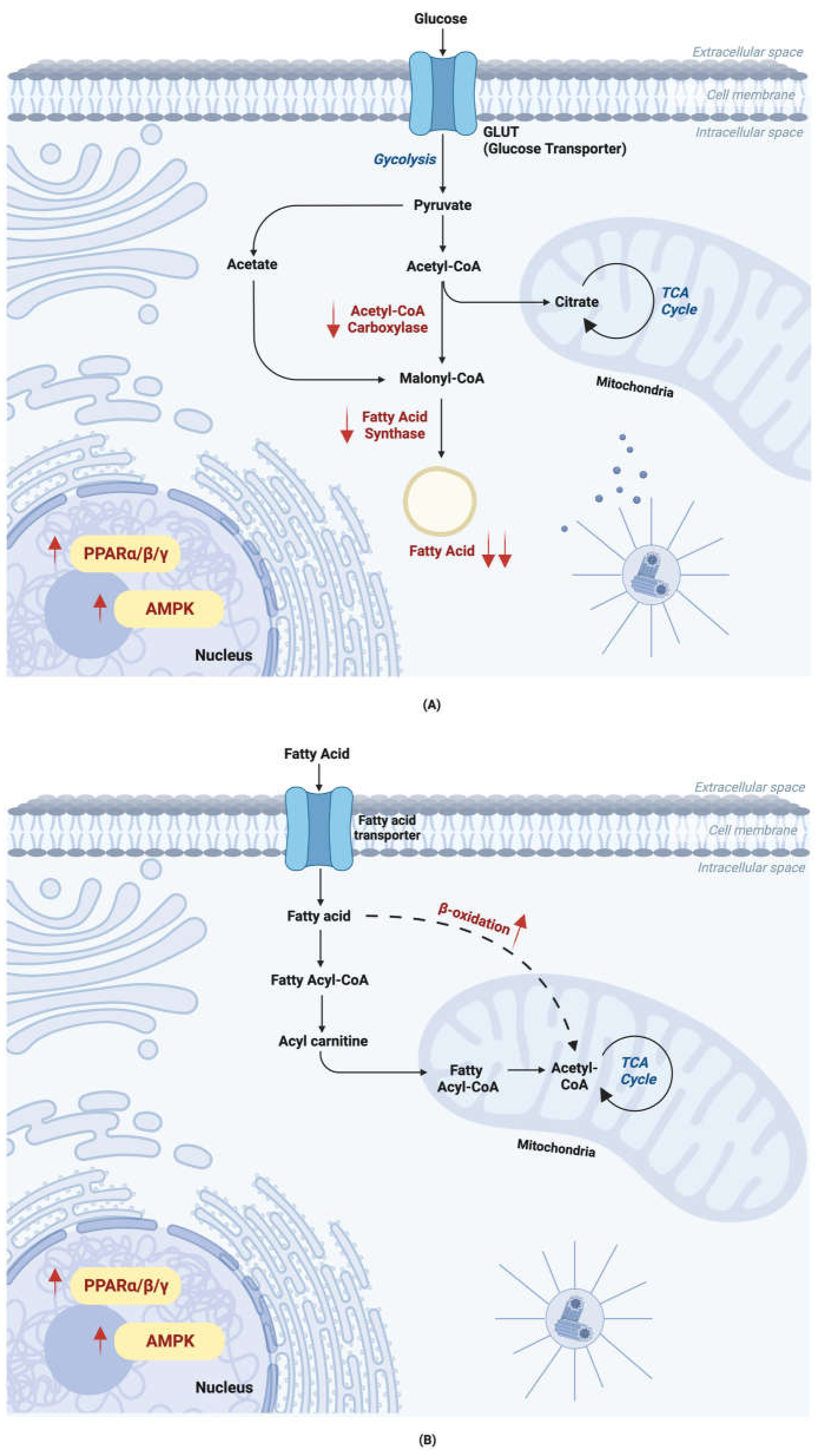
3.2. Fucoxanthin Alters Lipid Metabolism
3.3. Anti-Inflammatory Activity of Fucoxanthin
| Inflammatory Cytokines | Experimental Model | Dose | References |
|---|---|---|---|
| ↓ IL-1β | High-fat-diet-induced obese mice | 0.2, 0.4, or 0.6% | [79] |
| RAW 264.7 macrophages | I. okamurae-extracted fucoxanthin; 12.5, 25, or 50 μM | [86] | |
| RAW 264.7 macrophages | 5, 10, or 20 μM | [87] | |
| ↓ IL-4 ↓ IL-5 | OVA-stimulated (OVA) mice | 10 or 30 μM | [90] |
| Bronchoalveolar lavage fluid (BALF) from asthmatic mice | 10 mg/kg or 30 mg/kg | ||
| OVA-stimulated (OVA) mice | 10 or 30 μM | ||
| ↓ IL-6 | RAW 264.7 macrophages cells | I. okamurae-extracted fucoxanthin; 12.5, 25, or 50 μM | [86] |
| BEAS-2B cells | 3, 10, or 30 μM | [90] | |
| Bronchoalveolar lavage fluid (BALF) from asthmatic mice | 10 mg/kg or 30 mg/kg | ||
| RAW 264.7 macrophages | 5, 10, or 20 μM | [87] | |
| ↓ IL-8 | BEAS-2B cells | 3, 10, or 30 μM | [90] |
| Bronchoalveolar lavage fluid (BALF) from asthmatic mice | 10 mg/kg or 30 mg/kg | ||
| ↓ IL-10 | RAW 264.7 macrophages | 5, 10, or 20 μM | [87] |
| ↓ IL-13 | Bronchoalveolar lavage fluid (BALF) from asthmatic mice | 10 mg/kg or 30 mg/kg | [90] |
| OVA-stimulated (OVA) mice | 10 or 30 μM | ||
| ↓ TNF-α | High-fat-diet-induced obese mice | 0.2, 0.4, 0.6% | [79] |
| RAW 264.7 macrophages cells | I. okamurae-extracted fucoxanthin; 12.5, 25, or 50 μM | [86] | |
| Bronchoalveolar lavage fluid (BALF) from asthmatic mice | 10 mg/kg or 30 mg/kg | [90] | |
| OVA-stimulated (OVA) mice | 10 or 30 μM | ||
| ↓ COX-2 | High-fat-diet-induced obese mice | 0.2, 0.4, or 0.6% | [79] |
| RAW 264.7 macrophages | 5, 10, or 20 μM | [87] | |
| ↓ iNOS |
3.4. Anti-Oxidant Activity of Fucoxanthin against NAFLD
4. Preventive Effect of Fucoxanthin on NASH Development
5. Signaling Pathways Altered by Fucoxanthin
6. Conclusions and Further Potential of Fucoxanthin against NAFLD
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.C. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar]
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin. Gastroenterol. Hepatol. 2020, 18, 2650–2666. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Ali, A.H.; Ibdah, J.A. Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 7280. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; De Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. Petalonia binghamiae extract and its constituent fucoxanthin ameliorate high-fat diet-induced obesity by activating AMP-activated protein kinase. J. Agric. Food Chem. 2012, 60, 3389–3395. [Google Scholar] [CrossRef]
- Koo, S.Y.; Hwang, J.H.; Yang, S.H.; Um, J.I.; Hong, K.W.; Kang, K.; Pan, C.H.; Hwang, K.T.; Kim, S.M. Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef]
- Woo, M.N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. -Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, H.; Shi, C.; Gao, Y.; Zhang, Y.; Xu, X.; Ding, H.; Tang, L.; Xing, Y. Distribution of heavy metals and metalloids in bulk and particle size fractions of soils from coal-mine brownfield and implications on human health. Chemosphere 2017, 172, 505–515. [Google Scholar] [CrossRef]
- Mikami, K.; Hosokawa, M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int. J. Mol. Sci. 2013, 14, 13763–13781. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.; García-Oliveira, P.; Prieto, M.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Murase, W.; Kamakura, Y.; Kawakami, S.; Yasuda, A.; Wagatsuma, M.; Kubota, A.; Kojima, H.; Ohta, T.; Takahashi, M.; Mutoh, M.; et al. Fucoxanthin Prevents Pancreatic Tumorigenesis in C57BL/6J Mice That Received Allogenic and Orthotopic Transplants of Cancer Cells. Int. J. Mol. Sci. 2021, 22, 13620. