Genetic Insight into the Interaction of IBDV with Host—A Clue to the Development of Novel IBDV Vaccines
Abstract
:1. Introduction
2. Epidemics and Pathogenesis of Different IBDV Strains
2.1. Classical IBDV (cIBDV)
2.2. Variant IBDV (varIBDV)
2.3. Very Virulent IBDV (vvIBDV)
2.4. Novel Variant IBDV (nVarIBDV)
2.5. Other Strain Types of IBDV
3. Classification of IBDV Based on Genomic Phylogenetic Evolution
3.1. Recent Scheme for IBDV Genomic Classification
3.2. Revised Proposal for IBDV Genomic Classification
4. Genetic Factors Affecting IBDV–Host Interactions
4.1. Mutations in IBDV
| Protein | Residues | Site | Impact | Refs. |
|---|---|---|---|---|
| VP2 | 213 D | PBC | Immune escape | [112] |
| 219 Q | PBC | Virus assembly | [115] | |
| 222 | PBC | Immune escape; Virus replication and virulence-related | [30,56,59,106] | |
| 223 | PBC | Immune escape | [106] | |
| 234–236 (IDA) | PBC | Intermolecular interactions | [78] | |
| 249 | PDE | Immune escape; virus replication and virulence-related | [53,77] | |
| 253 | PDE | Cellular adaptability; virulence-related | [69,70,114,119] | |
| 254 | PDE | Immune escape | [30] | |
| 256 | PDE | Virus replication and virulence-related | [77] | |
| D279N | PFG | Cellular adaptability | [113,118] | |
| 284 | PFG | Cellular adaptability | [69,70,113,114] | |
| 286 I | PFG | Immune escape | [112] | |
| 318 D | PHI | Immune escape | [56,59,106,117] | |
| 321 A | PHI | Immune escape; virulence-related | [56,72] | |
| 323 | PHI | Immune escape | [117] | |
| 324 | PHI | Immune escape; virus assembly | [106,115] | |
| VP1 | A276T | N/A a | Intermolecular interactions | [72] |
| V4I | N/A | Virus replication and virulence-related | [79] | |
| 145/146/147 (TDN b, TEG or NEG) | N/A | Virus replication and virulence-related | [121] | |
| A163V | N/A | virulence-related(undetermined) | [14] | |
| R186A | N/A | Polymerase activity related | [124] | |
| R426A | N/A | Virus replication and polymerase activity related | [125] | |
| VP3 | 235(C-terminal) | N/A | Virus replication | [126,127] |
4.2. Gene Reassortment and Recombination in IBDV
5. Role of Reverse Genetics in Research on IBDV Genomic Function and Vaccine Development
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becht, H. Infectious Bursal Disease Virus. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 1980; pp. 107–121. ISBN 978-3-642-67717-5. [Google Scholar]
- Berg, T.P.V.D. Acute infectious bursal disease in poultry: A review. Avian Pathol. 2000, 29, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Islam, M.R.; Raue, R. Research on infectious bursal disease—The past, the present and the future. Vet. Microbiol. 2003, 97, 153–165. [Google Scholar] [CrossRef]
- McFerran, J.; McNulty, M.; McKillop, E.; Connor, T.; McCracken, R.; Collins, D.; Allan, G. Isolation and serological studies with infectious bursal disease viruses from fowl, turkeys and ducks: Demonstration of a second serotype. Avian Pathol. 1980, 9, 395–404. [Google Scholar] [CrossRef]
- Jackwood, D.J. Advances in vaccine research against economically important viral diseases of food animals: Infectious bursal disease virus. Vet. Microbiol. 2017, 206, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wu, T.; Hussain, A.; Gao, Y.; Zeng, X.; Wang, Y.; Gao, L.; Li, K.; Wang, Y.; Liu, C.; et al. Novel variant strains of infectious bursal disease virus isolated in China. Vet. Microbiol. 2019, 230, 212–220. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Gao, Y.; Qi, X. The Over-40-Years-Epidemic of Infectious Bursal Disease Virus in China. Viruses 2022, 14, 2253. [Google Scholar] [CrossRef] [PubMed]
- Zachar, T.; Popowich, S.; Goodhope, B.; Knezacek, T.; Ojkic, D.; Willson, P.; Ahmed, K.A.; Gomis, S. A 5-year study of the incidence and economic impact of variant infectious bursal disease viruses on broiler production in Saskatchewan, Canada. Can. J. Vet. Res. 2016, 80, 255–261. [Google Scholar]
- Myint, O.; Suwanruengsri, M.; Araki, K.; Izzati, U.Z.; Pornthummawat, A.; Nueangphuet, P.; Fuke, N.; Hirai, T.; Jackwood, D.J.; Yamaguchi, R. Bursa atrophy at 28 days old caused by variant infectious bursal disease virus has a negative economic impact on broiler farms in Japan. Avian Pathol. 2021, 50, 6–17. [Google Scholar] [CrossRef]
