Oxidative Stress, Inflammatory, Angiogenic, and Apoptotic molecules in Proliferative Diabetic Retinopathy and Diabetic Macular Edema Patients
Abstract
1. Introduction
2. Results
2.1. Sociodemographic and Patient Characteristics
2.2. Ophthalmologic Examination
2.3. Bioanalytical Testing
2.3.1. The Blood Parameters of the Study Groups Are Shown in Table 3 and Figure 1, Figure 2 and Figure 3
| SCG | PDRG | DMEG | |
|---|---|---|---|
| Glucose (mg/dL) | 83.47 ± 7.95 | 138.71 ± 42.45 * | 184.35 ± 42.11 * |
| HbA1c (%) | 5.48 ± 0.36 | 7.04 ± 0.76 | 7.76 ± 0.96 |
| Total Cholesterol (mg/dL) | 158.73 ± 20.21 | 220.35 ± 40.41 | 235.51 ± 39.44 * |
| C-Reactive Protein (mg/dL) | 1.73 ± 7.95 | 3.24 ± 0.71 * | 4.08 ± 0.79 * |
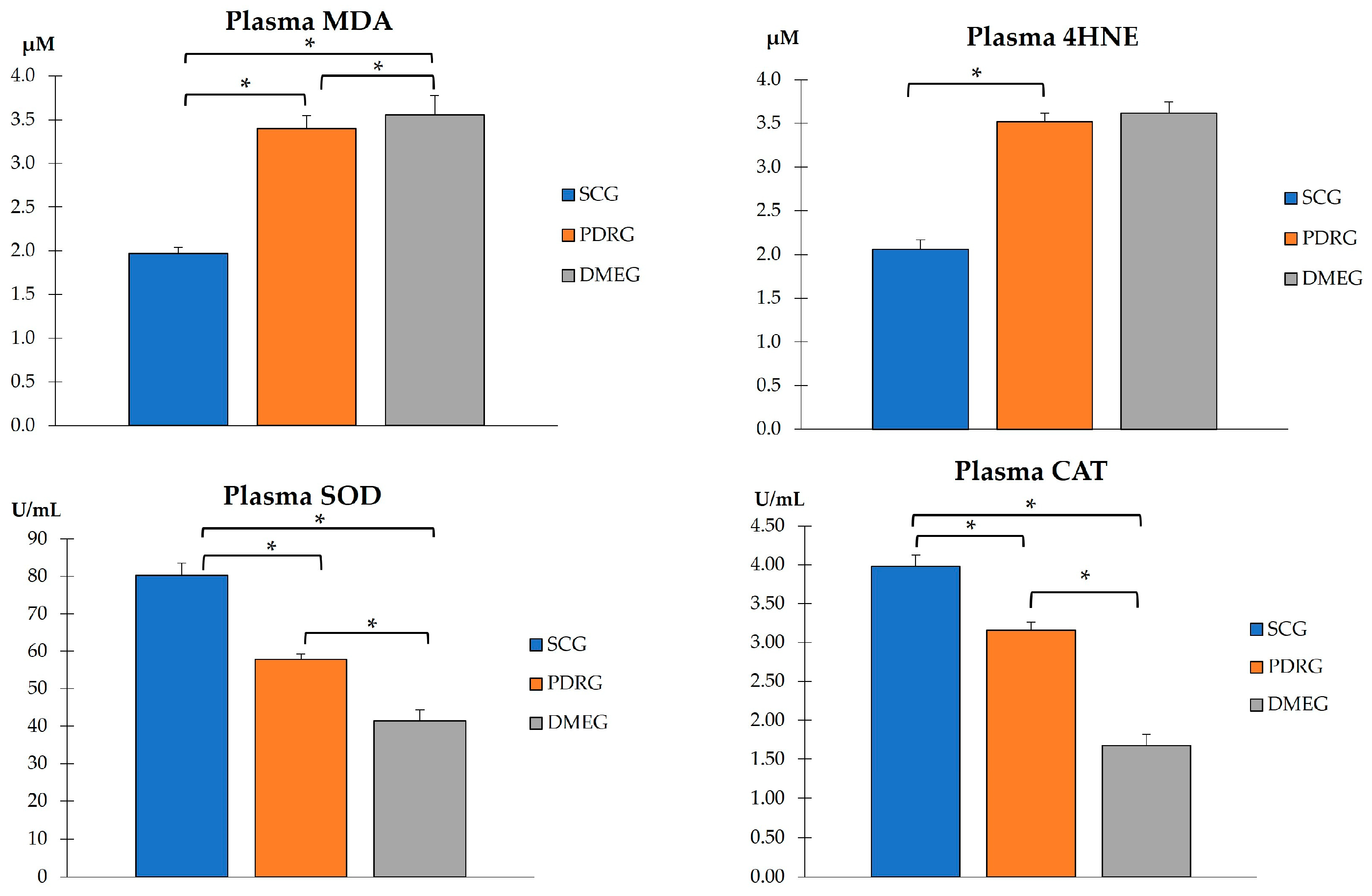
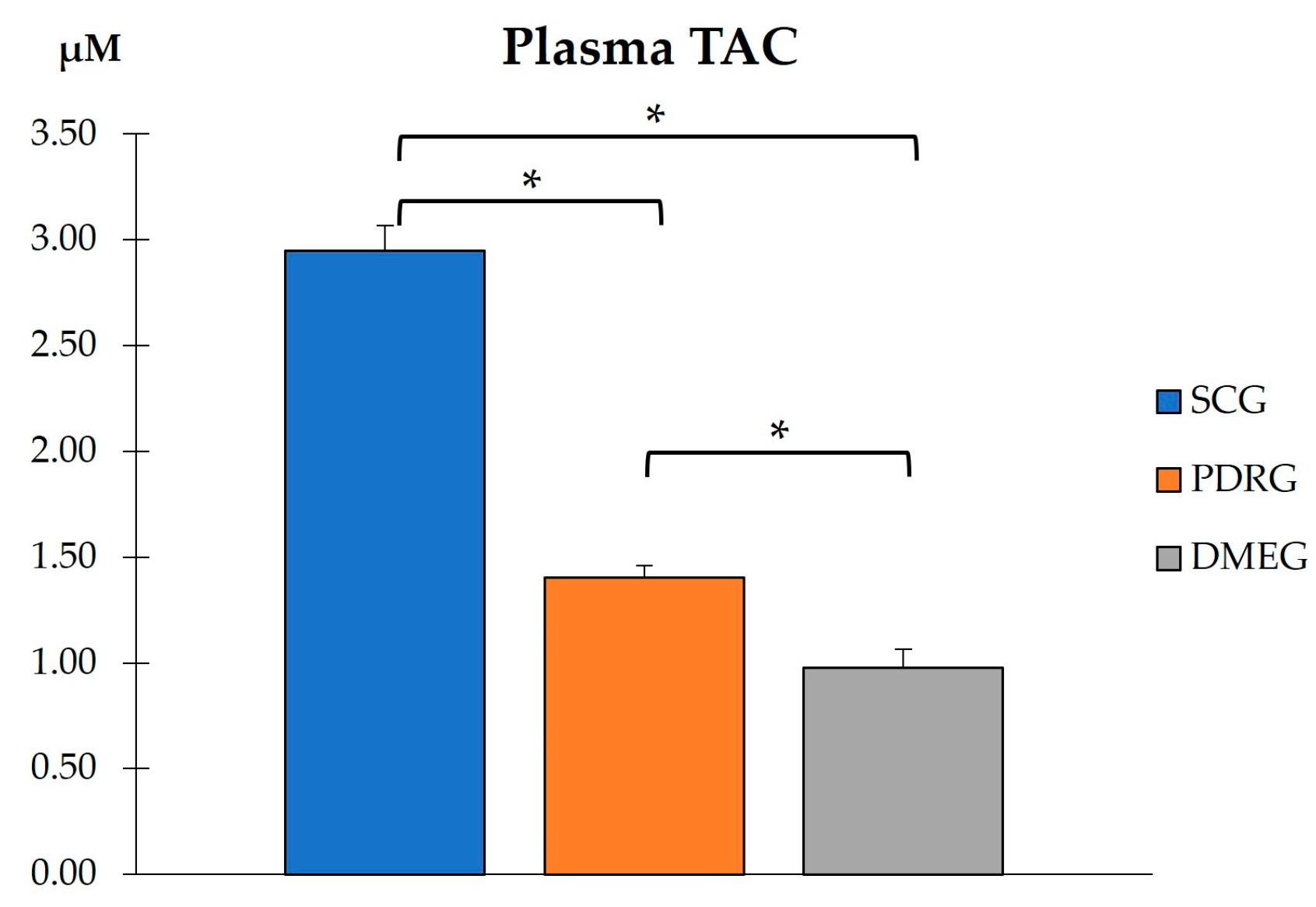
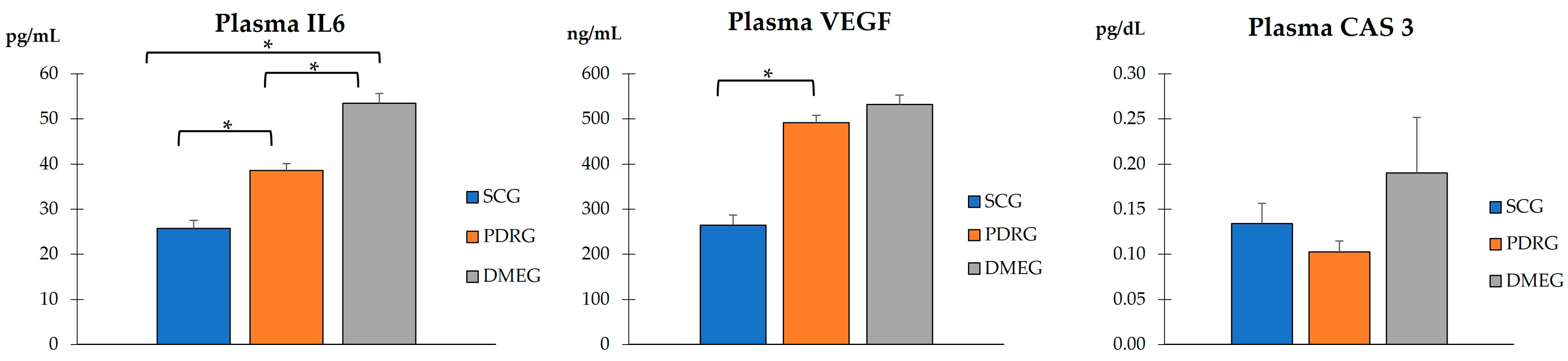
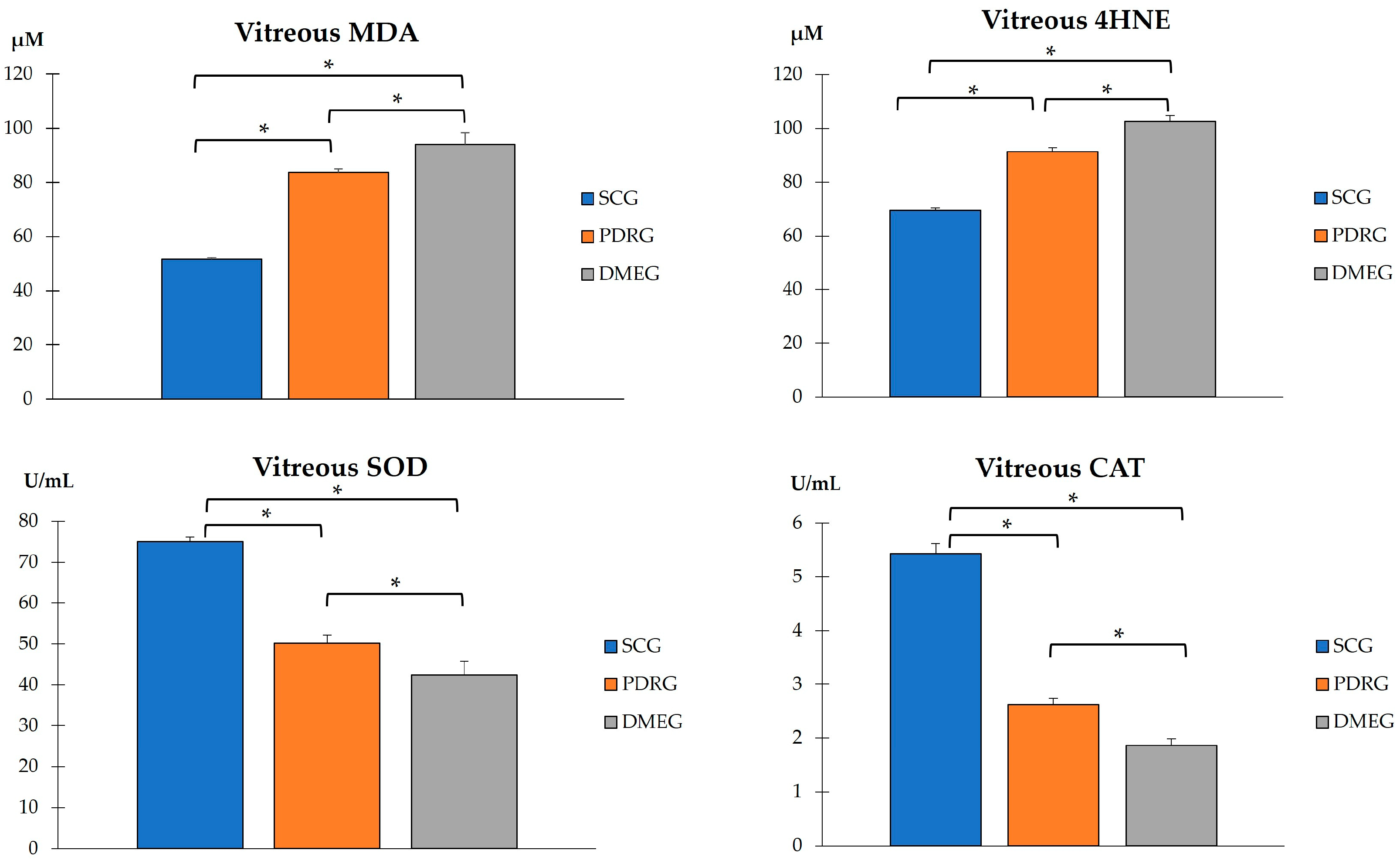
2.3.2. Plasma Parameters of the Study Groups
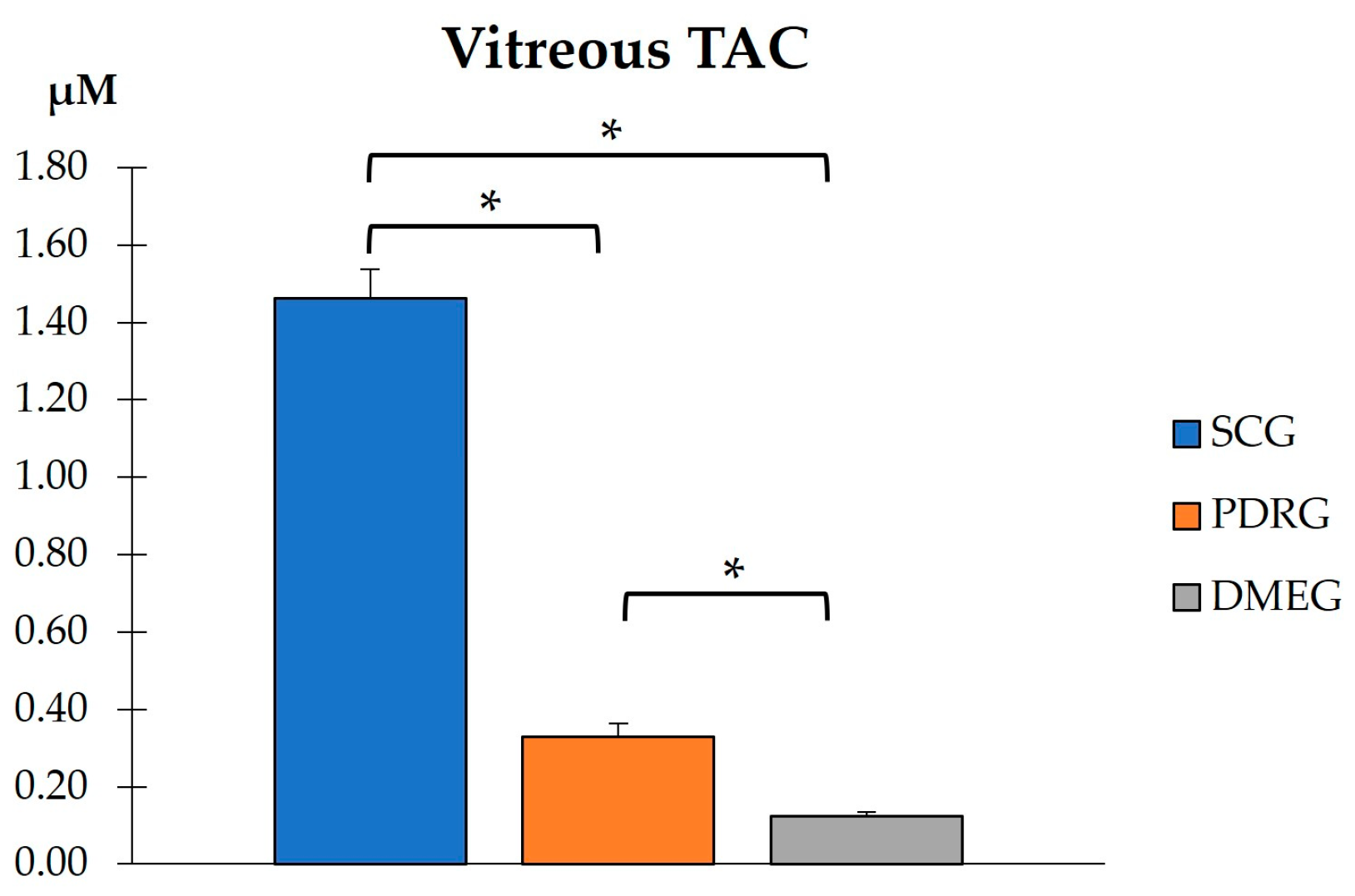
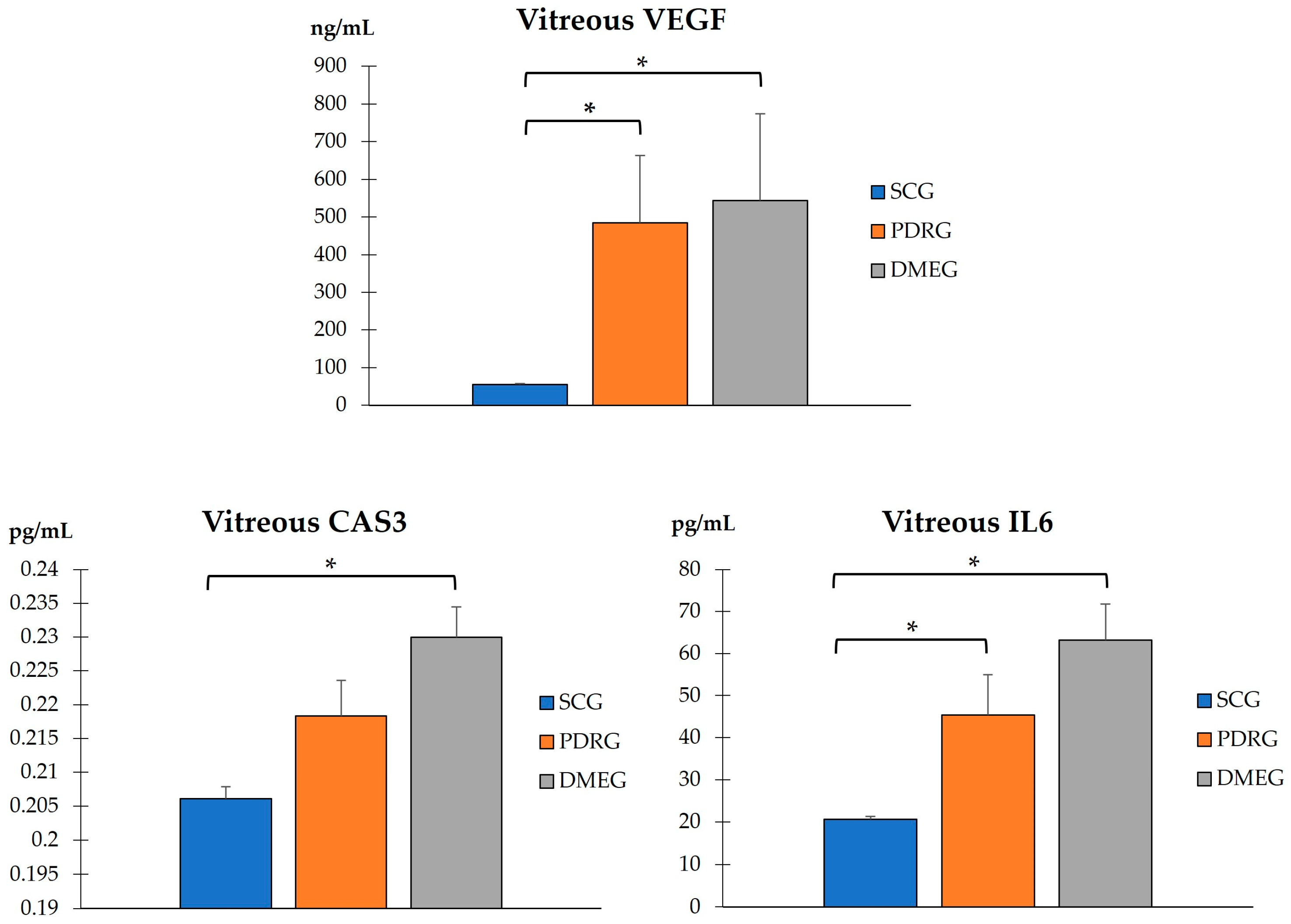
2.3.3. Vitreous Body Parameters
2.3.4. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Participants
4.3. Ophthalmic Examination
4.4. Sampling Procedures
4.4.1. Blood Sampling
4.4.2. Vitreous Body Sampling
4.5. Analytical Laboratory Procedures
4.5.1. Total Blood Samples
4.5.2. Plasma Samples
- (1)
- Lipid peroxidation (LPO) byproducts:
- MDA was quantified using the colorimetric TBARS Assay kit (Ref: 10009055, Cayman Chemical Company, Ann Arbor, MI, USA) a thiobarbituric acid (TBA)-based assessment. In the presence of this acidic reactive, MDA forms what is known as TBA reactive substances (TBARS), the amount of which can be quantified by colorimetric methods. The assay was conducted on 100 µL of plasma following the protocol provided by the manufacturer, with the use of a boiling water bath to reach the required temperature (90–100 °C). The reaction product was measured using a spectrophotometer with a light wavelength of 525 nm. The concentration was calculated by extrapolating all standard curve data, as published elsewhere [30,31,33,61].