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Kubota, A.; Kojima, H.; Maeda, H.; Miyashita, K.; Kawagoe, C.; Mutoh, M.; Tanaka, T. Fucoxanthin and Colorectal Cancer Prevention. Cancers 2021, 13, 2379. [Google Scholar] [CrossRef]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, T.; Okumura, S.; Iwashita, T.; Kosumi, D.; Hashimoto, H.; Katsumura, S. Stereocontrolled total synthesis of fucoxanthin and its polyene chain-modified derivative. Org. Lett. 2012, 14, 808–811. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Conn, P.F.; Schalch, W.; Truscott, T.G. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. B Biol. 1991, 11, 41–47. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of dietary fucoxanthin from Himanthalia elongata brown seaweed. Food Res. Int. 2017, 99 Pt 3, 995–1001. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Maeda, H. Anti-obesity and anti-diabetic activities of algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 453–472. [Google Scholar]
- Soriano, E.; Fernandez, I. Allenes and computational chemistry: From bonding situations to reaction mechanisms. Chem. Soc. Rev. 2014, 43, 3041–3105. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.K.; Bhaskar, N.; Divakar, S.; Baskaran, V. Bioavailability and metabolism of fucoxanthin in rats: Structural characterization of metabolites by LC-MS (APCI). Mol. Cell Biochem. 2010, 333, 299–310. [Google Scholar] [CrossRef]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: Formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Hashimoto, T.; Kanazawa, K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. Biochim. Biophys Acta 2008, 1780, 743–749. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef]
- Iio, K.; Okada, Y.; Ishikura, M. Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats. J. Food Hyg. Soc. Jpn. 2011, 52, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Spagolla Napoleao Tavares, R.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Schafer-Korting, M.; Marx, U.; Gaspar, L.R.; Zoschke, C. Skin Irritation Testing beyond Tissue Viability: Fucoxanthin Effects on Inflammation, Homeostasis, and Metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bugianesi, E.; Forlani, G.; Cerrelli, F.; Lenzi, M.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; Melchionda, N.; et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003, 37, 917–923. [Google Scholar] [CrossRef]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Smith, U. Abdominal obesity: A marker of ectopic fat accumulation. J. Clin. Investig. 2015, 125, 1790–1792. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.W.; Yan, H.Y.; Wang, Z.Y.; Zhao, S.H.; Wang, B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: Evidence from a meta-analysis of 21 cohort studies. Obes. Rev. 2016, 17, 510–519. [Google Scholar] [CrossRef]
- Xue, K.; Wu, D.; Wang, Y.; Zhao, Y.; Shen, H.; Yao, J.; Huang, X.; Li, X.; Zhou, Z.; Wang, Z.; et al. The mitochondrial calcium uniporter engages UCP1 to form a thermoporter that promotes thermogenesis. Cell Metab. 2022, 34, 1325–1341.e6. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chen, Y.L.; Huang, W.C.; Liou, C.J. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem. Biophys Res. Commun. 2018, 495, 197–203. [Google Scholar] [CrossRef]
- Gusdon, A.M.; Song, K.X.; Qu, S. Nonalcoholic Fatty liver disease: Pathogenesis and therapeutics from a mitochondria-centric perspective. Oxidative Med. Cell. Longev. 2014, 2014, 637027. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Zhang, H.X.; Guo, J.R.; Lam, C.W.K.; Wang, C.Y.; Zhang, W. Mitochondria-Mediated Pathogenesis and Therapeutics for Non-Alcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019, 63, e1900043. [Google Scholar] [CrossRef]
- Mitchell, P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: Power transmission by proticity. Biochem. Soc. Trans. 1976, 4, 399–430. [Google Scholar] [CrossRef]
