Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation
Abstract
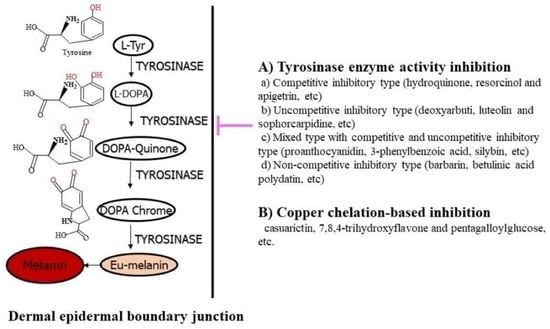
1. Importance of Tyrosinase and Melanin Synthesis
 indicates the increase of synthesis.
indicates the increase of synthesis.
 indicates the increase of synthesis.
indicates the increase of synthesis.
2. Inhibition of Melanin Biosynthesis and Natural Tyrosinase Inhibitor Sources
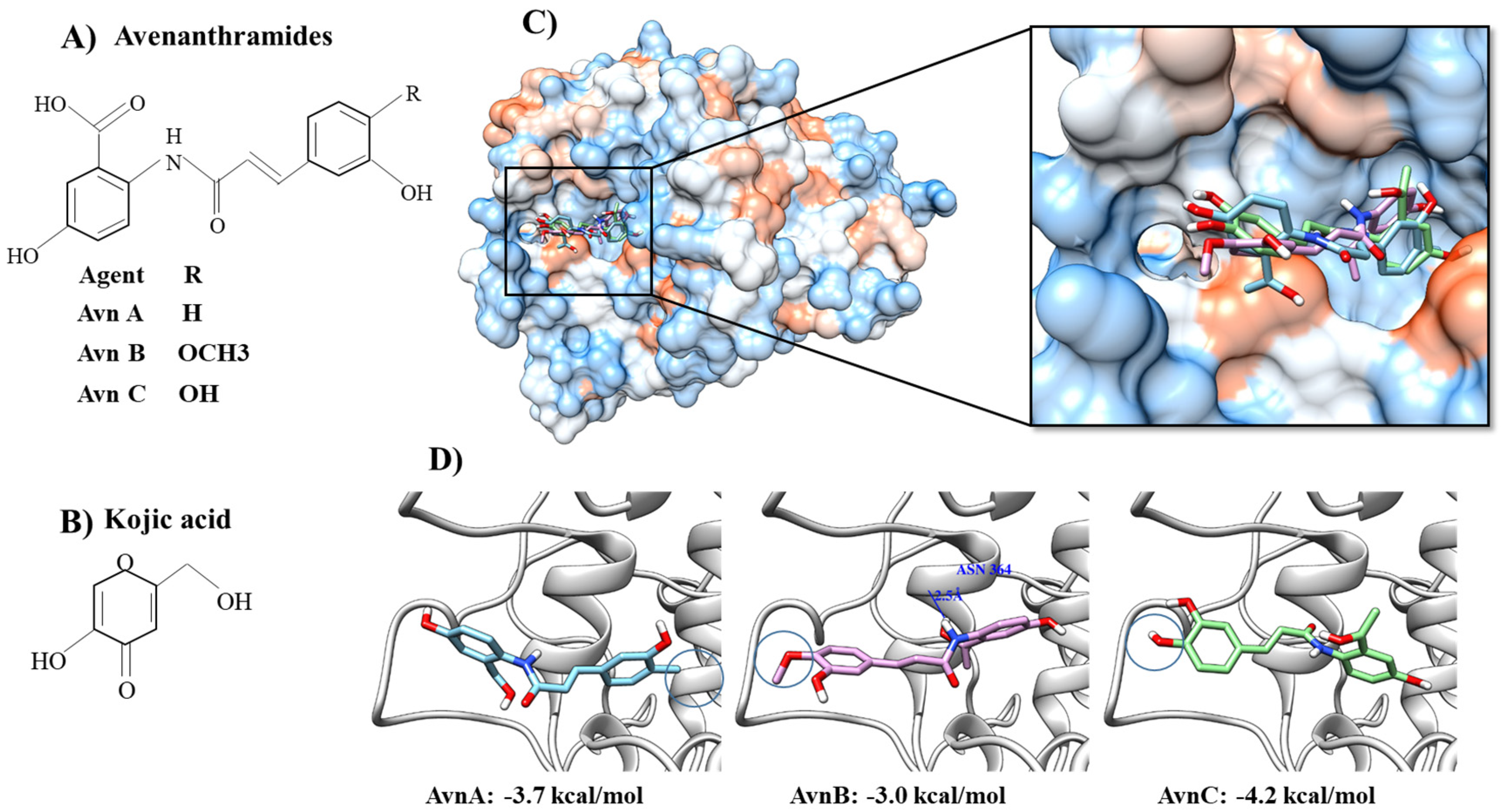
2.1. Molecular Docking Simulation of Compounds with Tyrosinase and Structure–Activity Relationship (SAR) of Inhibitors
2.2. Derek Nexus for Prediction of Skin Sensitization
3. Tyrosinase Inhibitor Types Based on Action Mechanism
3.1. Classical Tyrosinase Inhibitor Types
3.2. Tyrosinase Inhibitor Types Based on Action Mechanism
3.2.1. Competitive Inhibitory Type
3.2.2. Uncompetitive Inhibitory Type
3.2.3. Mixed Type Inhibitors with Competitive and Uncompetitive Modes
3.2.4. Noncompetitive inhibitory type
4. Tyrosinase Inhibitor Types Based on Copper Chelation and Melanogenic Downregulation
4.1. Properties of Metalloenzyme Tyrosinase
4.2. Copper Chelating Tyrosinase Inhibitors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Videira, I.F.; Dos, S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin pigments and much more-types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Kothari, S.; Chavan, B.; Spencer, J.D. Regulation of melanogenesis—Controversies and new concepts. Exp. Dermatol. 2008, 17, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef]
- Hassan, M.; Abbas, Q.; Ashraf, Z.; Moustafa, A.A.; Seo, S.Y. Pharmacoinformatics exploration of polyphenol oxidases leading to novel inhibitors by virtual screening and molecular dynamic simulation study. Comput. Biol. Chem. 2017, 68, 131–142. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, H.J.; Choi, K.H.; Chung, J.H.; Kim, K.H.; Park, K.C. UVB-induced GM-CSF production is suppressed by dexamethasone in HaCaT cells. Photodermatol. Photoimmunol. Photomed. 2001, 17, 121–125. [Google Scholar] [CrossRef]
- Demunter, A.; De Wolf-Peeters, C.; Degreef, H.; Stas, M.; van den Oord, J.J. Expression of the endothelin-B receptor in pigment cell lesions of the skin. Evidence for its role as tumor progression marker in malignant melanoma. Virchows Arch. 2001, 438, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, S.; Yasumoto, K.; Amae, S.; Udono, T.; Watanabe, K.; Saito, H.; Takeda, K. Regulation of pigment cell specific gene expression by MITF. Pigment Cell Res. 2000, 13 (Suppl. 8), 98–102. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Setaluri, V. Role of microphanthalmia transcription factor in the regulation of melanocyte differentiation marker TRP-1. Biochem. Biophy. Res. Commun. 1999, 256, 657–663. [Google Scholar] [CrossRef]
- Algahtani, H.; Marghalani, S.; Satti, M.; Shirah, B. Levetiracetam-Induced Skin Hyperpigmentation: An Extremely Rare Undesirable Side Effect. J. Epilepsy Res. 2017, 7, 106. [Google Scholar] [CrossRef]
- Chawla, S.; Kvalnes, K.; Wickett, R.; Manga, P.; Boissy, R.E. Deoxyarbutin and its derivatives inhibit tyrosinase activity and melanin synthesis without inducing reactive oxygen species or apoptosis. J. Drugs Dermatol. 2012, 11, e28–e34. [Google Scholar] [PubMed]
- Tasaka, K.; Kamei, C.; Nakano, S.; Takeuchi, Y.; Yamato, M. Effects of certain resorcinol derivatives on the tyrosinase activity and the growth of melanoma cells. Methods Find. Exp. Clin. Pharmacol. 1998, 20, 99–109. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Seo, S.-Y.; Babar, M.M.; Zaidi, N.-U.S. Synthesis, kinetic mechanism and docking studies of vanillin derivatives as inhibitors of mushroom tyrosinase. Bioorg. Med. Chem. 2015, 23, 5870–5880. [Google Scholar] [CrossRef]
- Cabanes, J.; Chazarra, S.; GARCIA-CARMONA, F. Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase. J. Pharm. Pharmacol. 1994, 46, 982–985. [Google Scholar] [CrossRef]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.A.; Berna, J.; Rodriguez-Lopez, J.N.; Tudela, J.; Garcia-Canovas, F. Action of tyrosinase on alpha and beta-arbutin: A kinetic study. PLoS ONE 2017, 12, e0177330. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, H.J.; Park, T.; Lee, M.J.; Lim, H.S.; Yang, W.S.; Hwang, C.W.; Park, D.; Kim, C.H. Inhibitory Effect of Avenanthramides (Avn) on Tyrosinase Activity and Melanogenesis in α-MSH-Activated SK-MEL-2 Cells: In Vitro and In Silico Analysis. Int. J. Mol. Sci. 2021, 22, 7814. [Google Scholar] [CrossRef]
- Alam, J.; Sharma, L. Potential Enzymatic Targets in Alzheimer’s: A Comprehensive Review. Curr Drug Targets. 2019, 20, 316–339. [Google Scholar] [CrossRef]
- Gao, H. Predicting tyrosinase inhibition by 3D QSAR pharmacophore models and designing potential tyrosinase inhibitors from traditional Chinese medicine database. Phytomedicine 2018, 38, 145–157. [Google Scholar] [CrossRef]