- The 4HNE concentration was analyzed using the BIOXYTECH® LPO-586™ Colorimetric Assay for Lipid Peroxidation Markers (Ref: 21012, OXIS Health Products, Inc. Portland, OR; USA). The assay, based on the reaction of a chromogenic reagent, N-methyl-2-phenylindole (R1), with 4-hydroxyalkenals, was conducted following the protocol provided by the manufacturer, using 140 µL of plasma. The reaction occurred under 45 °C of temperature, and the formed product was measured using a spectrophotometer with a light wavelength of 586 nm. The final levels were calculated by extrapolating the standard curve data, as reported before. Since this kit quantifies the concentration of both MDA and 4HNE, the MDA value obtained from the previous kit was subtracted from the value obtained with this kit [61].
- (2)
- Antioxidant molecules:
- SOD activity was measured according to the techniques described in previous works, based on the ability of SOD to inhibit a superoxide-driven reaction in the presence of EDTA, Mn, Cl, and mercaptoethanol [61].
- CAT activity was determined using the absorbance technique per unit of time and is a measure of CAT described by analyzing differences between groups using the SPSS/decrease in absorbance at 240 nm [59].
- The TAC, which is a measure of the combined activities of all of the antioxidants in a sample including vitamins, proteins, lipids, glutathione, and uric acid, was measured in the plasma samples using the colorimetric Antioxidant Assay Kit (Ref: 709001, Cayman Chemical Company, Ann Arbor, MI, USA) based on the antioxidant capacity of the sample to inhibit the 2,2′-azino-di-[3-ethylbenzthiazoline sulphonate] oxidation to 2,2′-azino-di-[3-ethylbenzthiazoline sulphonate] radical solution by the metmyoglobin, as reported. The assay was conducted at room temperature following the protocol provided by the manufacturer, using 10 µL of plasma sample, and the reaction product was measured at a light wavelength of 405 nm using a plate reader. The concentration was calculated by extrapolating all standard curve data [30,49,50,69].
- (3)
- Pro-inflammatory molecules:
- The IL6 expression was calculated in PLS samples by using the Human IL-6 ELISA Kit (Ref: EH2IL6, Invitrogen, Vienna, Austria), an ELISA-based assay. The assay was conducted at room temperature following the protocol provided by the manufacturer, except that the samples were diluted by 1/2 (25 μL of sample and 25 μL of standard diluent). The reaction product was read twice using a plate reader; first at a light wavelength of 450 nm and then at 550 nm. Then, the 550 nm values were subtracted from the 450 nm values to obtain a corrected value and reduce the interference caused by optical imperfections in the microplate. The concentration of IL6 was calculated by extrapolation of the standard curve data [63].
- (4)
- Pro-angiogenic VEGF.
- The PLS levels of the VEGF were measured using the Human VEGF ELISA Kit (Ref: KHG0111, Invitrogen, Vienna, Austria), an ELISA-based assay. The assay was conducted at room temperature following the protocol provided by the manufacturer except that the samples were diluted by 1/2 (50 μL of sample and 50 μL of standard diluent). The reaction product was read with a spectrophotometer at a light wavelength of 450 nm, and the final concentration of VEGF was calculated by extrapolation of the standard curve data [41,45,69].
- (5)
- Pro-apoptotic CAS3.
- The PLS concentration of CAS3 was quantified using the Human Caspase-3 (active) ELISA Kit (Ref: KHO1091, Invitrogen, Vienna, Austria), an enzyme-linked immunosorbent assay (ELISA). The assay was conducted at room temperature following the protocol provided by the manufacturer, using 100 µL of vitreous body sample and reading the reaction product with a plate reader at a light wavelength of 450 nm. The concentration was calculated by extrapolating all standard curve data [50].
4.5.3. Vitreous Body Samples
- (1)
- Pro-oxidants and antioxidants.
- (2)
- Furthermore, the following molecules were assayed in the VIT samples of the study participants:
- Pro-inflammatory IL6. To calculate the concentration of this molecule in the VIT samples, we used the Human IL-6 ELISA Kit (Ref: EH2IL6, Invitrogen, Vienna, Austria), an ELISA-based assay. The assay was conducted at room temperature following the protocol provided by the manufacturer, except that the samples were diluted by 1/2 (25 μL of sample and 25 μL of standard diluent). The reaction product was read twice using a plate reader; first at a light wavelength of 450 nm and then at 550 nm. Then, the 550 nm values were subtracted from the 450 nm values to obtain a corrected value and reduce the interference caused by optical imperfections in the microplate. The concentration of IL6 was calculated by extrapolation of the standard curve data [60].
- Pro-angiogenic VEGF. The VIT levels of the VEGF were measured using the Human VEGF ELISA Kit (Ref: KHG0111, Invitrogen, Vienna, Austria), an ELISA-based assay. The assay was conducted at room temperature following the protocol provided by the manufacturer except that the samples were diluted by 1/2 (50 μL of sample and 50 μL of standard diluent). The reaction product was read with a spectrophotometer at a light wavelength of 450 nm, and the final concentration of VEGF was calculated by extrapolation of the standard curve data [41,45,69].
- Pro-apoptotic CAS3. The concentration of CAS3 was quantified using the Human Caspase-3 (active) ELISA Kit (Ref: KHO1091, Invitrogen, Vienna, Austria), an enzyme-linked immunosorbent assay (ELISA). The assay was conducted at room temperature following the protocol provided by the manufacturer, using 100 µL of vitreous body sample and reading the reaction product with a plate reader at a light wavelength of 450 nm. The concentration was calculated by extrapolating all standard curve data [50].