- Dey, S.; Pathak, D.; Ramamurthy, N.; Maity, H.K.; Chellappa, M.M. Infectious bursal disease virus in chickens: Prevalence, impact, and management strategies. VMRR 2019, 10, 85–97. [Google Scholar] [CrossRef]
- von Einem, U.I.; Gorbalenya, A.E.; Schirrmeier, H.; Behrens, S.-E.; Letzel, T.; Mundt, E. VP1 of infectious bursal disease virus is an RNA-dependent RNA polymerase. J. Gen. Virol. 2004, 85, 2221–2229. [Google Scholar] [CrossRef]
- Domingo, E.; Holland, J. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 1997, 51, 151–178. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Zierenberg, K.; Müller, H. The genome segment B encoding the RNA-dependent RNA polymerase protein VP1 of very virulent infectious bursal disease virus (IBDV) is phylogenetically distinct from that of all other IBDV strains. Arch. Virol. 2001, 146, 2481–2492. [Google Scholar] [CrossRef]
- Wang, W.; He, X.; Zhang, Y.; Qiao, Y.; Shi, J.; Chen, R.; Chen, J.; Xiang, Y.; Wang, Z.; Chen, G.; et al. Analysis of the global origin, evolution and transmission dynamics of the emerging novel variant IBDV (A2dB1b): The accumulation of critical aa-residue mutations and commercial trade contributes to the emergence and transmission of novel variants. Transbounding Emerg. Dis 2022, 69, e2832–e2851. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, J.; Zheng, J.; Xu, H.; Li, L.; Yu, L. Genetic reassortment of infectious bursal disease virus in nature. Biochem. Biophys. Res. Commun. 2006, 350, 277–287. [Google Scholar] [CrossRef]
- Van den Berg, T.; Morales, D.; Eterradossi, N.; Rivallan, G.; Toquin, D.; Raue, R.; Zierenberg, K.; Zhang, M.; Zhu, Y.; Wang, C. Assessment of genetic, antigenic and pathotypic criteria for the characterization of IBDV strains. Avian Pathol. 2004, 33, 470–476. [Google Scholar] [CrossRef]
- Xu, M.; Lin, S.; Zhao, Y.; Jin, J.; Tang, N.; Zhang, G. Characteristics of very virulent infectious bursal disease viruses isolated from Chinese broiler chickens (2012–2013). Acta Trop. 2015, 141, 128–134. [Google Scholar] [CrossRef]
- Xu, A.; Pei, Y.; Zhang, K.; Xue, J.; Ruan, S.; Zhang, G. Phylogenetic analyses and pathogenicity of a variant infectious bursal disease virus strain isolated in China. Virus Res. 2020, 276, 197833. [Google Scholar] [CrossRef] [PubMed]
- Tomás, G.; Marandino, A.; Courtillon, C.; Amelot, M.; Keita, A.; Pikula, A.; Hernández, M.; Hernández, D.; Vagnozzi, A.; Panzera, Y. Antigenicity, pathogenicity and immunosuppressive effect caused by a South American isolate of infectious bursal disease virus belonging to the “distinct” genetic lineage. Avian Pathol. 2019, 48, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Tomás, G.; Hernández, M.; Marandino, A.; Hernández, D.; Techera, C.; Grecco, S.; Panzera, Y.; Pérez, R. Genome sequence of a distinct infectious bursal disease virus. Genome Announc. 2015, 3, e01061-15. [Google Scholar] [CrossRef]
- Michel, L.O.; Jackwood, D.J. Classification of infectious bursal disease virus into genogroups. Arch. Virol. 2017, 162, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, D.J.; Schat, K.A.; Michel, L.O.; de Wit, S. A proposed nomenclature for infectious bursal disease virus isolates. Avian Pathol. 2018, 47, 576–584. [Google Scholar] [CrossRef]
- Islam, M.R.; Nooruzzaman, M.; Rahman, T.; Mumu, T.T.; Rahman, M.M.; Chowdhury, E.H.; Eterradossi, N.; Müller, H. A unified genotypic classification of infectious bursal disease virus based on both genome segments. Avian Pathol. 2021, 50, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, L.; Jiang, N.; Gao, L.; Li, K.; Gao, Y.; Liu, C.; Cui, H.; Pan, Q.; Zhang, Y.; et al. An improved scheme for infectious bursal disease virus genotype classification based on both genome-segments A and B. J. Integr. Agric. 2021, 20, 1372–1381. [Google Scholar] [CrossRef]
- Eterradossi, N.; Saif, Y. Infectious Bursal Disease (Gumboro Disease), Chapter 3.3.12. OIE Terrestrial Manual. Available online: http://www.oie.int/en/standard-setting/terrestrial-manual/access-online/ (accessed on 1 February 2023).