- Smith, R.E.; Roberts, J.C.; Hittelman, K.J. Nonphosphorylating respiration of mitochondria from brown adipose tissue of rats. Science 1966, 154, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Divakaruni, A.S.; Brand, M.D. The regulation and physiology of mitochondrial proton leak. Physiology 2011, 26, 192–205. [Google Scholar] [CrossRef]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Essays Biochem. 2010, 47, 53–67. [Google Scholar]
- Huang, E. Different Types of Membrane Transport. 2022. Available online: https://app.biorender.com/biorender-templates/t-6329c98fbae8d5e25bed4fed-different-types-of-membrane-transport (accessed on 23 November 2022).
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Smorlesi, A.; Frontini, A.; Giordano, A.; Cinti, S. The adipose organ: White-brown adipocyte plasticity and metabolic inflammation. Obes. Rev. 2012, 13 (Suppl. S2), 83–96. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef]
- Symonds, M.E. Brown adipose tissue growth and development. Scientifica 2013, 2013, 305763. [Google Scholar] [CrossRef]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med.Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef]
- Enerback, S. Human brown adipose tissue. Cell Metab. 2010, 11, 248–252. [Google Scholar] [CrossRef]
- Seale, P. Transcriptional control of brown adipocyte development and thermogenesis. Int. J. Obes. 2010, 34 (Suppl. S1), S17–S22. [Google Scholar] [CrossRef]
- Miyashita, K. Anti-obesity therapy by food component: Unique activity of marine carotenoid, fucoxanthin. Obes. Control. 2014, 1, 4. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Effect of medium-chain triacylglycerols on anti-obesity effect of fucoxanthin. J. Oleo. Sci. 2007, 56, 615–621. [Google Scholar] [CrossRef]
- Ahn, J.; Ha, T.Y.; Ahn, J.; Jung, C.H.; Seo, H.D.; Kim, M.J.; Kim, Y.S.; Jang, Y.J. Undaria pinnatifida extract feeding increases exercise endurance and skeletal muscle mass by promoting oxidative muscle remodeling in mice. FASEB J. 2020, 34, 8068–8081. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.J.; Tian, Y.B.; Cao, Z.H.; Tao, L.L.; Zhang, X.; Gao, S.Z.; Ge, C.R.; Lin, Q.Y.; Jois, M. The polymorphisms of UCP1 genes associated with fat metabolism, obesity and diabetes. Mol. Biol. Rep. 2010, 37, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020, 42, 101092. [Google Scholar] [CrossRef]
- Hodson, L.; Gunn, P.J. The regulation of hepatic fatty acid synthesis and partitioning: The effect of nutritional state. Nat. Rev. Endocrinol. 2019, 15, 689–700. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Muradian, K.; Vaiserman, A.; Min, K.J.; Fraifeld, V.E. Fucoxanthin and lipid metabolism: A minireview. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 891–897. [Google Scholar] [CrossRef]
- Woo, M.N.; Jeon, S.M.; Shin, Y.C.; Lee, M.K.; Kang, M.A.; Choi, M.S. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol. Nutr. Food Res 2009, 53, 1603–1611. [Google Scholar] [CrossRef]
- Takenaka, A.; Nakamura, S.; Mitsunaga, F.; Inoue-Murayama, M.; Udono, T.; Suryobroto, B. Human-specific SNP in obesity genes, adrenergic receptor beta2 (ADRB2), Beta3 (ADRB3), and PPAR gamma2 (PPARG), during primate evolution. PLoS ONE 2012, 7, e43461. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Lawitz, E.; Noureddin, M.; DeFronzo, R.; Shulman, G.I. GS-0976 (Firsocostat): An investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH). Expert Opin. Investig. Drugs 2020, 29, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Kim, M.H.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Park, J.G.; Ko, H.C.; Lee, N.H.; Chung, W.S.; Kim, S.J. A water-soluble extract of Petalonia binghamiae inhibits the expression of adipogenic regulators in 3T3-L1 preadipocytes and reduces adiposity and weight gain in rats fed a high-fat diet. J. Nutr. Biochem. 2010, 21, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, W.; Huang, X.; Zhao, Y.; Ren, Q.; Hong, Z.; Huang, M.; Xing, X. Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/Akt and AMPK pathways. J. Funct. Foods 2018, 48, 515–524. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, M.K.; Park, Y.B.; Shin, Y.C.; Choi, M.S. Beneficial effects of Undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6J mice. Food Chem. Toxicol. 2011, 49, 727–733. [Google Scholar] [CrossRef]