4.6. Statistical Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| AGEs | advanced glycation end products |
| BCVA | best-corrected visual acuity |
| BRB | blood retinal barrier |
| CAS3 | cysteine protease 3 |
| CAT | antioxidant catalase |
| CAT | cube average thickness on OCT |
| CSFT | central subfield foveal thickness on OCT |
| DM | diabetes mellitus |
| DME | diabetic macular edema |
| DMEG | diabetic macular edema group |
| DR | diabetic retinopathy |
| ELISA | enzyme-linked immunosorbent assays |
| IL | interleukin |
| IL6 | interleukin 6 |
| IOP | intraocular pressure |
| LE | left eye |
| LogMAR | logarithm of the minimum angle of resolution. |
| LPO | lipid peroxidation. |
| MAPK | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| NADH/NAD | nicotine adenine dinucleotide |
| NF-κB | nuclear factor kappa B |
| NINF | neuroinflammation/neuroinflammatory |
| NVC | neurovascular couple |
| OCT | optical coherence tomography |
| OCTA | optical coherence tomography angiography |
| OS | oxidative stress |
| PARP | poly-adenyl-ribose-polymerase |
| PDR | proliferative diabetic retinopathy. |
| PDRG | proliferative diabetic retinopathy group |
| PLS | plasma samples |
| PRP | panretinal photocoagulation |
| RE | right eye |
| ROS | reactive oxygen species |
| SCG | surrogate control group |
| SOD | antioxidant superoxide dismutase |
| TAC | total antioxidant capacity |
| TNF-α | tumor necrosis factor alpha |
| T2DM | type 2 diabetes mellitus |
| VEGF | vascular endothelial growth factor |
| VIT | vitreous body samples |
| 4HNE | 4-hydroxynonenal. |
References
- Magliano, D.J.; Boyko, E.J.; Balkau, B.; Barengo, N.; Barr, E.; Basit, A.; Bhata, D.; Bommer, C.; Booth, G.; Cariou, B.; et al. International Diabetes Federation Atlas, 10th ed.; Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/ (accessed on 15 March 2023).
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Chung, Y.C.; Xu, T.; Tung, T.H.; Chen, M.; Chen, P.E. Early Screening for Diabetic Retinopathy in Newly Diagnosed Type 2 Diabetes and Its Effectiveness in Terms of Morbidity and Clinical Treatment: A Nationwide Population-Based Cohort. Front. Public Health. 2022, 10, 771862. [Google Scholar] [CrossRef]
- Riva, C.E.; Falsini, B.; Logean, E. Flicker-evoked responses of human optic nerve head blood flow: Luminance versus chromatic modulation. Investig. Ophthalmol. Vis. Sci. 2001, 42, 756–762. [Google Scholar]
- Rector, D.M.; Yao, X.; Harper, R.M.; George, J.S. In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity. In In Vivo Optical Imaging of Brain Function, 2nd ed.; Frostig, R.D., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; Volume 5. [Google Scholar]
- Yin, L.; Zhang, D.; Ren, Q.; Su, X.; Sun, Z. Prevalence and risk factors of diabetic retinopathy in diabetic patients: A community based cross-sectional study. Medicine 2020, 99, e19236. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Beatson, B. Pan-American Collaborative Retina Study Group (PACORES). Lesson learned from PACORES in proliferative diabetic retinopathy management. Data from Latin America and Spain: The Asbury Lecture 2020. Retina 2022, 42, 4–10. [Google Scholar] [PubMed]
- Shah, S.; Feher, M.; McGovern, A.; Sherlock, J.; Whyte, M.B.; Munro, N.; Hinton, W.; Gatenby, P.; de Lusignan, S. Diabetic retinopathy in newly diagnosed Type 2 diabetes mellitus: Prevalence and predictors of progression; a national primary network study. Diabetes Res. Clin. Pract. 2021, 175, 108776. [Google Scholar] [CrossRef] [PubMed]
- Bandello, F.; Cicinelli, M.V. 19th EURETINA Congress Keynote Lecture: Diabetic Retinopathy Today. Ophthalmologica 2020, 243, 163–171. [Google Scholar] [CrossRef]
- Wang, S.Y.; Andrews, C.A.; Herman, W.H.; Gardner, T.W.; Stein, J.D. Incidence and Risk Factors for Developing Diabetic Retinopathy among Youths with Type 1 or Type 2 Diabetes throughout the United States. Ophthalmology 2017, 124, 424–430. [Google Scholar] [CrossRef]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.; et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, G.S.; Chang, J.S. Management of Complications and Vision Loss from Proliferative Diabetic Retinopathy. Curr. Diab. Rep. 2021, 21, 33. [Google Scholar] [CrossRef]
- Jacoba, C.M.P.; Doan, D.; Salongccay, R.P.; Aquino, L.A.C.M.; Silva, J.P.Y.; Salva, C.M.G.; Zhang, D.; Alog, G.P.; Zhang, K.; Locaylocay, K.B.M.; et al. Performance of Automated Machine Learning for Diabetic Retinopathy Image Classification from Multi-field Handheld Retinal Images. Ophthalmol. Retina 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.I.; Regillo, C.D.; Sadda, S.R.; Ipp, E.; Bhaskaranand, M.; Ramachandra, C.; Solanki, K. Artificial Intelligence Detection of Diabetic Retinopathy: Subgroup Comparison of the EyeArt System with Ophthalmologists’ Dilated Examinations. Ophthalmol. Sci. 2022, 3, 100228. [Google Scholar] [CrossRef]
- Bek, T. Systemic risk factors contribute differently to the development of proliferative diabetic retinopathy and clinically significant macular oedema. Diabetologia 2020, 63, 2462–2470. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.S.; Rasmussen, M.; Grauslund, J.; Subhi, Y.; Cehofski, L.J. Proteomic analysis of vitreous humor of eyes with diabetic macular oedema: A systematic review. Acta Ophthalmol. 2022, 100, e1043–e1051. [Google Scholar] [CrossRef]
- Srinivasan, S.; Raman, R.; Kulothungan, V.; Swaminathan, G.; Sharma, T. Influence of serum lipids on the incidence and progression of diabetic retinopathy and macular oedema: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular genetics Study-II. Clin. Exp. Ophthalmol. 2017, 45, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Yokoyama, T.; Takeda, N.; Katai, N.; Yoshida-Hata, N.; Nakamura, Y.; Yamamoto, S.; Noda, M.; Mizoue, T.; Nakagawa, T. A comparison in the ability to detect diabetic retinopathy between fasting plasma glucose and HbA1c levels in a longitudinal study. Endocrinol. Diabetes Metab. 2020, 4, e00196. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Y.; Fang, S.; Chen, Y.; Zhang, W.; Xia, F.; Wang, N.; Lu, Y. Associations between the Neutrophil-to-Lymphocyte Ratio and Diabetic Complications in Adults with Diabetes: A Cross-Sectional Study. J. Diabetes Res. 2020, 2020, 6219545. [Google Scholar] [CrossRef]