- Müller, H.; Mundt, E.; Eterradossi, N.; Islam, M.R. Current status of vaccines against infectious bursal disease. Avian Pathol. 2012, 41, 133–139. [Google Scholar] [CrossRef]
- Rautenschlein, S.; Kraemer, C.; Vanmarcke, J.; Montiel, E. Protective efficacy of intermediate and intermediate plus infectious bursal disease virus (IBDV) vaccines against very virulent IBDV in commercial broilers. Avian Dis. 2005, 49, 231–237. [Google Scholar] [PubMed]
- Block, H.; Meyer-Block, K.; Rebeski, D.E.; Scharr, H.; de Wit, S.; Rohn, K.; Rautenschlein, S. A field study on the significance of vaccination against infectious bursal disease virus (IBDV) at the optimal time point in broiler flocks with maternally derived IBDV antibodies. Avian Pathol. 2007, 36, 401–409. [Google Scholar] [CrossRef]
- Snyder, D.; Vakharia, V.; Savage, P. Naturally occurring-neutralizing monoclonal antibody escape variants define the epidemiology of infectious bursal disease viruses in the United States. Arch. Virol. 1992, 127, 89–101. [Google Scholar] [CrossRef]
- Jackwood, D.J.; Sommer-Wagner, S.E. Amino acids contributing to antigenic drift in the infectious bursal disease Birnavirus (IBDV). Virology 2011, 409, 33–37. [Google Scholar] [CrossRef]
- Hou, B.; Wang, C.; Luo, Z.; Shao, G. Commercial vaccines used in China do not protect against a novel infectious bursal disease virus variant isolated in Fujian. Vet. Rec. 2022, 191, e1840. [Google Scholar] [CrossRef]
- Ingrao, F.; Rauw, F.; Lambrecht, B.; van den Berg, T. Infectious Bursal Disease: A complex host–pathogen interaction. Dev. Comp. Immunol. 2013, 41, 429–438. [Google Scholar] [CrossRef]
- Trapp, J.; Rautenschlein, S. Infectious bursal disease virus’ interferences with host immune cells: What do we know? Avian Pathol. 2022, 51, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zheng, S. Infectious Bursal Disease Virus-Host Interactions: Multifunctional Viral Proteins that Perform Multiple and Differing Jobs. Int. J. Mol. Sci. 2017, 18, 161. [Google Scholar] [CrossRef]
- Li, J.; Zheng, S.J. Role of MicroRNAs in host defense against infectious bursal disease virus (IBDV) infection: A hidden front line. Viruses 2020, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Kasanga, C.; Yamaguchi, T.; Wambura, P.; Maeda-Machang’u, A.; Ohya, K.; Fukushi, H. Molecular characterization of infectious bursal disease virus (IBDV): Diversity of very virulent IBDV in Tanzania. Arch. Virol. 2007, 152, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, D.H.; Saif, Y.M. Antigenic diversity of infectious bursal disease viruses. Avian Dis. 1987, 31, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Mundt, E.; de Haas, N.; van Loon, A.A. Development of a vaccine for immunization against classical as well as variant strains of infectious bursal disease virus using reverse genetics. Vaccine 2003, 21, 4616–4624. [Google Scholar] [CrossRef]
- Gao, L.; Qi, X.; Li, K.; Gao, H.; Gao, Y.; Qin, L.; Wang, Y.; Wang, X. Development of a tailored vaccine against challenge with very virulent infectious bursal disease virus of chickens using reverse genetics. Vaccine 2011, 29, 5550–5557. [Google Scholar] [CrossRef]
- Qi, X.; Wang, Y.; Gao, L.; Gao, H.; Gao, Y.; Wang, X. Development and Application of the Reverse Genetic Technologies for Infectious Bursal Disease Virus. Bing Du Xue Bao Chin. J. Virol. 2015, 31, 326–331. [Google Scholar]
- Yang, H.; Ye, C. Reverse genetics approaches for live-attenuated vaccine development of infectious bursal disease virus. Curr. Opin. Virol. 2020, 44, 139–144. [Google Scholar] [CrossRef]
- Cosgrove, A. An apparently new disease of chickens: Avian nephrosis. Avian Dis. 1962, 6, 385–389. [Google Scholar] [CrossRef]
- Sharma, J.M.; Kim, I.J.; Rautenschlein, S.; Yeh, H.Y. Infectious bursal disease virus of chickens: Pathogenesis and immunosuppression. Dev. Comp. Immunol. 2000, 24, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.C.; Lam, K.M. Apoptosis induced by infectious bursal disease virus. J. Gen. Virol. 1994, 75, 1803–1806. [Google Scholar] [CrossRef]