- Wilm, A.; Kühnl, J.; Kirchmair, J. Computational approaches for skin sensitization prediction. Crit. Rev. Toxicol. 2018, 48, 738–760. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.E.; Dean, P.M. Three-dimensional hydrogen-bond geometry and probability information from a crystal survey. J. Comput. Aided Mol. Des. 1996, 10, 607–622. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Haghbeen, K.; Saboury, A.A.; Karbassi, F. Substrate share in the suicide inactivation of mushroom tyrosinase. Biochim. Biophys. Acta 2004, 1675, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Garcia-Ruiz, P.A.; Saura-Sanmartin, A.; Berna, J.; Garcia-Canovas, F.; Rodri-guez-Lopez, J.N. Structural and kinetic considerations on the catalysis of deoxyarbutin by tyrosinase. PLoS ONE 2017, 12, e0187845. [Google Scholar] [CrossRef]
- Avonto, C.; Wang, Y.H.; Avula, B.; Wang, M.; Rua, D.; Khan, I.A. Comparative studies on the chemical and enzymatic stability of alpha- and beta-arbutin. Int. J. Cosmet. Sci. 2016, 38, 187–193. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakajima, T.; Iwadate, T.; Nihei, K.I. Chemical synthesis and tyrosinase-inhibitory activity of isotachioside and its related glycosides. Carbohydr. Res. 2018, 465, 22–28. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Lee, N.K.; Son, K.H.; Chang, H.W.; Kang, S.S.; Park, H.; Heo, M.Y.; Kim, H.P. Prenylated flavonoids as tyrosinase inhibitors. Arch. Pharm. Res. 2004, 27, 1132–1135. [Google Scholar] [CrossRef]
- Lou, S.N.; Yu, M.W.; Ho, C.T. Tyrosinase inhibitory components of immature calamondin peel. Food Chem. 2012, 35, 1091–1096. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I. Flavonols from saffron flower: Tyrosinase inhibitory activity and inhibition mechanism. J. Agric. Food Chem. 1999, 47, 4121–4125. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Cesar, T.B.; Jackson, E.; Mertens-Talcott, S. Pharmacokinetic study of nobiletin and tangeretin in rat serum by high-performance liquid chromatography-electrospray ionization-mass spectrometry. J. Agric. Food Chem. 2011, 59, 145–151. [Google Scholar] [CrossRef]
- Gao, H.; Nishida, J.; Saito, S.; Kawabata, J. Inhibitory effects of 5,6,7-trihydroxyflavones on tyrosinase. Molecules 2007, 12, 86–97. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, G.; Chen, J.; Zheng, Z.P. Characterization of a new flavone and tyrosinase inhibition constituents from the Twigs of Morus alba L. Molecules 2016, 21, 1130. [Google Scholar] [CrossRef]
- Demirkiran, O.; Sabudak, T.; Ozturk, M.; Topcu, G. Antioxidant and tyrosinase inhibitory activities of flavonoids from Trifolium nigrescens Subsp. petrisavi. J. Agric. Food Chem. 2013, 61, 12598–12603. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cheng, X.; Wang, L.; Wang, S.; Ren, G. Mushroom tyrosinase inhibitors from mung bean (Vigna radiatae L.) extracts. Int. J. Food Sci. Nutr. 2012, 63, 358–361. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Ha, T.J.; Curtis-Long, M.J.; Ryu, H.W.; Gal, S.W.; Park, K.H. Inhibitory effects on mushroom tyrosinase by flavones from the stem barks of Morus lhou (S.) Koidz. J. Enzym. Inhib. Med. Chem. 2008, 23, 922–930. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, I.S.; So, Y.K.; Kim, H.-H.; Kim, Y.H. Kushenol a and 8-prenylkaempferol, tyrosinase inhibitors, derived from Sophora flavescens. J. Enzym. Inhib. Med. Chem. 2018, 33, 1048–1054. [Google Scholar] [CrossRef]
- Jeong, S.H.; Ryu, Y.B.; Curtis-Long, M.J.; Ryu, H.W.; Baek, Y.S.; Kang, J.E.; Lee, W.S.; Park, K.H. Tyrosinase inhibitory polyphenols from roots of Morus lhou. J. Agric. Food Chem. 2009, 57, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, D.H.; Lee, J.K.; Lee, J.Y.; Kim, D.H.; Kim, H.K.; Lee, H.J.; Kim, H.C. Natural ortho-dihydroxyisoflavone derivatives from aged Korean fermented soybean paste as potent tyrosinase and melanin formation inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1162–1164. [Google Scholar] [CrossRef]
- Chang, T.S.; Ding, H.Y.; Lin, H.C. Identifying 6,7,4’-trihydroxyisoflavone as a potent tyrosinase inhibitor. Biosci. Biotechnol. Biochem. 2005, 69, 1999–2001. [Google Scholar] [CrossRef]
- Chang, T.S. Two potent suicide substrates of mushroom tyrosinase: 7,8,4’-trihydroxyisoflavone and 5,7,8,4’-tetrahydroxyisoflavone. J. Agric. Food Chem. 2007, 55, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.Y.; Kim, Y.M.; Lee, J.; Park, S.H.; Kim, J.; Park, H.M.; Lee, C.H. Desmodianone H and uncinanone B, potential tyrosinase inhibitors obtained from Lespedeza maximowiczii by using bioactivity-guided isolation. Biosci. Biotechnol. Biochem. 2014, 78, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Seo, S.H.; Lee, B.G.; Lee, Y.S. Identification of tyrosinase inhibitors from Glycyrrhiza uralensis. Planta Med. 2005, 71, 785–787. [Google Scholar] [CrossRef]