- Sharma, T.; Raman, R. Relationship between triglyceride glucose index, retinopathy and nephropathy in Type 2 diabetes. Endocrinol. Diabetes Metab. 2020, 4, e00151. [Google Scholar]
- Sun, Y.; Zou, H.; Li, X.; Xu, S.; Liu, C. Plasma Metabolomics Reveals Metabolic Profiling For Diabetic Retinopathy and Disease Progression. Front. Endocrinol. 2021, 12, 757088. [Google Scholar] [CrossRef]
- Sheetz, M.J.; King, G.L. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 2002, 288, 2579–2588. [Google Scholar] [CrossRef]
- Jarab, A.S.; Al-Qerem, W.; Alqudah, S.; Abu Heshmeh, S.R.; Mukattash, T.L.; Alzoubi, K.H. Blood pressure control and its associated factors in patients with hypertension and type 2 diabetes. Electron. J. General Med. 2023, 20, em477. [Google Scholar] [CrossRef]
- Kropp, M.; Golubnitschaja, O.; Mazurakova, L.; Koklesova, A.; Sargheini, N.; Vo, T.-T.K.S.; de Clerck, E.; Polivka Jr., J.; Potuznik, P.; Polivka, J.; et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications—Risks and mitigation. EPMA J. 2023, 14, 21–42. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, D.; Tsubota, K.; Negishi, K.; Kurihara, T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J. Clin. Med. 2021, 10, 4666. [Google Scholar] [CrossRef] [PubMed]
- Amoaku, W.M.; Ghanchi, F.; Bailey, C.; Banerjee, S.; Downey, L.; Gale, R.; Hamilton, R.; Khunti, K.; Posner, E.; Quhill, F.; et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye 2020, 34 (Suppl. S1), 1–51. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Yeh, S.; Maguire, M.G.; Smith, J.R.; Mruthyunjaya, P.; Jain, N.; Kim, L.A.; Weng, C.Y.; Flaxel, C.J.; Schoenberger, S.D.; et al. Intravitreal Pharmacotherapies for Diabetic Macular Edema: A Report by the American Academy of Ophthalmology. Ophthalmology 2022, 129, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Davis, M.D.; Danis, R.P.; Lovato, J.F.; Perdue, L.H.; Greven, C.l. The effects of medical management on the Progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes. (ACCORD) Eye Study. Ophthalmology 2014, 121, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Tetsumoto, A.; Inoue, S.; Takano, F.; Yamada, H.; Hayashida, M.; Otsuka, K.; Miki, A.; Kusuhara, S.; Nakamura, M. Intraoperative Three-Dimensional Fluorescein Angiography-Guided Pars Plana Vitrectomy for the Treatment of Proliferative Diabetic Retinopathy: The Maximized Utility of the Digital Assisted Vitrectomy. Retina 2023, 43, 359–362. [Google Scholar] [CrossRef]
- Roig-Revert, M.J.; Lleó-Pérez, A.; Zanon-Moreno, V.; Vivar-Llopis, B.; Marín-Montiel, J.; Dolz-Marco, R.; Alonso-Muñoz, L.; Albert-Fort, M.; López-Gálvez, M.I.; Galarreta-Mira, D.; et al. Valencia Study on Diabetic Retinopathy (VSDR). Enhanced Oxidative Stress and Other Potential Biomarkers for Retinopathy in Type 2 Diabetics: Beneficial Effects of the Nutraceutic Supplements. Biomed. Res. Int. 2015, 2015, 408180. [Google Scholar] [CrossRef]
- Pinazo-Duran, M.D.; Gallego-Pinazo, R.; García-Medina, J.J.; Zanón-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martínez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Brzović-Šarić, V.; Landeka, I.; Šarić, B.; Barberić, M.; Andrijašević, L.; Cerovski, B.; Oršolić, N.; Đikić, D. Levels of selected oxidative stress markers in the vitreous and serum of diabetic retinopathy patients. Mol. Vis. 2015, 21, 649. [Google Scholar] [PubMed]
- Gehl, Z.; Bakondi, E.; Resch, M.D.; Hegedűs, C.; Kovács, K.; Lakatos, P.; Szabó, A.; Nagy, Z.; Virág, L. Diabetes-induced oxidative stress in the vitreous humor. Redox. Biol. 2016, 9, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yao, T.; Okumura, K.; Seko, Y.; Kitano, S. Elevation of the vitreous body concentrations of oxidative stress-responsive apoptosis-inducing protein (ORAIP) in proliferative diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1519–1525. [Google Scholar] [CrossRef]
- Ankamah, E.; Sebag, J.; Ng, E.; Nolan, J.M. Vitreous antioxidants, degeneration, and vitreo-retinopathy: Exploring the links. Antioxidants 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Sahjapal, N.S.; Goel, R.K.M.; Chaubey, A.; Aurora, R.; Jain, S.K. Pathological perturbations in diabetic retinopathy, hyperglycemia, AGEs, Oxidative stress and inflammartory pathways. Curr. Protein Pept. Sci. 2019, 20, 92–113. [Google Scholar] [CrossRef]
- Suzuki, Y.; Suzuki, Y.; Nakazawa, M.; Suzuki, K.; Yamazaki, H.; Miyagawa, Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn. J. Ophthalmol. 2011, 55, 256–263. [Google Scholar] [CrossRef]
- Koskela, U.E.; Kuusisto, S.M.; Nissinen, A.E.; Savolainen, M.J.; Liinamaa, M.J. High vitreous concentration of IL-6 and IL-8, but not of adhesion molecules in relation to plasma concentrations in proliferative diabetic retinopathy. Ophthalmic. Res. 2013, 49, 108–114. [Google Scholar] [CrossRef]
- Mesquita, J.; Castro de Sousa, J.P.; Vaz-Pereira, S.; Neves, A.; Tavares-Ratado, P.; Santos, F.A.; Passarinha, L.T.; Tomaz, C. VEGF-B levels in the vitreous of diabetic and non-diabetic patients with ocular diseases and its correlation with structural parameters. Med. Sci. 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A. Cross Talks between Oxidative Stress, Inflammation and Epigenetics in Diabetic Retinopathy. Cells 2023, 12, 300. [Google Scholar] [CrossRef]
- Oshitari, T. Diabetic retinopathy: Neurovascular disease requiring neuroprotective and regenerative therapies. Neural Reg. Res. 2022, 17, 795–796. [Google Scholar] [CrossRef] [PubMed]
- Hanaguri, J.; Yokota, H.; Watanabe, M.; Yamagami, S.; Kushiyama, A.; Kuo, L.; Nagaoka, T. Retinal blood flow dysregulation precedes neural retinal dysfunction in type 2 diabetic mice. Sci. Rep. 2021, 11, 18401. [Google Scholar] [CrossRef] [PubMed]