- Aricibasi, M.; Jung, A.; Heller, E.D.; Rautenschlein, S. Differences in genetic background influence the induction of innate and acquired immune responses in chickens depending on the virulence of the infecting infectious bursal disease virus (IBDV) strain. Vet. Immunol. Immunopathol. 2010, 135, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Scholtissek, C.; Becht, H. The genome of infectious bursal disease virus consists of two segments of double-stranded RNA. J. Virol. 1979, 31, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Mundt, E.; Müller, H. Complete Nucleotide Sequences of 5′- and 3′-Noncoding Regions of Both Genome Segments of Different Strains of Infectious Bursal Disease Virus. Virology 1995, 209, 10–18. [Google Scholar] [CrossRef]
- Chen, F.; Liu, J.; Yan, Z.; Liu, D.; Ji, J.; Qin, J.; Li, H.; Ma, J.; Bi, Y.; Xie, Q. Complete genome sequence analysis of a natural reassortant infectious bursal disease virus in China. J. Virol. 2012, 86, 11942. [Google Scholar] [CrossRef]
- Raja, P.; Senthilkumar, T.; Parthiban, M.; Thangavelu, A.; Gowri, A.M.; Palanisammi, A.; Kumanan, K. Complete genome sequence analysis of a naturally reassorted infectious bursal disease virus from India. Genome Announc. 2016, 4, e00709-16. [Google Scholar] [CrossRef]
- Banda, A.; Villegas, P. Genetic characterization of very virulent infectious bursal disease viruses from Latin America. Avian Dis. 2004, 48, 540–549. [Google Scholar] [CrossRef]
- Hernández, M.; Tomás, G.; Marandino, A.; Iraola, G.; Maya, L.; Mattion, N.; Hernández, D.; Villegas, P.; Banda, A.; Panzera, Y. Genetic characterization of South American infectious bursal disease virus reveals the existence of a distinct worldwide-spread genetic lineage. Avian Pathol. 2015, 44, 212–221. [Google Scholar] [CrossRef]
- Rosenberger, J.; Cloud, S. Isolation and characterization of variant infectious bursal disease viruses. J. Am. Vet. Med. Assoc. 1986, 189, 357. [Google Scholar]
- Vakharia, V.N.; He, J.; Ahamed, B.; Snyder, D.B. Molecular basis of antigenic variation in infectious bursal disease virus. Virus Res. 1994, 31, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Eterradossi, N.; Toquin, D.; Rivallan, G.; Guittet, M. Modified activity of a VP2-located neutralizing epitope on various vaccine, pathogenic and hypervirulent strains of infectious bursal disease virus. Arch. Virol. 1997, 142, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, C.D.; Spies, U.; Shaw, K.; Peters, R.W.; Papageorgiou, A.; Muller, H.; Boursnell, M.E.G. A comparison of the sequences of segment A of four infectious bursal disease virus strains and identification of a variable region in VP2. J. Gen. Virol. 1990, 71, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Letzel, T.; Coulibaly, F.; Rey, F.A.; Delmas, B.; Jagt, E.; van Loon, A.A.M.W.; Mundt, E. Molecular and Structural Bases for the Antigenicity of VP2 of Infectious Bursal Disease Virus. J. Virol. 2007, 81, 12827–12835. [Google Scholar] [CrossRef]
- Van der Marel, P.; Snyder, D.; Lütticken, D. Antigenic characterization of IBDV field isolates by their reactivity with a panel of monoclonal antibodies. DTW. Dtsch. Tierarztl. Wochenschr. 1990, 97, 81–83. [Google Scholar]
- Jackwood, D.J. Molecular epidemiologic evidence of homologous recombination in infectious bursal disease viruses. Avian Dis. 2012, 56, 574–577. [Google Scholar] [CrossRef]
- Jackwood, D.; Cookson, K.; Sommer-Wagner, S.; Galludec, H.L.; De Wit, J. Molecular characteristics of infectious bursal disease viruses from asymptomatic broiler flocks in Europe. Avian Dis. 2006, 50, 532–536. [Google Scholar] [CrossRef]
- Chettle, N.; Stuart, J.; Wyeth, P. Outbreak of virulent infectious bursal disease in East Anglia. Vet. Rec. 1989, 125, 271. [Google Scholar] [CrossRef]
- Van den Berg, T.; Gonze, M.; Meulemans, G. Acute infectious bursal disease in poultry: Isolation and characterisation of a highly virulent strain. Avian Pathol. 1991, 20, 133–143. [Google Scholar] [CrossRef]
- Di Fabio, J.; Rossini, L.; Eterradossi, N.; Toquin, M.; Gardin, Y. European-like pathogenic infectious bursal disease viruses in Brazil. Vet. Rec. 1999, 145, 203–204. [Google Scholar]
- Mawgod, S.A.; Arafa, A.S.; Hussein, H.A. Molecular genotyping of the infectious bursal disease virus (IBDV) isolated from broiler flocks in Egypt. Int. J. Vet. Sci. Med. 2014, 2, 46–52. [Google Scholar] [CrossRef]
- Barathidasan, R.; Singh, S.; Kumar, M.A.; Desingu, P.; Palanivelu, M.; Singh, M.; Dhama, K. Recurrent outbreaks of infectious bursal disease (IBD) in a layer farm caused by very virulent IBD virus (vvIBDV) in India: Pathology and molecular analysis. South Asian J. Exp. Biol. 2013, 3, 200–206. [Google Scholar] [CrossRef]