- Shih, P.H.; Shiue, S.J.; Chen, C.N.; Cheng, S.W.; Lin, H.Y.; Wu, L.W.; Wu, M.S. Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.B.; Bae, M.; Lee, Y.; Kang, H.; Hu, S.; Pham, T.X.; Park, Y.K.; Lee, J.Y. Consumption of Low Dose Fucoxanthin Does Not Prevent Hepatic and Adipose Inflammation and Fibrosis in Mouse Models of Diet-Induced Obesity. Nutrients 2022, 14, 2280. [Google Scholar] [CrossRef]
- Tan, C.P.; Hou, Y.H. First evidence for the anti-inflammatory activity of fucoxanthin in high-fat-diet-induced obesity in mice and the antioxidant functions in PC12 cells. Inflammation 2014, 37, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Moustakas, I.I.; Pyrina, I.; Lembessis, P.; Koutsilieris, M.; Chatzigeorgiou, A. Metabolic inflammation as an instigator of fibrosis during non-alcoholic fatty liver disease. World J. Gastroenterol. 2020, 26, 1993–2011. [Google Scholar] [CrossRef] [PubMed]
- Świderska, M.; Maciejczyk, M.; Zalewska, A.; Pogorzelska, J.; Flisiak, R.; Chabowski, A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic. Res. 2019, 53, 841–850. [Google Scholar] [CrossRef]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef]
- Hajighasem, A.; Farzanegi, P.; Mazaheri, Z. Effects of combined therapy with resveratrol, continuous and interval exercises on apoptosis, oxidative stress, and inflammatory biomarkers in the liver of old rats with non-alcoholic fatty liver disease. Arch. Physiol. Biochem. 2019, 125, 142–149. [Google Scholar] [CrossRef]
- El-Din, S.H.; Sabra, A.N.; Hammam, O.A.; Ebeid, F.A.; El-Lakkany, N.M. Pharmacological and antioxidant actions of garlic and.or onion in non-alcoholic fatty liver disease (NAFLD) in rats. J. Egypt. Soc. Parasitol. 2014, 44, 295–308. [Google Scholar]
- Clark, R.B. The role of PPARs in inflammation and immunity. J. Leukoc. Biol. 2002, 71, 388–400. [Google Scholar] [CrossRef]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-kappaB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharm. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, R.; Liu, K.; Li, M.; Luo, H.; Cui, L.; Huang, L.; Luo, L. Fucoxanthin attenuates LPS-induced acute lung injury via inhibition of the TLR4/MyD88 signaling axis. Aging 2020, 13, 2655–2667. [Google Scholar] [CrossRef]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.L.; Wang, G.; Chen, L.; et al. Fucoxanthin, a Marine Xanthophyll Isolated From Conticribra weissflogii ND-8: Preventive Anti-Inflammatory Effect in a Mouse Model of Sepsis. Front. Pharmacol. 2019, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, N.H.; Kim, S.J.; Lee, H.J.; Kim, S. Fucoxanthin Inhibits the Inflammation Response in Paw Edema Model through Suppressing MAPKs, Akt, and NFkappaB. J. Biochem. Mol. Toxicol. 2016, 30, 111–119. [Google Scholar] [CrossRef]
- Wu, S.J.; Liou, C.J.; Chen, Y.L.; Cheng, S.C.; Huang, W.C. Fucoxanthin Ameliorates Oxidative Stress and Airway Inflammation in Tracheal Epithelial Cells and Asthmatic Mice. Cells 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Cruz, C.M.; Rinna, A.; Forman, H.J.; Ventura, A.L.; Persechini, P.M.; Ojcius, D.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007, 282, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, L.; Del Ben, M.; Baratta, F.; Perri, L.; Albanese, F.; Pastori, D.; Violi, F.; Angelico, F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J. Hepatol. 2015, 7, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Fukuda, S.; Izumi, H.; Saga, N. Anti-Oxidant and Fucoxanthin Contents of Brown Alga Ishimozuku (Sphaerotrichia divaricata) from the West Coast of Aomori, Japan. Mar. Drugs 2018, 16, 255. [Google Scholar] [CrossRef]