- Capitão, M.; Soares, R. Angiogenesis and Inflammation Crosstalk in Diabetic Retinopathy. J. Cell Biochem. 2016, 117, 2443–2453. [Google Scholar] [CrossRef]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Sachdeva. M.M. Retinal Neurodegeneration in Diabetes: An Emerging Concept in Diabetic Retinopathy. Curr. Diab. Rep. 2021, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Verdejo, C.; Marco, P.; Renau-Piqueras, J.; Pinazo-Durán, M.D. Lipid peroxidation in proliferative vitreoretinopathies. Eye 1999, 13, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Mancino, R.; Di Pierro, D.; Varesi, C.; Cerulli, A.; Feraco, A.; Cedrone, C.; Pinazo-Duran, M.D.; Coletta, M.; Nucci, C. Lipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samples from patients with diabetic retinopathy. Mol. Vis. 2011, 17, 1298–1304. [Google Scholar] [PubMed]
- Sanz-González, S.M.; García-Medina, J.J.; Zanón-Moreno, V.; López-Gálvez, M.I.; Galarreta-Mira, D.; Duarte, L.; Valero-Velló, M.; Ramírez, A.I.; Arévalo, J.F.; Pinazo-Durán, M.D. On Behalf of The Valencia Study Group on Diabetic Retinopathy (VSDR) Report Number. Clinical and Molecular-Genetic Insights into the Role of Oxidative Stress in Diabetic Retinopathy: Antioxidant Strategies and Future Avenues. Antioxidants 2020, 9, 1101. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Albarral, J.A.; de Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Salobrar-García, E.; Pinazo-Durán, M.D.; Ramírez, J.M.; Salazar, J.J. Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regen Res. 2020, 15, 1408–1416. [Google Scholar]
- Fernández-Albarral, J.A.; de Julián-López, E.; Soler-Domínguez, C.; de Hoz, R.; López-Cuenca, I.; Salobrar-García, E.; Ramírez, J.M.; Pinazo-Durán, M.D.; Salazar, J.J.; Ramírez, A.I. The Role of Autophagy in Eye Diseases. Life 2021, 11, 189. [Google Scholar] [CrossRef]
- Fragiotta, S.; Pinazo-Durán, M.D.; Scuderi, G. Understanding Neurodegeneration from a Clinical and Therapeutic Perspective in Early Diabetic Retinopathy. Nutrients 2022, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015, 49, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Mondal, L.K.; Pramanik, S.; Chowdhury, S.; Bose, C.; Bera, D.; Saha, A.; Bhattacharjee, K. Do different lipid components accelerate the pathogenesis and severity of Diabetic Retinopathy? Int. J. Retin. Vitr. 2022, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Rudraraju, M.; Narayanan, S.P.; Somanath, P.R. Regulation of blood-retinal barrier cell-junctions in diabetic retinopathy. Pharmacol. Res. 2020, 161, 105115. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Koval, M.; Ranganathan, S.; Fanayan, S.; Hancock, W.S.; Lundberg, E.K.; Beavis, R.C.; Lane, L.; Duek, P.; McQuade, L.; et al. Systems Proteomics View of the Endogenous Human Claudin Protein Family. J. Proteome Res. 2015, 15, 339–359. [Google Scholar] [CrossRef]
- Rosa, A.C.; Bruni, N.; Meineri, G.; Corsi, D.; Cavi, N.; Gastaldi, D.; Dosio, F. Strategies to expand the therapeutic potential of superoxide dismutase by exploiting delivery approaches. Int. J. Biol. Macromol. 2021, 168, 846–865. [Google Scholar] [CrossRef]
- Tovmasyan, A.; Maia, C.G.; Weitner, T.; Carballal, S.; Sampaio, R.S.; Lieb, D.; Ghazaryan, R.; Ivanovic-Burmazovic, I.; Ferrer-Sueta, G.; Radi, R.; et al. A comprehensive evaluation of catalase-like activity of different classes of redox-active therapeutics. Free Radic. Biol. Med. 2015, 86, 308–321. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Del-Rio-Vellosillo, M. Update on the Effects of Antioxidants on Diabetic Retinopathy: In Vitro Experiments, Animal Studies and Clinical Trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef]
- Likidlilid, A.; Patchanans, N.; Peerapatdit, T.; Sriratanasathavorn, C. Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. J. Med. Assoc. Thail. 2010, 93, 682–693. [Google Scholar]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox. Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Xia, H.Q.; Yang, J.R.; Zhang, K.X.; Dong, R.L.; Yuan, H.; Wang, Y.C.; Zhou, H.; Li, X.M. Molecules related to diabetic retinopathy in the vitreous and involved pathways. Int. J. Ophthalmol. 2022, 15, 1180–1189. [Google Scholar] [CrossRef]
- Tang, L.; Xu, G.-T.; Zhang, J.-F. Inflammation in diabetic retinopathy: Possible roles in pathogenesis and potential implications for therapy. Neural Regen Res. 2023, 18, 976–982. [Google Scholar] [PubMed]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Shimura, M. Involvement of Cytokines in the Pathogenesis of Diabetic Macular Edema. Int. J. Mol. Sci. 2021, 22, 3427. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Silva, P.S.; Stittm, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Botto, L.; Beretta, E.; Daffara, R.; Miserocchi, G.; Palestini, P. Biochemical and morphological changes in endothelial cells in response to hypoxic interstitial edema. Respir. Res. 2006, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.J.; Joglekar, M.V.; Hardikar, A.A.; Keech, A.C.; O’Neal, D.N.; Januszewski, A.S. Biomarkers in Diabetic Retinopathy. Rev. Diabet. Stud. 2015, 12, 159–195. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Jiang, F.; You, C.; Mao, C.; Yu, J.; Han, J.; Zhang, Z.; Yan, H. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS ONE 2014, 9, e110531. [Google Scholar] [CrossRef]
- Bosma, E.K.; van Noorden, C.J.F.; Schlingemann, R.O.; Klaassen, I. The role of plasmalemma vesicle-associated protein in pathological breakdown of blood-brain and blood-retinal barriers: Potential novel therapeutic target for cerebral edema and diabetic macular edema. Fluids Barriers CNS 2018, 15, 24. [Google Scholar] [CrossRef]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, H.; Bao, S.; Wang, N.; Gillies, M.C. Diabetic macular edema: New concepts in pathophysiology and treatment. Cell Biosci. 2014, 4, 27. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, I.M.; Rezzola, S.; Cancarini, A.; Russo, A.; Costagliola, C.; Semeraro, F.; Presta, M. Human vitreous in proliferative diabetic retinopathy: Characterization and translational implications. Prog. Retin. Eye Res. 2019, 72, 100756. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Engle, J.T.; Griffin, E.A.; Miller, J.P.; Chu, W.; Zhou, D.; Mach, R.H. Imaging caspase-3 activation as a marker of apoptosis-targeted treatment response in cancer. Mol. Imaging Biol. 2015, 17, 384–393. [Google Scholar] [CrossRef]