- Hernández, M.; Banda, A.; Hernández, D.; Panzera, F.; Pérez, R. Detection of very virulent strains of infectious bursal disease virus (vvIBDV) in commercial broilers from Uruguay. Avian Dis. 2006, 50, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Hon, C.-C.; Lam, T.-Y.; Drummond, A.; Rambaut, A.; Lee, Y.-F.; Yip, C.-W.; Zeng, F.; Lam, P.-Y.; Ng, P.T.W.; Leung, F.C.C. Phylogenetic Analysis Reveals a Correlation between the Expansion of Very Virulent Infectious Bursal Disease Virus and Reassortment of Its Genome Segment B. J. Virol. 2006, 80, 8503–8509. [Google Scholar] [CrossRef]
- Brown, M.D.; Skinner, M.A. Coding sequences of both genome segments of a European ‘very virulent’infectious bursal disease virus. Virus Res. 1996, 40, 1–15. [Google Scholar] [CrossRef]
- Eterradossi, N.; Arnauld, C.; Tekaia, F.; Toquin, D.; Le Coq, H.; Rivallan, G.; Guittet, M.; Domenech, J.; Van den Berg, T.; Skinner, M. Antigenic and genetic relationships between European very virulent infectious bursal disease viruses and an early West African isolate. Avian Pathol. 1999, 28, 36–46. [Google Scholar] [CrossRef]
- Mundt, E. Tissue culture infectivity of different strains of infectious bursal disease virus is determined by distinct amino acids in VP2. J. Gen. Virol. 1999, 80, 2067–2076. [Google Scholar] [CrossRef]
- Qi, X.; Gao, H.; Gao, Y.; Qin, L.; Wang, Y.; Gao, L.; Wang, X. Naturally occurring mutations at residues 253 and 284 in VP2 contribute to the cell tropism and virulence of very virulent infectious bursal disease virus. Antivir. Res. 2009, 84, 225–233. [Google Scholar] [CrossRef]
- Qi, X.; Lu, Z.; Wang, N.; Chen, Y.; Zhang, L.; Gao, L.; Li, K.; Ren, X.; Wang, Y.; Gao, H.; et al. Analysis of the function of D279N mutation of VP2 of infectious bursal disease virus. J. Integr. Agric. 2015, 14, 2618–2625. [Google Scholar] [CrossRef]
- Escaffre, O.; Nouën, C.L.; Amelot, M.; Ambroggio, X.; Ogden, K.M.; Guionie, O.; Toquin, D.; Müller, H.; Islam, M.R.; Eterradossi, N. Both Genome Segments Contribute to the Pathogenicity of Very Virulent Infectious Bursal Disease Virus. J. Virol. 2013, 87, 14. [Google Scholar] [CrossRef]
- Boot, H.J.; ter Huurne, A.A.H.M.; Hoekman, A.J.W.; Peeters, B.P.H.; Gielkens, A.L.J. Rescue of Very Virulent and Mosaic Infectious Bursal Disease Virus from Cloned cDNA: VP2 Is Not the Sole Determinant of the Very Virulent Phenotype. J. Virol. 2000, 74, 6701–6711. [Google Scholar] [CrossRef] [PubMed]
- Boot, H.J.; Hoekman, A.J.W.; Gielkens, A.L.J. The enhanced virulence of very virulent infectious bursal disease virus is partly determined by its B-segment. Arch. Virol. 2005, 150, 137–144. [Google Scholar] [CrossRef]
- Jackwood, D.J.; Sommer-Wagner, S.E.; Crossley, B.M.; Stoute, S.T.; Woolcock, P.R.; Charlton, B.R. Identification and pathogenicity of a natural reassortant between a very virulent serotype 1 infectious bursal disease virus (IBDV) and a serotype 2 IBDV. Virology 2011, 420, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Nouën, C.L.; Rivallan, G.; Toquin, D.; Darlu, P.; Morin, Y.; Beven, V.; de Boisseson, C.; Cazaban, C.; Comte, S.; Gardin, Y.; et al. Very virulent infectious bursal disease virus: Reduced pathogenicity in a rare natural segment-B-reassorted isolate. J. Gen. Virol. 2006, 87, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, L.; Chen, Y.; Gao, L.; Wu, G.; Qin, L.; Wang, Y.; Ren, X.; Gao, Y.; Gao, H.; et al. Mutations of Residues 249 and 256 in VP2 Are Involved in the Replication and Virulence of Infectious Bursal Disease Virus. PLoS ONE 2013, 8, e70982. [Google Scholar] [CrossRef]
- Delgui, L.; Oña, A.; Gutiérrez, S.; Luque, D.; Navarro, A.; Castón, J.R.; Rodríguez, J.F. The capsid protein of infectious bursal disease virus contains a functional α4β1 integrin ligand motif. Virology 2009, 386, 360–372. [Google Scholar] [CrossRef]
- Yu, F.; Ren, X.; Wang, Y.; Qi, X.; Song, J.; Gao, Y.; Qin, L.; Gao, H.; Wang, X. A single amino acid V4I substitution in VP1 attenuates virulence of very virulent infectious bursal disease virus (vvIBDV) in SPF chickens and increases replication in CEF cells. Virology 2013, 440, 204–209. [Google Scholar] [CrossRef]