- Kim, J.M.; Ko, R.K.; Jung, D.S.; Kim, S.S.; Lee, N.H. Tyrosinase inhibitory constituents from the stems of Maackia fauriei. Phytother. Res. 2010, 24, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Kang, S.; Kim, J.-B.; Kim, Y.; Jin, C. Chemical constituents from Apios americana and their inhibitory activity on tyrosinase. Molecules 2018, 23, 232. [Google Scholar] [CrossRef]
- Si, Y.X.; Wang, Z.J.; Park, D.; Chung, H.Y.; Wang, S.F.; Yan, L.; Yang, J.M.; Qian, G.Y.; Yin, S.J.; Park, Y.D. Effect of hesperetin on tyrosinase: Inhibition kinetics integrated computational simulation study. Int. J. Biol. Macromol. 2012, 50, 257–262. [Google Scholar] [CrossRef]
- Chiari, M.E.; Vera, D.M.; Palacios, S.M.; Carpinella, M.C. Tyrosinase inhibitory activity of a 6-isoprenoid-substituted flavanone isolated from Dalea elegans. Bioorg. Med. Chem. 2011, 19, 3474–3482. [Google Scholar] [CrossRef]
- Hu, X.; Yu, M.-H.; Yan, G.-R.; Wang, H.-Y.; Hou, A.-J.; Lei, C. Isoprenylated phenolic compounds with tyrosinase inhibition from Morus nigra. J. Asian Nat. Prod. Res. 2018, 20, 488–493. [Google Scholar] [CrossRef]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 200. [Google Scholar] [CrossRef]
- Chai, W.M.; Lin, M.Z.; Wang, Y.X.; Xu, K.L.; Huang, W.Y.; Pan, D.D.; Zou, Z.R.; Peng, Y.Y. Inhibition of tyrosinase by cherimoya pericarp proanthocyanidins: Structural characterization, inhibitory activity and mechanism. Food Res. Int. 2017, 100, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Chung, J.E.; Kurisawa, M.; Uyama, H.; Kobayashi, S. New tyrosinase inhibitors, (+)-catechin-aldehyde polycondensates. Biomacromolecules 2004, 5, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Lin-Tao Wang, Z.T.; Wei, D.Z.; Xiang, H.B. Mechanism and inhibitory effect of galangin and its flavonoid mixture from Alpinia officinarum on mushroom tyrosinase and B16 murine melanoma cells. J. Enzym. Inhib. Med. Chem. 2007, 22, 433–438. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Yan, J.; Gong, D. Inhibitory effect of morin on tyrosinase: Insights from spectroscopic and molecular docking studies. Food Chem. 2014, 163, 226–233. [Google Scholar] [CrossRef]
- Jhan, J.K.; Chung, Y.C.; Chen, G.H.; Chang, C.H.; Lu, Y.C.; Hsu, C.K. Anthocyanin contents in the seed coat of black soya bean and their anti-human tyrosinase activity and antioxidative activity. Int. J. Cosmet. Sci. 2016, 38, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Du, Z.; Xue, G.; Chen, Q.; Lu, Y.; Zheng, X.; Conney, A.H.; Zhang, K. Synthesis and biological evaluation of unsymmetrical curcumin analogues as tyrosinase inhibitors. Molecules 2013, 18, 3948–3961. [Google Scholar] [CrossRef] [PubMed]
- Le-Thi-Thu, H.; Casañola-Martín, G.M.; Marrero-Ponce, Y.; Rescigno, A.; Saso, L.; Parmar, V.S.; Torrens, F.; Abad, C. Novel coumarin-based tyrosinase inhibitors discovered by OECD principles-validated QSAR approach from an enlarged, balanced database. Mol. Divers. 2011, 15, 507–520. [Google Scholar] [CrossRef]
- Pintus, F.; Matos, M.J.; Vilar, S.; Hripcsak, G.; Varela, C.; Uriarte, E.; Santana, L.; Borges, F.; Medda, R.; Di Petrillo, A.; et al. New insights into highly potent tyrosinase inhibitors based on 3-heteroarylcoumarins: Anti-melanogenesis and antioxidant activities, and computational molecular modeling studies. Bioorg. Med. Chem. 2017, 25, 1687–1695. [Google Scholar] [CrossRef]
- Masamoto, Y.; Ando, H.; Murata, Y.; Shimoishi, Y.; Tada, M.; Takahata, K. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci. Biotechnol. Biochem. 2003, 67, 631–634. [Google Scholar] [CrossRef]
- Asthana, S.; Zucca, P.; Vargiu, A.V.; Sanjust, E.; Ruggerone, P.; Rescigno, A. Structure–activity relationship study of hydroxycoumarins and mushroom tyrosinase. J. Agric. Food Chem. 2015, 63, 7236–7244. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Hou, A.; Wang, H. Inhibitory effect of 2,4,2’,4’-tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone on tyrosinase activity and melanin biosynthesis. Biol. Pharm. Bull. 2009, 32, 86–90. [Google Scholar] [CrossRef]
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef]
- Okombi, S.; Rival, D.; Bonnet, S.; Mariotte, A.-M.; Perrier, E.; Boumendjel, A. Discovery of benzylidenebenzofuran-3(2H)-one (aurones) as inhibitors of tyrosinase derived from human melanocytes. J. Med. Chem. 2006, 49, 329–333. [Google Scholar] [CrossRef]
- Hu, X.; Wu, J.W.; Wang, M.; Yu, M.H.; Zhao, Q.S.; Wang, H.Y.; Hou, A.J. 2-Arylbenzofuran, flavonoid, and tyrosinase inhibitory constituents of Morus yunnanensis. J. Nat. Prod. 2012, 75, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Seong, S.H.; Zhou, Y.; Shrestha, S.; Jung, H.A.; Choi, J.S. Structure(−)activity relationship of the tyrosinase inhibitors kuwanon G, mulberrofuran G, and albanol B from Morus species: A kinetics and molecular docking study. Molecules 2018, 23, 1413. [Google Scholar] [CrossRef]