- Zalewska, R.; Zalewski, B.; Reszec, J.; Mariak, Z.; Zimnoch, L.; Proniewska-Skretek, E. The expressions of Fas and caspase-3 in human glaucomatous optic nerve axons. Med. Sci. Monit. 2008, 14, BR274-8. [Google Scholar] [PubMed]
- Tian, M.; Liu, S.; Liu, L.; Zhang, E.K.; Wang, H.W.; Deng, Y.; Yue, Y.K. Correlations of the severity of diabetic retinopathy with EPO, Caspase-3 expression and oxidative stress. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9707–9713. [Google Scholar]
- Matthews, D.R.; Stratton, I.M.; Aldington, S.J.; Holman, R.R.; Kohner, E.M. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch. Ophthalmol. 2004, 122, 1631–1640. [Google Scholar]
- Majumdar, S.; Tripathy, K. Macular Hole. 22 August 2022. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- International Council of Ophthalmology. ICO Guidelines for Diabetic Eye Care; International Council of Ophthalmology: San Francisco, CA, USA, 2017. [Google Scholar]
- Mohammad, G.; Alam, K.; Nawaz, M.I.; Siddiquei, M.M.; Mousa, A.; Abu El-Asrar, A.M. Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J. Physiol. Biochem. 2015, 71, 359–372. [Google Scholar] [CrossRef]
- Fung, A.T.; Galvin, J.; Tran, T. Epiretinal membrane: A review. Clin. Exp. Ophthalmol. 2021, 49, 289–308. [Google Scholar] [CrossRef]


| SCG | PDRG | DMEG | |
|---|---|---|---|
| Age (years) | 60 ± 9 | 63 ± 13 | 61 ± 8 |
| Sex (Males/Females) | 4/12 | 14/15 | 5/10 |
| Affected/Operated RE (%) | 45 | 58 | 44 |
| Affected/Operated LE (%) | 55 | 42 | 56 |
| DM Duration (years) | - | 19 ± 6 | 16 ± 3 |
| SCG | PDRG | DMEG | |
|---|---|---|---|
| BCVA Log MAR (RE/LE) | 0.08/0.09 | 0.48/0.54 * | 0.66/0.62 * |
| IOP mm Hg (RE/LE) | 15 ± 2/14 ± 2 | 16 ± 1/15 ± 2 | 19 ± 1/19 ± 2 * |
| CSFT μm (RE/LE) | 256 ± 18/247 ± 23 | 370 ± 46/397 ± 44 * | 262 ± 10/254 ± 39 * |
| CAT μm (RE/LE) | 264 ± 18/- | 284 ± 39/290 ± 36 * | 395 ± 32/399 ± 42 * |
| DATA COMPARISON SCG vs. PDRG | ||||||||
| Biomarker Units | MDA μM | 4HNE μM | SOD U/mL | CAT U/mL | TAC μM | IL6 pg/mL | VEGF ng/mL | CAS3 pg/mL |
| Biological Sample | Plasma | Plasma | Plasma | Plasma | Plasma | Plasma | Plasma | Plasma |
| p-value | 2.70 × 10−7 | 1.17 × 10−3 | 1.42 × 10−6 | 6.45 × 10−5 | 4.16 × 10−14 | 1.39 × 10−4 | 8.89 × 10−10 | 0.36 |
| Biological Sample | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body |
| p-value | 1.06 × 10−22 | 8.96 × 10−14 | 9.01 × 10−13 | 9.67 × 10−18 | 9.74 × 10−8 | 5.72 × 10−6 | 2.5 × 10−3 | 0.126 |
| DATA COMPARISON SCG vs. DMEG | ||||||||
| Biomarker Units | MDA μM | 4HNE μM | SOD U/mL | CAT U/mL | TAC μM | IL6 pg/mL | VEGF ng/mL | CAS3 pg/mL |
| Biological Sample | Plasma | Plasma | Plasma | Plasma | Plasma | Plasma | Plasma | Plasma |
| p-value | 4.11 × 10−6 | 3.24 × 10−10 | 3.32 × 10−9 | 1.28 × 10−11 | 6.10 × 10−13 | 3.87 × 10−10 | 2.45 × 10−9 | 0.53 |
| Biological Sample | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body |
| p-value | 3.06 × 10−5 | 4.44 × 10−16 | 6.26 × 10−6 | 2.30 × 10−6 | 1.57 × 10−6 | 9.09 × 10−3 | 2.59 × 10−3 | 5.44 × 10−3 |
| DATA COMPARISON PDRG vs. DMEG | ||||||||
| Biomarker Units | MDA μM | 4HNE μM | SOD U/mL | CAT U/mL | TAC μM | IL6 pg/mL | VEGF ng/mL | CAS3 pg/mL |
| Biological Sample | Plasma | Plasma | Plasma | Plasma | Plasma | Plasma | Plasma | Plasma |
| p-value | 0.2455 | 0.3021 | 3.06 × 10−5 | 1.67 × 10−9 | 1.44 × 10−4 | 2.04 × 10−6 | 0.12 | 0.12 |
| Biological Sample | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body | Vitreous Body |
| p-value | 7.336 × 10−4 | 1.75 × 10−4 | 0.02 | 1.3 × 10−4 | 8.76 × 10−6 | 0.2956 | 0.5677 | 0.1354 |
| INCLUSION | EXCLUSION |
|---|---|
| Individuals aged between 40 and 80 years, inclusive. | Individuals aged younger than 40 years or older than 80 years. |
| Accurate diagnosis of PDR/DME for the corresponding group of T2DM participants (PDRG). | Other DM or DR type. |
| Non-diabetic individuals for the comparative group of participants (CG). These can include patients suffering from macular hole (MH), epiretinal membrane (EPM), or rhegmatogenous retinal detachment (RRD). | Patients experiencing other ophthalmological diseases and/or comorbidities. Patients receiving local or systemic treatment that may interfere with the study. Eye/laser surgery in the previous 12 months. |
| Precise and complete data of medical history. | History including any diagnoses that do not fit the study purpose. |
| Adequate psycho-physical status for participating in the study. | Unfeasibility of having a thorough and complete clinical history. Unable to participate. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés-Blasco, I.; Gallego-Martínez, A.; Machado, X.; Cruz-Espinosa, J.; Di Lauro, S.; Casaroli-Marano, R.; Alegre-Ituarte, V.; Arévalo, J.F.; Pinazo-Durán, M.D. Oxidative Stress, Inflammatory, Angiogenic, and Apoptotic molecules in Proliferative Diabetic Retinopathy and Diabetic Macular Edema Patients. Int. J. Mol. Sci. 2023, 24, 8227. https://doi.org/10.3390/ijms24098227
Andrés-Blasco I, Gallego-Martínez A, Machado X, Cruz-Espinosa J, Di Lauro S, Casaroli-Marano R, Alegre-Ituarte V, Arévalo JF, Pinazo-Durán MD. Oxidative Stress, Inflammatory, Angiogenic, and Apoptotic molecules in Proliferative Diabetic Retinopathy and Diabetic Macular Edema Patients. International Journal of Molecular Sciences. 2023; 24(9):8227. https://doi.org/10.3390/ijms24098227
Chicago/Turabian StyleAndrés-Blasco, Irene, Alex Gallego-Martínez, Ximena Machado, Javier Cruz-Espinosa, Salvatore Di Lauro, Ricardo Casaroli-Marano, Víctor Alegre-Ituarte, José Fernando Arévalo, and María Dolores Pinazo-Durán. 2023. "Oxidative Stress, Inflammatory, Angiogenic, and Apoptotic molecules in Proliferative Diabetic Retinopathy and Diabetic Macular Edema Patients" International Journal of Molecular Sciences 24, no. 9: 8227. https://doi.org/10.3390/ijms24098227
APA StyleAndrés-Blasco, I., Gallego-Martínez, A., Machado, X., Cruz-Espinosa, J., Di Lauro, S., Casaroli-Marano, R., Alegre-Ituarte, V., Arévalo, J. F., & Pinazo-Durán, M. D. (2023). Oxidative Stress, Inflammatory, Angiogenic, and Apoptotic molecules in Proliferative Diabetic Retinopathy and Diabetic Macular Edema Patients. International Journal of Molecular Sciences, 24(9), 8227. https://doi.org/10.3390/ijms24098227