- Thai, T.N.; Jang, I.; Kim, H.-A.; Kim, H.-S.; Kwon, Y.-K.; Kim, H.-R. Characterization of antigenic variant infectious bursal disease virus strains identified in South Korea. Avian Pathol. 2021, 50, 174–181. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, N.; Fan, L.; Niu, X.; Zhang, W.; Huang, M.; Gao, L.; Li, K.; Gao, Y.; Liu, C. Identification and pathogenicity evaluation of a novel reassortant Infectious Bursal Disease Virus (genotype A2dB3). Viruses 2021, 13, 1682. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Y.; Zhang, Y.; Qiao, Y.; Deng, Q.; Chen, R.; Chen, J.; Huang, T.; Wei, T.; Mo, M. The emerging naturally reassortant strain of IBDV (genotype A2dB3) having segment A from Chinese novel variant strain and segment B from HLJ 0504-like very virulent strain showed enhanced pathogenicity to three-yellow chickens. Transbound. Emerg. Dis. 2022, 69, e566–e579. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, Y.; Zhang, W.; Niu, X.; Huang, M.; Gao, Y.; Liu, A.; Gao, L.; Li, K.; Pan, Q. Genotyping and molecular characterization of infectious bursal disease virus identified in important poultry-raising areas of China during 2019 and 2020. Front. Vet. Sci. 2021, 8, 759861. [Google Scholar] [CrossRef]
- Thai, T.N.; Yoo, D.-S.; Jang, I.; Kwon, Y.-K.; Kim, H.-R. Dynamics of the Emerging Genogroup of Infectious Bursal Disease Virus Infection in Broiler Farms in South Korea: A Nationwide Study. Viruses 2022, 14, 1604. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, H.B.; Hair-Bejo, M.; Omar, A.R.; Ideris, A. Genetic diversity of recent infectious bursal disease viruses isolated from vaccinated poultry flocks in Malaysia. Front. Vet. Sci. 2021, 8, 643976. [Google Scholar] [CrossRef]
- Fan, L.; Wu, T.; Wang, Y.; Hussain, A.; Jiang, N.; Gao, L.; Li, K.; Gao, Y.; Liu, C.; Cui, H.; et al. Novel variants of infectious bursal disease virus can severely damage the bursa of fabricius of immunized chickens. Vet. Microbiol. 2020, 240, 108507. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kuang, H.; Guo, H.; Cai, L.; Chu, D.; Wang, X.; Hu, J.; Rong, J. Development of a recombinant VP2 vaccine for the prevention of novel variant strains of infectious bursal disease virus. Avian Pathol. 2020, 49, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, Y.; Jiang, N.; Gao, L.; Li, K.; Gao, Y.; Cui, H.; Pan, Q.; Liu, C.; Zhang, Y.; et al. A reassortment vaccine candidate of the novel variant infectious bursal disease virus. Vet. Microbiol. 2020, 251, 108905. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Wang, Z.; Xu, Z.; Pang, Y.; Leng, M.; Tang, S.; Zhang, X.; Qin, J.; Chen, F.; Lin, W. Pathogenicity and molecular characterization of infectious bursal disease virus in China. Poult. Sci. 2022, 101, 101502. [Google Scholar] [CrossRef]
- Sun, J.; Lu, P.; Yan, Y.; Hua, X.; Jiang, J.; Zhao, Y. Sequence and analysis of genomic segment A and B of very virulent infectious bursal disease virus isolated from China. J. Vet. Med. Ser. B 2003, 50, 148–154. [Google Scholar] [CrossRef]
- Pikuła, A.; Lisowska, A.; Jasik, A.; Śmietanka, K. Identification and assessment of virulence of a natural reassortant of infectious bursal disease virus. Vet. Res. 2018, 49, 89. [Google Scholar] [CrossRef]
- Cui, P.; Ma, S.-J.; Zhang, Y.-G.; Li, X.-S.; Gao, X.-Y.; Cui, B.-A.; Chen, H.-Y. Genomic sequence analysis of a new reassortant infectious bursal disease virus from commercial broiler flocks in Central China. Arch. Virol. 2013, 158, 1973–1978. [Google Scholar] [CrossRef]
- Hon, C.-C.; Lam, T.T.-Y.; Yip, C.-W.; Wong, R.T.-Y.; Shi, M.; Jiang, J.; Zeng, F.; Leung, F.C.-C. Phylogenetic evidence for homologous recombination within the family Birnaviridae. J. Gen. Virol. 2008, 89, 3156–3164. [Google Scholar] [CrossRef] [PubMed]
- He, C.-Q.; Ma, L.-Y.; Wang, D.; Li, G.-R.; Ding, N.-Z. Homologous recombination is apparent in infectious bursal disease virus. Virology 2009, 384, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhu, N.; Cui, Y.; Hou, L.; Zhou, J.; Qiu, Y.; Yang, X.; Liu, C.; Wang, D.; Guo, J.; et al. Characterization and pathogenicity of a naturally reassortant and recombinant infectious bursal disease virus in China. Transbounding Emerg. Dis. 2022, 69, e746–e758. [Google Scholar] [CrossRef]
- He, X.; Xiong, Z.; Yang, L.; Guan, D.; Yang, X.; Wei, P. Molecular epidemiology studies on partial sequences of both genome segments reveal that reassortant infectious bursal disease viruses were dominantly prevalent in southern China during 2000–2012. Arch. Virol. 2014, 159, 3279–3292. [Google Scholar] [CrossRef]