- Seo, M.J.; Seo, Y.J.; Pan, C.H.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Fucoxanthin Suppresses Lipid Accumulation and ROS Production During Differentiation in 3T3-L1 Adipocytes. Phytother. Res. 2016, 30, 1802–1808. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Zhang, L.; Zhao, G.-X.; Chen, Y.; Sun, K.-L.; Wang, B. Fucoxanthin attenuates doxorubicin-induced cardiotoxicity via anti-oxidant and anti-apoptotic mechanisms associated with p38, JNK and p53 pathways. J. Funct. Foods 2019, 62, 103542. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Park, Y.K.; Lee, J.Y. Food components with antifibrotic activity and implications in prevention of liver disease. J. Nutr. Biochem. 2018, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kokeny, G.; Nemeth, A.; Kopp, J.B.; Chen, W.; Oler, A.J.; Manzeger, A.; Rosivall, L.; Mozes, M.M. Susceptibility to kidney fibrosis in mice is associated with early growth response-2 protein and tissue inhibitor of metalloproteinase-1 expression. Kidney Int. 2022, 102, 337–354. [Google Scholar] [CrossRef]
- Kim, M.B.; Bae, M.; Hu, S.; Kang, H.; Park, Y.K.; Lee, J.Y. Fucoxanthin exerts anti-fibrogenic effects in hepatic stellate cells. Biochem. Biophys. Res. Commun. 2019, 513, 657–662. [Google Scholar] [CrossRef]
- Michel, L.Y.M.; Farah, C.; Balligand, J.L. The Beta3 Adrenergic Receptor in Healthy and Pathological Cardiovascular Tissues. Cells 2020, 9, 2584. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Hosokawa, M.; Miyashita, K. Fucoxanthin promotes translocation and induction of glucose transporter 4 in skeletal muscles of diabetic/obese KK-A(y) mice. Phytomedicine 2012, 19, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.I.; Ko, H.C.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Lee, N.H.; Kim, S.J. Fucoxanthin exerts differing effects on 3T3-L1 cells according to differentiation stage and inhibits glucose uptake in mature adipocytes. Biochem. Biophys. Res. Commun. 2011, 409, 769–774. [Google Scholar] [CrossRef]
- Yim, M.J.; Hosokawa, M.; Mizushina, Y.; Yoshida, H.; Saito, Y.; Miyashita, K. Suppressive effects of Amarouciaxanthin A on 3T3-L1 adipocyte differentiation through down-regulation of PPARgamma and C/EBPalpha mRNA expression. J. Agric. Food Chem. 2011, 59, 1646–1652. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, X.; Zhang, W.; Zheng, P.; Huang, F.; Ding, G.; Yang, Z. Protective Effects of Fucoxanthin against Alcoholic Liver Injury by Activation of Nrf2-Mediated Antioxidant Defense and Inhibition of TLR4-Mediated Inflammation. Mar. Drugs 2019, 17, 552. [Google Scholar] [CrossRef]
- Zheng, D.; Chen, L.; Li, G.; Jin, L.; Wei, Q.; Liu, Z.; Yang, G.; Li, Y.; Xie, X. Fucoxanthin ameliorated myocardial fibrosis in STZ-induced diabetic rats and cell hypertrophy in HG-induced H9c2 cells by alleviating oxidative stress and restoring mitophagy. Food Funct. 2022, 13, 9559–9575. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Kim, S.C.; Lee, J.H.; Lee, J.R.; Kim, I.K.; Baek, S.Y.; Kim, Y.W. Fucoxanthin, the constituent of Laminaria japonica, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress. BMC Complement. Altern. Med. 2018, 18, 97. [Google Scholar] [CrossRef]