- Liang, C.P.; Chang, C.H.; Liang, C.C.; Hung, K.Y.; Hsieh, C.W. In vitro antioxidant activities, free radical scavenging capacity, and tyrosinase inhibitory of flavonoid compounds and ferulic acid from Spiranthes sinensis (Pers.). AMES Mol. 2014, 19, n4681–n4694. [Google Scholar] [CrossRef]
- Lin, Y.F.; Hu, Y.H.; Lin, H.T.; Liu, X.; Chen, Y.H.; Zhang, S.; Chen, Q.X. Inhibitory effects of propyl gallate on tyrosinase and its application in controlling pericarp browning of harvested longan fruits. J. Agric. Food Chem. 2013, 61, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Ishiguro, K.; Kubo, I. Tyrosinase inhibitory p-coumaric acid from ginseng leaves. Phytother. Res. 1999, 13, 371–375. [Google Scholar] [CrossRef]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Garcia-Ruiz, P.A.; Saura-Sanmartin, A.; Berna, J.; Rodríguez-López, J.N.; Garcia-Canovas, F. Action of tyrosinase on caffeic acid and its n-nonyl ester. Catalysis and suicide inactivation. Int. J. Biol. Macromol. 2018, 107, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-H.; Chen, Q.-X.; Cui, Y.; Gao, H.-J.; Xu, L.; Yu, X.-Y.; Wang, Y.; Yan, C.-L.; Wang, Q. 4-Hydroxy cinnamic acid as mushroom preservation: Anti-tyrosinase activity kinetics and application. Int. J. Biol. Macromol. 2016, 86, 489–495. [Google Scholar] [CrossRef]
- Iwai, K.; Kishimoto, N.; Kakino, Y.; Mochida, K.; Fujita, T. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J. Agric. Food Chem. 2004, 52, 4893–4898. [Google Scholar] [CrossRef]
- Park, J.; Boo, Y.C. Isolation of resveratrol from Vitis Viniferae caulis and its potent inhibition of human tyrosinase. Evid. Based Complement Alternat. Med. 2013, 2013, 645257. [Google Scholar] [CrossRef]
- Shin, N.H.; Ryu, S.Y.; Choi, E.J.; Kang, S.H.; Chang, I.M.; Min, K.R.; Kim, Y. Oxyresveratrol as the potent inhibitor on DOPA oxidase activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 1998, 243, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.M.; Ha, Y.M.; Kim, J.-A.; Chung, K.W.; Uehara, Y.; Lee, K.J.; Chun, P.; Byun, Y.; Chung, H.Y.; Moon, H.R. Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 7451–7455. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, M.; Yoshimatsu, M.; Inoue, A.; Kanno, T.; Tatefuji, T.; Hashimoto, K. Inhibitory effect of gnetin c, a resveratrol dimer from melinjo (Gnetum gnemon), on tyrosinase activity and melanin biosynthesis. Biol. Pharm. Bull. 2012, 35, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, J.H.; Baek, S.H.; Seo, J.H.; Kho, Y.H.; Oh, T.K.; Lee, C.H. Enhancement of tyrosinase inhibition of the extract of Veratrum patulum using cellulase. Biotechnol. Bioeng. 2004, 87, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Kubo, I. Resveratrol as a kcat type inhibitor for tyrosinase: Potentiated melanogenesis inhibitor. Bioorg. Med. Chem. 2012, 20, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, Z.; Rafiq, M.; Nadeem, H.; Hassan, M.; Afzal, S.; Waseem, M.; Afzal, K.; Latip, J. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLoS ONE 2017, 12, e0178069. [Google Scholar] [CrossRef]
- Tan, C.; Zhu, W.; Lu, Y. Aloin, cinnamic acid and sophorcarpidine are potent inhibitors of tyrosinase. Chin. Med. J. 2002, 115, 1859–1862. [Google Scholar]
- Leu, Y.L.; Hwang, T.L.; Hu, J.W.; Fang, J.Y. Anthraquinones from Polygonum cuspidatum as tyrosinase inhibitors for dermal use. Phytother. Res. 2008, 22, 552–556. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hu, G.; Zhang, Q.; Yang, Y.X.; Li, Q.Q.; Hu, Y.J.; Chen, H.; Yang, F.Q. Screening and characterizing tyrosinase inhibitors from Salvia miltiorrhiza and Carthamus tinctorius by spectrum-effect relationship analysis and molecular docking. J. Anal. Methods Chem. 2018, 2018, 2141389. [Google Scholar] [CrossRef] [PubMed]
- Kahn, V. Effect of kojic acid on the oxidation of DL-dopa, norepinephrine, and dopamine by mushroom tyrosinase. Pigment Cell Res. 1995, 8, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Wagle, A.; Seong, S.H.; Park, H.J.; Jung, H.A.; Choi, J.S. A New Tyrosinase Inhibitor from the Red Alga Symphyocladia latiuscula (Harvey) Yamada (Rhodomelaceae). Mar. Drugs. 2019, 17, 295. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Li, Y.; Chen, M.; Wong, V.K.W.; Zhang, K.; Zheng, X.; Liu, W. Inhibition of plant essential oils and their interaction in binary combinations against tyrosinase. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.C.; Lin, H.H.; Tsai, K.C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 7995. [Google Scholar] [CrossRef]
- Hashim, F.J.; Vichitphan, S.; Han, J.; Vichitphan, K. Alternative Approach for Specific Tyrosinase Inhibitor Screening: Uncompetitive Inhibition of Tyrosinase by Moringa oleifera. Molecules 2021, 26, 4576. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; He, X.; Teng, B.; McRae, J.M. Valonea Tannin: Tyrosinase Inhibition Activity, Structural Elucidation and Insights into the Inhibition Mechanism. Molecules 2021, 26, 2747. [Google Scholar] [CrossRef] [PubMed]