- Alfonso-Morales, A.; Rios, L.; Martínez-Pérez, O.; Dolz, R.; Valle, R.; Perera, C.L.; Bertran, K.; Frías, M.T.; Ganges, L. Evaluation of a Phylogenetic Marker Based on Genomic Segment B of Infectious Bursal Disease Virus: Facilitating a Feasible Incorporation of this Segment to the Molecular Epidemiology Studies for this Viral Agent. PLoS ONE 2015, 10, e0125853. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Gao, L.; Qin, L.; Deng, X.; Wu, G.; Zhang, L.; Yu, F.; Ren, X.; Gao, Y.; Gao, H. Genomic sequencing and molecular characteristics of a very virulent strain of infectious bursal disease virus isolated in China. Agric. Sci. Technol.-Hunan 2011, 12, 1946–1949. [Google Scholar]
- Tammiranta, N.; Ek-Kommonen, C.; Rossow, L.; Huovilainen, A. Circulation of very virulent avian infectious bursal disease virus in Finland. Avian Pathol. 2018, 47, 520–525. [Google Scholar] [CrossRef]
- Pikuła, A.; Śmietanka, K.; Perez, L.J. Emergence and expansion of novel pathogenic reassortant strains of infectious bursal disease virus causing acute outbreaks of the disease in Europe. Transbound. Emerg. Dis. 2020, 67, 1739–1744. [Google Scholar] [CrossRef]
- Nwagbo, I.O.; Shittu, I.; Nwosuh, C.I.; Ezeifeka, G.O.; Odibo, F.J.; Michel, L.O.; Jackwood, D.J. Molecular characterization of field infectious bursal disease virus isolates from Nigeria. Vet. World 2016, 9, 1420. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, S. Host Combats IBDV Infection at Both Protein and RNA Levels. Viruses 2022, 14, 2309. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Zheng, S.J. Advances on Innate Immune Evasion by Avian Immunosuppressive Viruses. Front. Immunol. 2022, 13, 901913. [Google Scholar] [CrossRef]
- Zheng, S.J. Infectious Bursal Disease Virus, Chapter 7. In Avian Virology: Current Research and Future Trends; Caister Academic Press: Poole, UK, 2019; ISBN 978-1-912530-10-6. [Google Scholar]
- Eterradossi, N.; Arnauld, C.; Toquin, D.; Rivallan, G. Critical amino acid changes in VP2 variable domain are associated with typical and atypical antigenicity in very virulent infectious bursal disease viruses. Arch. Virol. 1998, 143, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, F.; Chevalier, C.; Gutsche, I.; Pous, J.; Navaza, J.; Bressanelli, S.; Delmas, B.; Rey, F.A. The Birnavirus Crystal Structure Reveals Structural Relationships among Icosahedral Viruses. Cell 2005, 120, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Becht, H.; Müller, H.; Müller, H.K. Comparative studies on structural and antigenic properties of two serotypes of infectious bursal disease virus. J. Gen. Virol. 1988, 69, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Eterradossi, N.; Saif, Y.M. Infectious Bursal Disease. In Diseases of Poultry; Wiley: Hoboken, NJ, USA, 2020; pp. 257–283. ISBN 978-1-119-37116-8. [Google Scholar]
- Lee, C.-C.; Ko, T.-P.; Chou, C.-C.; Yoshimura, M.; Doong, S.-R.; Wang, M.-Y.; Wang, A.H.-J. Crystal structure of infectious bursal disease virus VP2 subviral particle at 2.6Å resolution: Implications in virion assembly and immunogenicity. J. Struct. Biol. 2006, 155, 74–86. [Google Scholar] [CrossRef]
- Garriga, D.; Querol-Audí, J.; Abaitua, F.; Saugar, I.; Pous, J.; Verdaguer, N.; Castón, J.R.; Rodriguez, J.F. The 2.6-Angstrom Structure of Infectious Bursal Disease Virus-Derived T=1 Particles Reveals New Stabilizing Elements of the Virus Capsid. J. Virol. 2006, 80, 6895–6905. [Google Scholar] [CrossRef]
- Heine, H.-G.; Haritou, M.; Failla, P.; Fahey, K.; Azad, A. Sequence Analysis and Expression of the Host-protective Immunogen VP2 of a Variant Strain of Infectious Bursal Disease Virus Which Can Circumvent Vaccination with Standard Type I Strains. J. Gen. Virol. 1991, 72, 1835–1843. [Google Scholar] [CrossRef]
- Lim, B.-L.; Cao, Y.; Yu, T.; Mo, C.-W. Adaptation of very virulent infectious bursal disease virus to chicken embryonic fibroblasts by site-directed mutagenesis of residues 279 and 284 of viral coat protein VP2. J. Virol. 1999, 73, 2854–2862. [Google Scholar] [CrossRef]
- Brandt, M.; Yao, K.; Liu, M.; Heckert, R.A.; Vakharia, V.N. Molecular determinants of virulence, cell tropism, and pathogenic phenotype of infectious bursal disease virus. J. Virol. 2001, 75, 11974–11982. [Google Scholar] [CrossRef]
- Bao, K.; Qi, X.; Li, Y.; Gong, M.; Wang, X.; Zhu, P. Cryo-EM structures of infectious bursal disease viruses with different virulences provide insights into their assembly and invasion. Sci. Bull. 2022, 67, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.L.C. The PyMOL Molecular Graphics System, Version 1.8. 2015. Available online: https://www.pymol.org (accessed on 3 March 2023).