- Ma, S.Y.; Park, W.S.; Lee, D.S.; Choi, G.; Yim, M.J.; Lee, J.M.; Jung, W.K.; Park, S.G.; Seo, S.K.; Park, S.J.; et al. Fucoxanthin inhibits profibrotic protein expression in vitro and attenuates bleomycin-induced lung fibrosis in vivo. Eur. J. Pharm. 2017, 811, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Gruzman, A.; Babai, G.; Sasson, S. Adenosine Monophosphate-Activated Protein Kinase (AMPK) as a New Target for Antidiabetic Drugs: A Review on Metabolic, Pharmacological and Chemical Considerations. Rev. Diabet. Stud. 2009, 6, 13–36. [Google Scholar] [CrossRef]
- Garcia, D.; Hellberg, K.; Chaix, A.; Wallace, M.; Herzig, S.; Badur, M.G.; Lin, T.; Shokhirev, M.N.; Pinto, A.F.M.; Ross, D.S.; et al. Genetic Liver-Specific AMPK Activation Protects against Diet-Induced Obesity and NAFLD. Cell Rep. 2019, 26, 192–208.e6. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Rawson, R.B.; Brown, M.S. Mutant mammalian cells as tools to delineate the sterol regulatory element-binding protein pathway for feedback regulation of lipid synthesis. Arch. Biochem. Biophys. 2002, 397, 139–148. [Google Scholar] [CrossRef]
- Kim, Y.R.; Lee, E.J.; Shin, K.O.; Kim, M.H.; Pewzner-Jung, Y.; Lee, Y.M.; Park, J.W.; Futerman, A.H.; Park, W.J. Hepatic triglyceride accumulation via endoplasmic reticulum stress-induced SREBP-1 activation is regulated by ceramide synthases. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Peters, J.M.; Shah, Y.M.; Gonzalez, F.J. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer 2012, 12, 181–195. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [PubMed]
- Matsusue, K.; Kusakabe, T.; Noguchi, T.; Takiguchi, S.; Suzuki, T.; Yamano, S.; Gonzalez, F.J. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008, 7, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Ilchovska, D.D.; Barrow, D.M. An Overview of the NF-kB mechanism of pathophysiology in rheumatoid arthritis, investigation of the NF-kB ligand RANKL and related nutritional interventions. Autoimmun. Rev. 2021, 20, 102741. [Google Scholar] [CrossRef] [PubMed]
- Creed, T.J.; Probert, C.S. Review article: Steroid resistance in inflammatory bowel disease—Mechanisms and therapeutic strategies. Aliment. Pharm. 2007, 25, 111–122. [Google Scholar] [CrossRef]
- Sohrabi, M.; Gholami, A.; Amir Kalali, B.; Khoonsari, M.; Sahraei, R.; Nasiri Toosi, M.; Zamani, F.; Keyvani, H. Are Serum Levels of Nuclear Factor Kappa B and Forkhead Box Protein P3 in Patients with Non-Alcoholic Fatty Liver Disease Related to Severity of Fibrosis? Middle East J. Dig. Dis. 2021, 13, 356–362. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Lin, L.; Chen, P. Liraglutide in combination with human umbilical cord mesenchymal stem cell could improve liver lesions by modulating TLR4/NF-kB inflammatory pathway and oxidative stress in T2DM/NAFLD rats. Tissue Cell 2020, 66, 101382. [Google Scholar] [CrossRef]
- Mehta, M.; Duseja, A.; Gupta, S. Phyllanthin Regulates Expression of NFKB/PI3K/AKT Pathway and Improves Liver Histology in Animal Model of NAFLD: A Preliminary Study. J. Clin. Exp. Hepatol. 2022, 12, S7. [Google Scholar] [CrossRef]
- Peluso, I.; Yarla, N.S.; Ambra, R.; Pastore, G.; Perry, G. MAPK signalling pathway in cancers: Olive products as cancer preventive and therapeutic agents. Semin. Cancer Biol. 2019, 56, 185–195. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Zhao, Y.; Li, M.; Guo, L. Targeting DUSP7 signaling alleviates hepatic steatosis, inflammation and oxidative stress in high fat diet (HFD)-fed mice via suppression of TAK1. Free Radic. Biol. Med. 2020, 153, 140–158. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Gradinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ma, M.; Zhang, S. Recent advances in delivery systems of fucoxanthin. Food Chem. 2023, 404, 134685. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Shanmugapriya, K.; Gereniu, C.R.N.; Chae, S.-J.; Kang, H.W.; Woo, H.-C.; Chun, B.-S. Ultrasound-mediated fucoxanthin rich oil nanoemulsions stabilized by κ-carrageenan: Process optimization, bio-accessibility and cytotoxicity. Ultrason. Sonochemistry 2019, 55, 105–116. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Zhao, L.; Yan, H.; Wang, S.; Wang, D. The stability and bioaccessibility of fucoxanthin in spray-dried microcapsules based on various biopolymers. RSC Adv. 2018, 8, 35139–35149. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ma, M.; Wang, D.; Xu, Y. A review of factors affecting the stability of zein-based nanoparticles loaded with bioactive compounds: From construction to application. Crit. Rev. Food Sci. Nutr. 2022, 1–17, Epub ahead of print. [Google Scholar] [CrossRef]
- Li, Y.; Dou, X.; Pang, J.; Liang, M.; Feng, C.; Kong, M.; Liu, Y.; Cheng, X.; Wang, Y.; Chen, X. Improvement of fucoxanthin oral efficacy via vehicles based on gum Arabic, gelatin and alginate hydrogel: Delivery system for oral efficacy enhancement of functional food ingredients. J. Funct. Foods 2019, 63, 103573. [Google Scholar] [CrossRef]
- Wang, C.; Ren, J.; Song, H.; Chen, X.; Qi, H. Characterization of whey protein-based nanocomplex to load fucoxanthin and the mechanism of action on glial cells PC12. Lwt 2021, 151, 112208. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Z.; Xue, C.; Tang, Q.; Zhang, T.; Chang, Y.; Wang, Y. Fucoxanthin-loaded nanoparticles composed of gliadin and chondroitin sulfate: Synthesis, characterization and stability. Food Chem. 2022, 379, 132163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, Z.; Liu, W.; Hou, Z.; Zhang, D.; Huang, L.; Zhang, Y. Oleic acid as a protein ligand improving intestinal absorption and ocular benefit of fucoxanthin in water through protein-based encapsulation. Food Funct. 2019, 10, 4381–4395. [Google Scholar] [CrossRef]
- Malgarim Cordenonsi, L.; Faccendini, A.; Catanzaro, M.; Bonferoni, M.C.; Rossi, S.; Malavasi, L.; Platcheck Raffin, R.; Scherman Schapoval, E.E.; Lanni, C.; Sandri, G.; et al. The role of chitosan as coating material for nanostructured lipid carriers for skin delivery of fucoxanthin. Int. J. Pharm. 2019, 567, 118487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, H.; Xu, Y.; Wang, D. Construction of fucoxanthin vector based on binding of whey protein isolate and its subsequent complex coacervation with lysozyme. J. Agric. Food Chem. 2019, 67, 2980–2990. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Kersten, S.; Mandard, S.; Tan, N.S.; Escher, P.; Metzger, D.; Chambon, P.; Gonzalez, F.J.; Desvergne, B.; Wahli, W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 2000, 275, 28488–28493. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Duan, Y.; Wei, H.; Ning, H.; Bi, C.; Zhao, Y.; Qin, Y.; Li, Y. Acetyl-CoA carboxylase (ACC) as a therapeutic target for metabolic syndrome and recent developments in ACC1/2 inhibitors. Expert Opin. Investig. Drugs 2019, 28, 917–930. [Google Scholar] [CrossRef]
- Qian, G.; Morral, N. Role of non-coding RNAs on liver metabolism and NAFLD pathogenesis. Hum. Mol. Genet. 2022, 31, R4–R21. [Google Scholar] [CrossRef]
- Shen, X.; Guo, H.; Xu, J.; Wang, J. Inhibition of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis by inhibiting the MAPK signaling pathway in rats with nonalcoholic fatty liver disease. J. Cell Physiol. 2019, 234, 18169–18179. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Xu, H.; Feng, X.; Lin, Y.; Huang, Y.; Peng, Y.; Gu, M. Obesity-induced upregulation of miR-361-5p promotes hepatosteatosis through targeting Sirt1. Metabolism 2018, 88, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Guo, J.; Fang, W.; Dou, L.; Li, M.; Huang, X.; Zhou, S.; Man, Y.; Tang, W.; Yu, L.; et al. Liver MicroRNA-291b-3p Promotes Hepatic Lipogenesis through Negative Regulation of Adenosine 5’-Monophosphate (AMP)-activated Protein Kinase alpha1. J. Biol. Chem. 2016, 291, 10625–10634. [Google Scholar] [CrossRef]