- Di Petrillo, A.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement Altern. Med. 2016, 16, 453. [Google Scholar] [CrossRef]
- Ding, H.Y.; Chang, T.S.; Shen, H.C.; Tai, S.S. Murine tyrosinase inhibitors from Cynanchum bungei and evaluation of in vitro and in vivo depigmenting activity. Exp. Dermatol, 2011; 20, 720–724. [Google Scholar] [CrossRef]
- Lin, R.D.; Chen, M.C.; Liu, Y.L.; Lin, Y.T.; Lu, M.K.; Hsu, F.L.; Lee, M.H. New Whitening Constituents from Taiwan-Native Pyracantha koidzumii: Structures and Tyrosinase Inhibitory Analysis in Human Epidermal Melanocytes. Int. J. Mol. Sci. 2015, 16, 28598–28613. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H. Ov-16 4-(3,4-dihydroxybenzoyloxymethyl)phenyl-O-β-D-glucopyranoside inhibits melanin synthesis by regulating expressions of melanogenesis-regulated gene and protein. Exp. Dermatol. 2011, 20, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M. Structure and inhibition mechanism of some synthetic compounds and phenolic derivatives as tyrosinase inhibitors: Review and new insight. J. Biomol. Struct. Dyn. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.J.; Si, Y.X.; Qian, G.Y. Inhibitory effect of phthalic acid on tyrosinase: The mixed-type inhibition and docking simulations. Enzym. Res. 2011, 2011, 294724. [Google Scholar] [CrossRef]
- Hridya, H.; Amrita, A.; Sankari, M.; Doss, C.G.P. Inhibitory effect of brazilein on tyrosinase and melanin synthesis: Kinetics and in silico approach. Int. J. Biol. Macromol. 2015, 81, 228–234. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Seo, S.-Y.; Kwon, K.S.; Babar, M.M.; Zaidi, N.-U.S. Kinetic and in silico studies of novel hydroxy-based thymol analogues as inhibitors of mushroom tyrosinase. Eur. J. Med. Chem. 2015, 98, 203–211. [Google Scholar] [CrossRef]
- Karbassi, F.; Saboury, A.A.; Khan, M.T.H.; Choudhary, M.I.; Saifi, Z.S. Mushroom tyrosinase inhibition by two potent uncompetitive inhibitors. J. Enzym. Inhib. Med. Chem. 2004, 19, 349–353. [Google Scholar] [CrossRef]
- Guo, N.; Wang, C.; Shang, C.; You, X.; Zhang, L.; Liu, W. Integrated study of the mechanism of tyrosinase inhibition by baicalein using kinetic, multispectroscopic and computational simulation analyses. Int. J. Biol. Macromol. 2018, 118, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.-M.; Huang, Q.; Lin, M.-Z.; Ou-Yang, C.; Huang, W.-Y.; Wang, Y.-X.; Xu, K.-L.; Feng, H.-L. Condensed tannins from longan bark as inhibitor of tyrosinase: Structure, activity, and mechanism. J. Agric. Food Chem. 2018, 66, 908–917. [Google Scholar] [CrossRef]
- Oyama, T.; Takahashi, S.; Yoshimori, A.; Yamamoto, T.; Sato, A.; Kamiya, T.; Abe, H.; Abe, T.; Tanuma, S.I. Discovery of a new type of scaffold for the creation of novel tyrosinase inhibitors. Bioorg. Med. Chem. 2016, 24, 4509–4515. [Google Scholar] [CrossRef]
- Micillo, R.; Pistorio, V.; Pizzo, E.; Panzella, L.; Napolitano, A.; D’Ischia, M. 2-S-Lipoylcaffeic Acid, a Natural Product-Based Entry to Tyrosinase Inhibition via Catechol Manipulation. Biomimetics 2017, 2, 15. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.Y.; Jenis, J.; Li, Z.P.; Ban, Y.J.; Baiseitova, A.; Park, K.H. Tyrosinase inhibitory study of flavonolignans from the seeds of Silybum marianum (Milk thistle). Bioorg. Med. Chem. 2019, 27, 2499–2507. [Google Scholar] [CrossRef]
- Ma, D.; Tu, Z.C.; Wang, H.; Zhang, L.; He, N.; McClements, D.J. Mechanism and kinetics of tyrosinase inhibition by glycolic acid: A study using conventional spectroscopy methods and hydrogen/deuterium exchange coupling with mass spectrometry. Food Funct. 2017, 8, 122–131. [Google Scholar] [CrossRef]
- Sheng, Z.; Ge, S.; Xu, X.; Zhang, Y.; Wu, P.; Zhang, K.; Xu, X.; Li, C.; Zhao, D.; Tang, X. Design, synthesis and evaluation of cinnamic acid ester derivatives as mushroom tyrosinase inhibitors. Medchemcomm 2018, 9, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.M.; Wei, Q.M.; Deng, W.L.; Zheng, Y.L.; Chen, X.Y.; Huang, Q.; Ou-Yang, C.; Peng, Y.Y. Anti-melanogenesis properties of condensed tannins from Vigna angularis seeds with potent antioxidant and A damage protection activities. Food Funct. 2019, 10, 99–111. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.L.; Dong, W.H.; Li, W.; Wang, P.; Cao, X.; Yuan, J.Z.; Chen, H.Q.; Mei, W.L.; Dai, H.F. Sesquiterpenoids and 2-(2-phenylethyl)chromones respectively acting as α-glucosidase and tyrosinase inhibitors from agarwood of an Aquilaria plant. J. Enzym. Inhib. Med. Chem. 2019, 34, 853–862. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Zhang, X.; Zheng, J.; Hu, W.; Teng, B. Hop Tannins as Multifunctional Tyrosinase Inhibitor: Structure Characterization, Inhibition Activity, and Mechanism. Antioxidants 2022, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Zhang, M.X.; Dong, X.W.; Hu, Y.Z.; Dai, X.Y.; Wei, X.; Hider, R.C.; Zhang, J.C.; Zhou, T. Design and synthesis of novel hydroxypyridinone derivatives as potential tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3103–3108. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Li, L.; Hu, S.Q. Molecular inhibitory mechanism of tricin on tyrosinase. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 107, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory mechanisms of glabridin on tyrosinase. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 168, 111–117. [Google Scholar] [CrossRef]
- Jirawattanapong, W.; Saifah, E.; Patarapanich, C. Synthesis of glabridin derivatives as tyrosinase inhibitors. Arch. Pharm. Res. 2009, 32, 647–654. [Google Scholar] [CrossRef]
- Li, L.; Cai, Y.; Sun, X.; Du, X.; Jiang, Z.; Ni, H.; Yang, Y.; Chen, F. Tyrosinase inhibition by p-coumaric acid ethyl ester identified from camellia pollen. Food Sci. Nutr. 2020, 9, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Saehlim, N.; Athipornchai, A.; Sirion, U.; Saeeng, R. New class of alkynyl glycoside analogues as tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127276. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, X.; Ni, H.; Du, X.; Chen, F.; Jiang, Z.; Li, Q. Identification and Characterization of the Tyrosinase Inhibitory Activity of Caffeine from Camellia Pollen. J. Agric. Food Chem. 2019, 67, 12741–12751. [Google Scholar] [CrossRef] [PubMed]
- Matos, P.; Paranhos, A.; Batista, M.T.; Figueirinha, A. Synergistic Effect of DIBOA and Verbascoside from Acanthus mollis Leaf on Tyrosinase Inhibition. Int. J. Mol.Sci. 2022, 23, 13536. [Google Scholar] [CrossRef]
- Biswas, R.; Chanda, J.; Kar, A.; Mukherjee, P.K. Tyrosinase inhibitory mechanism of betulinic acid from Dillenia indica. Food Chem. 2017, 232, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, L.; Zhai, J.; Han, N.; Liu, Z.; Li, S.; Yin, J. Characterization of antioxidant, α-glucosidase and tyrosinase inhibitors from the rhizomes of Potentilla anserina L. and their structure-activity relationship. Food Chem. 2021, 336, 127714. [Google Scholar] [CrossRef]
- Sari, S.; Barut, B.; Özel, A.; Kuruüzüm-Uz, A.; Şöhretoğlu, D. Tyrosinase and α-glucosidase inhibitory potential of compounds isolated from Quercus coccifera bark: In vitro and in silico perspectives. Bioorg. Chem. 2019, 86, 296–304. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wang, H.M.; Wen, Y.S.; Liu, W.; Li, P.H.; Chiu, C.C.; Chen, P.C.; Huang, C.Y.; Sheu, J.H.; Wen, Z.H. 4-(Phenylsulfanyl)butan-2-One Suppresses Melanin Synthesis and Melanosome Maturation In Vitro and In Vivo. Int. J. Mol. Sci. 2015, 16, 20240–20257. [Google Scholar] [CrossRef]
- Nihei, K.I.; Kubo, I. Substituent effect of benzaldehydes on tyrosinase inhibition. Plant Physiol. Biochem. 2017, 112, 278–282. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.M.; Zhang, J.; Zhang, Y.Q. An efficient preparation of mulberroside a from the branch bark of mulberry and its effect on the inhibition of tyrosinase activity. PLoS ONE 2014, 9, e109396. [Google Scholar] [CrossRef]
- Hemachandran, H.; Jain, F.; Mohan, S.; Kumar, D.T.; Priya Doss, C.G.; Ramamoorthy, S. Glandular hair constituents of Mallotus philippinensis Muell. fruit act as tyrosinase inhibitors: Insights from enzyme kinetics and simulation study. Int. J. Biol. Macromol 2018, 107 Pt B, 1675–1682. [Google Scholar] [CrossRef]
- Ketata, E.; Elleuch, H.; Neifar, A.; Mihoubi, W.; Ayadi, W.; Marrakchi, N.; Rezgui, F.; Gargouri, A. Anti-melanogenesis potential of a new series of Morita-Baylis-Hillman adducts in B16F10 melanoma cell line. Bioorg. Chem. 2019, 84, 17–23. [Google Scholar] [CrossRef]
- Goenka, S.; Ceccoli, J.; Simon, S.R. Anti-melanogenic activity of ellagitannin casuarictin in B16F10 mouse melanoma cells. Nat. Prod. Res. 2021, 35, 1830–1835. [Google Scholar] [CrossRef]
- Shang, C.; Zhang, Y.; You, X.; Guo, N.; Wang, Y.; Fan, Y.; Liu, W. The effect of 7,8,4’-trihydroxyflavone on tyrosinase activity and conformation: Spectroscopy and docking studies. Luminescence 2018, 33, 681–691. [Google Scholar] [CrossRef]
- Mu, Y.; Li, L.; Zhou, Y.; Wei, H.L.; Hu, S.Q. Inhibitory mechanism of red globe amaranth on tyrosinase. J. Cosmet. Sci. 2013, 64, 99–110. [Google Scholar]
- Kubo, I.; Kinst-Hori, I.; Chaudhuri, S.K.; Kubo, Y.; Sánchez, Y.; Ogura, T. Flavonols from Heterotheca limuloids: Tyrosinase inhibitory activity and structural criteria. Bioorg. Med. Chem. 2000, 8, 1749–1755. [Google Scholar] [CrossRef]
- Li, C.Y.; Lee, E.J.; Wu, T.S. Antityrosinase principles and constituents of the petals of Crocus sativus. J. Nat. Prod. 2004, 67, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Shi, Y.; Chai, W.M.; Feng, H.L.; Zhuang, J.X.; Chen, Q.X. Condensed tannins from Ficus virens as tyrosinase inhibitors: Structure, inhibitory activity and molecular mechanism. PLoS ONE 2014, 9, e91809. [Google Scholar] [CrossRef]