- Liu, W.; Cao, H.; Ye, C.; Chang, C.; Lu, M.; Jing, Y.; Zhang, D.; Yao, X.; Duan, Z.; Xia, H.; et al. Hepatic miR-378 targets p110alpha and controls glucose and lipid homeostasis by modulating hepatic insulin signalling. Nat. Commun. 2014, 5, 5684. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, H.; Liu, Z.; Sun, X.; Zhang, D.; Wang, S.; Xu, Y.; Zhang, G.; Wang, D. Modulation of Gut Microbiota by Fucoxanthin During Alleviation of Obesity in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2020, 68, 5118–5128. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Yang, B.; Pang, X.; Chen, T.; Chen, F.; Cheng, K.-W. Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food Funct. 2019, 10, 5644–5655. [Google Scholar] [CrossRef] [PubMed]
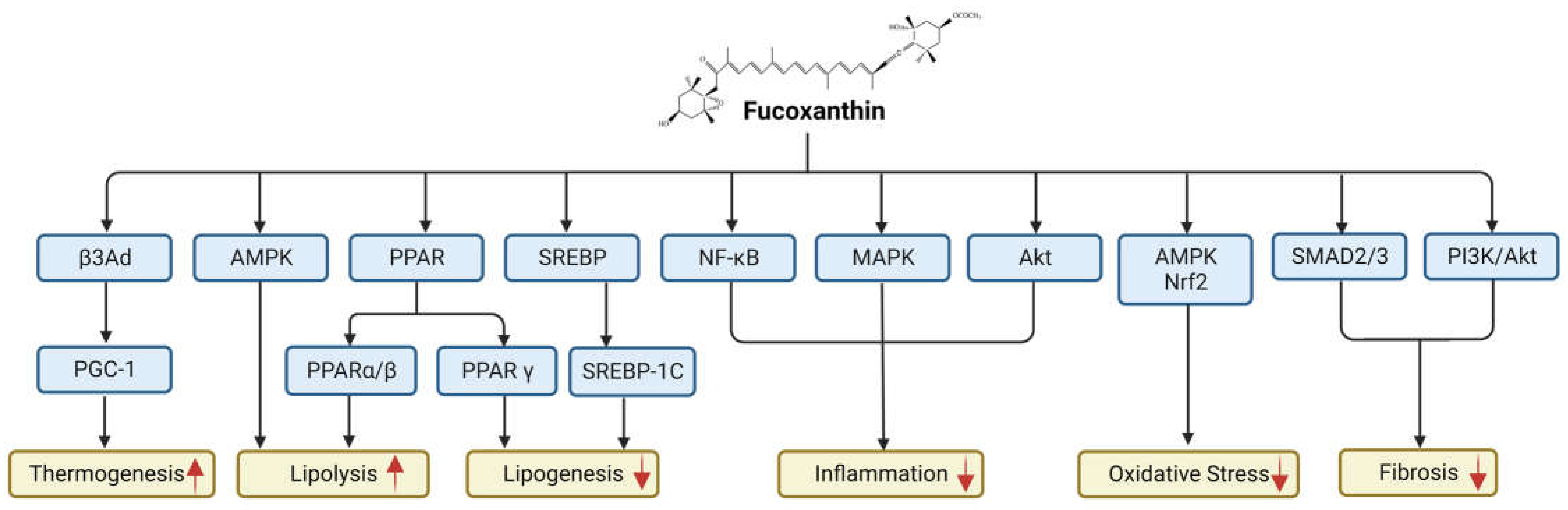
| Transcriptional Factors | Experimental Model | Dose | Reference(s) |
|---|---|---|---|
| Thermogenesis Related | |||
| ↑ PGC-1 | KK-Ay mice | Undaria pinnatifida-extracted fucoxanthin; 0.5% and 2% of control diet; | [51] |
| 0.2% fucoxanthin in control diet (AIN-93G) | [105] | ||
| High-fat-diet-induced obese mice | 0.69% Undaria pinnatifida-extracted fucoxanthin (2.9%) | [76] | |
| ↑ β3-adrenergic receptor (β3Ad) | KK-Ay mice | Undaria pinnatifida-extracted fucoxanthin; 0.5% and 2% of control diet; | [51] |
| High-fat-diet-induced obese mice | 1.06% or 2.22% in control diet (AIN-93G) | [57] | |
| Lipid Metabolism Related | |||
| ↑ AMPK | High-fat-diet-induced obese mice | Petalonia binghamiae-extracted fucoxanthin; 150 mg/kg/day | [7] |
| ↑ PPARα | Undaria pinnatifida-extracted fucoxanthin (2.9%); 0.69% w/w | [76] | |
| ↑ PPAR β | 0.05% and 0.2% fucoxanthin, w/w | [9] | |
| ↓ PPAR γ | High-fat-diet-induced obese mice | 0.05% and 0.2% fucoxanthin, w/w | [9] |
| Petalonia binghamiae-extracted fucoxanthin; 150 mg/kg/day | [7] | ||
| Undaria pinnatifida-extracted fucoxanthin (2.9%); 0.69% w/w | [76] | ||
| 3T3-L1 | Petalonia binghamiae-extracted fucoxanthin; 10 μM | [106] | |
| fucoxanthin, fucoxanthinol, and amarouciaxanthin extracted from U. pinnatifida; 10 μM | [107] | ||
| ↓ SREBP1c | High-fat-diet-induced obese mice | Petalonia binghamiae-extracted fucoxanthin; 150 mg/kg/day | [7] |
| Inflammation Related | |||
| ↓ NF-κB | RAW 264.7 macrophages | I. okamurae-extracted fucoxanthin; 12.5, 25, or 50 μM | [86] |
| Carr-induced paw edema in ICR mice | Undaria pinnatifida-extracted fucoxanthin; 4 and 8 mg/kg | [89] | |
| ↓ MAPK | Macrophage RAW 264.7 cells | I. okamurae-extracted fucoxanthin; 12.5, 25, or 50 μM | [86] |
| Carr-induced paw edema in ICR mice | Undaria pinnatifida-extracted fucoxanthin; 4 and 8 mg/kg | [89] | |
| ↓ Akt | |||
| Anti-Oxidant Related | |||
| ↑ Nrf2 | Alcoholic liver injury mouse model | 10, 20, 40 mg/kg b.w. | [108] |
| H9c2 cells | 1 μM | [109] | |
| ↑ AMPK | HepC2 cells | L. Japonica-extracted fucoxanthin; 30 μg/mL | [110] |
| Anti-Fibrogenic | |||
| ↓ SMAD2/3 | Human pulmonary fibroblasts (HPFs) | 5, 10, 20 μM | [111] |
| ↓ PI3K/Akt | |||
| ↓ MAPK | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winarto, J.; Song, D.-G.; Pan, C.-H. The Role of Fucoxanthin in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 8203. https://doi.org/10.3390/ijms24098203
Winarto J, Song D-G, Pan C-H. The Role of Fucoxanthin in Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2023; 24(9):8203. https://doi.org/10.3390/ijms24098203
Chicago/Turabian StyleWinarto, Jessica, Dae-Geun Song, and Cheol-Ho Pan. 2023. "The Role of Fucoxanthin in Non-Alcoholic Fatty Liver Disease" International Journal of Molecular Sciences 24, no. 9: 8203. https://doi.org/10.3390/ijms24098203
APA StyleWinarto, J., Song, D.-G., & Pan, C.-H. (2023). The Role of Fucoxanthin in Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences, 24(9), 8203. https://doi.org/10.3390/ijms24098203