- McDonald, M.I.; Scalbert, A. Precipitation of metal ions by plant polyphenols: Optimal conditions and origin of precipitation. J. Agric. Food Chem. 1996, 44, 599–606. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Zhang, L.; Wei, S.; Qin, Z.; Liang, D.; Ding, B.; Chen, H.; Song, W. Conformational changes of tyrosinase caused by pentagalloylglucose binding: Implications for inhibitory effect and underlying mechanism. Food Res. Int. 2022, 157, 111312. [Google Scholar] [CrossRef]
- da Silva, A.P.; Silva, N.F.; Andrade, E.H.A.; Gratieri, T.; Setzer, W.N.; Maia, J.G.S.; da Silva, J.K.R. Tyrosinase inhibitory activity, molecular docking studies and antioxidant potential of chemotypes of Lippia origanoides (Verbenaceae) essential oils. PLoS ONE 2017, 12, e0175598. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.G.; Gan, C.Y. Multifunctional Tyrosinase Inhibitor Peptides with Copper Chelating, UV-Absorption and Antioxidant Activities: Kinetic and Docking Studies. Foods 2021, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Kubglomsong, S.; Theerakulkait, C.; Reed, R.L.; Yang, L.; Maier, C.S.; Stevens, J.F. Isolation and Identification of Tyrosinase-Inhibitory and Copper-Chelating Peptides from Hydrolyzed Rice-Bran-Derived Albumin. J. Agric. Food Chem. 2018, 66, 8346–8354. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Cheng, S.; Li, H.; Xu, X.; Wang, Z.; Du, M. Tyrosinase inhibitory effects of the peptides from fish scale with the metal copper ions chelating ability. Food Chem. 2022, 390, 133146. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Tian, H.; Xiang, H.; Chen, S.; Li, L.; Hu, X. Copper chelating peptides derived from tilapia (Oreochromis niloticus) skin as tyrosinase inhibitor: Biological evaluation, in silico investigation and in vivo effects. Food Res. Int. 2023, 163, 112307. [Google Scholar] [CrossRef]
- Okajima, S.; Hamamoto, A.; Asano, M.; Isogawa, K.; Ito, H.; Kato, S.; Hirata, Y.; Furuta, K.; Takemori, H. Azepine derivative T4FAT, a new copper chelator, inhibits tyrosinase. Biochem. Biophys. Res. Commun. 2019, 509, 209–215. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Compound Class | Competitive Type | References |
|---|---|---|---|
| Hydroquinone | α-/β-arbutins | [1,26] | |
| 4-6-Hydroxy-2-naphthyl-1,3-bezendiol, resorcinol | resorcin | [13] | |
| Anthocyanidin, aurone, flavan-3,4-diol | flavonoid | [28] | |
| Kuwanon C, papyriflavonol A, sanggenon D, sophoflavescenol, flavonoid vinylation, lupinalbin, 7-O-gentibiosyl 2′-hydroxygenistein, gallocatechin, proanthocyanidins, (-)-8-Chlorocatechin, cyanidin, delphinidin, malvidin, pelargonidin, peonidin | flavonoid derivative | [29,36,47,52,53,56] | |
| 3-O-β-Galactosyl quercetin, 3′,5′-Di-C-β glucosyl phloretin, galactosyl-3-myricetin, potenserin C, 3-O-α-L-rhamnosyl quercetin-2-gallate | flavonoid glycoside | [30] | |
| Apigenin, chrysin, luteolin, baicalein, mormin, cyclomorusin, morusin, norartocarpetin | flavone | [31,37] | |
| Apigetrin, vitexin, baicalin, nobiletin, tangeretin, biflavone,7,8,4′-trihydroxy-isoflavone, 7,3′,4′-trihydroxy-isoflavone, 3-O-β-D-glucosyl 4,5,5,7,7-pentahydroxy 3,3-dimethoxy 3,4-O-biflavone, isovitexin, baicalein, 6-hydroxyapigenin, 6-hydroxy-kaempferol, 6-hydroxygalangin, tricin (5,7,4-trihydroxy-3,5-dimethoxyflavone), vitexin | flavone derivatives (C-glycosyl flavone, hydroxyflavone, flavone glucoside) | monophenolase/diphenolase | [32,33,34,35,36,40,42] |
| Quercetin, 4-O-β-D-glucosyl quercetin, β-D-glucosyl 3-O-6-O-malonyl quercetin, β-D-glucosyl 3-O-6-O-malonyl-kaempferol, morin, (±)2,3-cis-dihydromorin, 2,3-trans-dihydromorin, galangin, kaempferol, 8-prenylkaempferol, epicatechin, epigallocatechin, epicatechin gallate, epigallocatechin gallate, catechin, proanthocyanidin | flavonol | diphenolase | [38,39,50,51,54,55] |
| 6,7,4′-Trihydroxyisoflavone | hydroxyflavone | monophenolase | [41] |
| Daidzein, glyasperin C, formononetin, genistein, mirkoin, texasin, tectorigenin, odoratin | Isoflavone | [45] | |
| Eriodictyol, naringenin, hesperetin, hesperidin, liquiritin naringin, taxifolin, 6-isoprenoid flavanone, nigrasin K, sanggenon M/C/O, chalcomoracin, kuwanon J, sorocein H | flavanone | [48,49] | |
| Curcumin, desmethoxycurcumin, hydroxybenzoate, hydoxycinnamate | phenolic compound | [57] | |
| Esculetin, 8-epi-cleomiscosin, umbelliferone, thiophosphonic acid diamide, diazaphosphinane, resveratrol-hybrid | coumarin | [58,59,60,61] | |
| Butein, chalcone, flavan-3,4-diols, dihydroflavone, dihydrochalcone, 1,3-diphenyl-2-propen-1-one, carthamin, phloretin, sappan-chalcone, isoliquiritigenin, glabrene, 2,4,2,4-hydroxycalcone, 2,4,2′,4′-tetrahydroxychalcone 2,4,2′,4′-tetrahydroxy-3-3-methyl-2-butenyl-chalcone, vulpinoideol-B, dihydrochalcone, morachalcone-A, bavachinin | chalcone | [34,62] | |
| 2-Arylbenzofuran, 2R-2,3-dihydro-2-1-hydroxy-1-methylethyl-2,6-bibenzofuran-6,4-diol, benzofuran flavonoid mulberrofuran G, albanol B, macrourins E | aurone | [65,66] | |
| Resveratrol, oxyresveratrol, azo-resveratrol, azo-oxyresveratrol, E-2-2,4-dihydroxyphenyl, diazinyl, phenyl-4-methylbenzenesulfonate, trans-resveratrol, resveratrol dimer gnetin-C, hydroxystillbene | stilbenes | [56,73,74,75,76,77] | |
| Monoterpenoid phenol, carvacrol aand its derivatives, bakuchiol, iridoid glucoside, sylvestrosyl 7-O-caffeoyl-I, sylvestrosyl 7-O-p-coumaroyl-I | terpenoid | [79] |
| Compounds | Compound Class | Uncompetitive Type | References |
|---|---|---|---|
| Deoxyarbutin | [1] | ||
| Luteolin | diphenolase | [87] | |
| 2,5-Dihydroxyacetophenone (DHAP), 2,6-DHAP | [90] | ||
| β-D-Glucosyl 3,4-dihydroxy-5-methoxybiphenyl-2-O | [91] | ||
| β-D-Glucosyl Ov-16-4-3,4-dihydroxybenzoyloxymethyl phenyl-O | phenolic glycoside | [92] | |
| Sophorcarpidine | flavonoid glycoside | [30] |
| Compounds | Compound Class | Mixed Type | References |
|---|---|---|---|
| Cinnamic acid, aloin, hydroxypyridinone derivatives, phthalic acid derivatives | [93] | ||
| D-Arabinose, brazilein and thymol derivatives | diphenolase | [96,97] | |
| Baicalein | [98] | ||
| Proanthocyanidin, procyanidin, prodelphinidin, propelargonidin, and the acyl derivatives (galloyl benzoate, p-hydroxybenzoate | tannin | [99] | |
| 3-Phenylbenzoic acid | phenolic acid | [100] | |
| 2-S-Lipoyl-CA | CA-dihydrolipoic acid S-conjugate | [101] | |
| Isosilybin A/B, silydianin, 2,3-dihydrosilychristin, silybin, silychristin-A/-B | flavonolignan | monophenolase/diphenolase | [102] |
| Compounds | Compound Class | Noncompetitive Type | References |
|---|---|---|---|
| Barbarin, propanoic acid | [11] | ||
| 7,8,4-Trihydroxyflavone | diphenolase | [109] | |
| 8-Prenylkaempferol derivative Kushenol A, glabridin, 3,4-dihydroglabridin | isoflavone | [110] | |
| p-Coumaric acid ethyl ester | [112] | ||
| 4-Substituted resorcinol | [111] | ||
| Alkynyl glycoside analogues | [113] | ||
| Caffeine | [114] | ||
| Verbascoside and 2,4-dihydroxy-1,4-benzoxazin-3-one | [115] | ||
| Betulinic acid | [116] | ||
| 3-O-α-l-Rhamnosyl-2-gallate quercetin, biflavanols, potenserin-C/-D, 3-O-α-l-rhamnosyl-2-gallate quercetin, biflavanol | reversible/noncompetitive | [116,117] | |
| Polydatin, (-)-8-chlorocatechin, polydatin | [118] | ||
| 4-Phenylsulfanyl butan-2-one, 2-acetyl-5-methoxyphenyl-3-4-hydroxyphenyl acrylate, benzaldehyde | marine natural products | diphenolase | [104,119,120] |
| Oxyresveratrol, mulberroside A | marine natural products | diphenolase | [121] |
| Mallotophilippen A, B | marine natural products | monophenolase | [122] |
| 6-Oxocyclohex-1-en-1-yl ethyl acetate | marine natural products | [123] |
| Compounds | Compound Class | Type | References |
|---|---|---|---|
| Casuarictin | Ellagitannin | noncompetitive | [124] |
| 7,8,4-Trihydroxyflavone | reversible/non-competitive | [125] | |
| Ellagic acid | monophenolase/diphenolase | [126] | |
| Vanillic acid | monophenolase/diphenolase | [126] | |
| Galangin, kaempferol, quercetin | flavonol | competitive, monophenolase | [127,128] |
| Pentagalloylglucose | tannin | monophenolase/diphenolase | [129,130,131] |
| 1,8-Cineole, α-terpineol, thymol, α-/β-phellandrene, (E)-nerolidol, β-caryophyllene | sesquiterpenoid/essential oil | [132] | |
| GYSLGNWVCAAK | peptide | competitive | [133] |
| SSEYYGGEGSSSEQGYYGEG | peptide | [134] | |
| PFRMY, RGFTGM | peptide | reversible/noncompetitive | [136] |
| 5,6,7,8-Tetrahydro-4H-furo 3,2-c-azepine-4-thione | thioamide/azepine | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-D.; Choi, H.; Abekura, F.; Park, J.-Y.; Yang, W.-S.; Yang, S.-H.; Kim, C.-H. Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation. Int. J. Mol. Sci. 2023, 24, 8226. https://doi.org/10.3390/ijms24098226
Kim H-D, Choi H, Abekura F, Park J-Y, Yang W-S, Yang S-H, Kim C-H. Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation. International Journal of Molecular Sciences. 2023; 24(9):8226. https://doi.org/10.3390/ijms24098226
Chicago/Turabian StyleKim, Hee-Do, Hyunju Choi, Fukushi Abekura, Jun-Young Park, Woong-Suk Yang, Seung-Hoon Yang, and Cheorl-Ho Kim. 2023. "Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation" International Journal of Molecular Sciences 24, no. 9: 8226. https://doi.org/10.3390/ijms24098226
APA StyleKim, H.-D., Choi, H., Abekura, F., Park, J.-Y., Yang, W.-S., Yang, S.-H., & Kim, C.-H. (2023). Naturally-Occurring Tyrosinase Inhibitors Classified by Enzyme Kinetics and Copper Chelation. International Journal of Molecular Sciences, 24(9), 8226. https://doi.org/10.3390/ijms24098226