- Fan, L.; Wang, Y.; Jiang, N.; Gao, Y.; Niu, X.; Zhang, W.; Huang, M.; Bao, K.; Liu, A.; Wang, S.; et al. Residues 318 and 323 in capsid protein are involved in immune circumvention of the atypical epizootic infection of infectious bursal disease virus. Front. Microbiol. 2022, 13, 909252. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Ogawa, M.; Inoshima, Y.; Miyoshi, M.; Fukushi, H.; Hirai, K. Identification of Sequence Changes Responsible for the Attenuation of Highly Virulent Infectious Bursal Disease Virus. Virology 1996, 223, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, D.J.; Sreedevi, B.; LeFever, L.J.; Sommer-Wagner, S.E. Studies on naturally occurring infectious bursal disease viruses suggest that a single amino acid substitution at position 253 in VP2 increases pathogenicity. Virology 2008, 377, 110–116. [Google Scholar] [CrossRef]
- Lombardo, E.; Maraver, A.; Castón, J.R.; Rivera, J.; Fernández-Arias, A.; Serrano, A.; Carrascosa, J.L.; Rodriguez, J.F. VP1, the putative RNA-dependent RNA polymerase of infectious bursal disease virus, forms complexes with the capsid protein VP3, leading to efficient encapsidation into virus-like particles. J. Virol. 1999, 73, 6973–6983. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, K.; Qi, X.; Gao, H.; Gao, Y.; Qin, L.; Wang, Y.; Shen, N.; Kong, X.; Wang, X. Triplet amino acids located at positions 145/146/147 of the RNA polymerase of very virulent infectious bursal disease virus contribute to viral virulence. J. Gen. Virol. 2014, 95, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, K.; Qi, X.; Gao, Y.; Wang, Y.; Gao, H.; Wang, X. N-terminal domain of the RNA polymerase of very virulent infectious bursal disease virus contributes to viral replication and virulence. Sci. China Life Sci. 2018, 61, 1127–1129. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Y.; Ji, Z.; Chen, G.; Zhang, Y.; Qiao, Y.; Shi, M.; Li, M.; Huang, T.; Wei, T.; et al. The Full Region of N-Terminal in Polymerase of IBDV Plays an Important Role in Viral Replication and Pathogenicity: Either Partial Region or Single Amino Acid V4I Substitution Does Not Completely Lead to the Virus Attenuation to Three-Yellow Chickens. Viruses 2021, 13, 107. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Z.; Wu, X.; Ding, Z.; Zeng, Q.; Wu, H. An Improved, Dual-Direction, Promoter-Driven, Reverse Genetics System for the Infectious Bursal Disease Virus (IBDV). Viruses 2022, 14, 1396. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Z.; Wu, X.; Fu, Q.; Chen, Z.; Huang, Y.; Wu, H. PRMT5 Facilitates Infectious Bursal Disease Virus Replication through Arginine Methylation of VP1. J. Virol. 2023, 97, e01637-22. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, X.; Kang, Z.; Yu, F.; Qin, L.; Gao, H.; Gao, Y.; Wang, X. A single amino acid in the C-terminus of VP3 protein influences the replication of attenuated infectious bursal disease virus in vitro and in vivo. Antivir. Res. 2010, 87, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mosley, Y.-Y.C.; Wu, C.C.; Lin, T.L. A free VP3 C-terminus is essential for the replication of infectious bursal disease virus. Virus Res. 2017, 232, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, H.; Chen, G.; Liu, H.; Wang, S.; Xia, D.; Yu, Y.; Zhang, Y.; Jiang, J.; Ma, J.; et al. Identification and assessment of pathogenicity of a naturally reassorted infectious bursal disease virus from Henan, China. Poult. Sci. 2019, 98, 6433–6444. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, D.J.; Stoute, S.T.; Beate, M. Crossley Pathogenicity of Genome Reassortant Infectious Bursal Disease Viruses in Chickens and Turkeys. Avian Dis. 2016, 60, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Legnardi, M.; Franzo, G.; Tucciarone, C.M.; Koutoulis, K.; Duarte, I.; Silva, M.; Le Tallec, B.; Cecchinato, M. Detection and molecular characterization of a new genotype of infectious bursal disease virus in Portugal. Avian Pathol. 2022, 51, 97–105. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, W.; Chen, G.; Jiao, P.; Ji, Z.; Yang, L.; Wei, P. Serological study reveal different antigenic IBDV strains prevalent in southern China during the years 2000–2017 and also the antigenic differences between the field strains and the commonly used vaccine strains. Vet. Microbiol. 2019, 239, 108458. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, X.; Zheng, J.; Chu, W.; Xu, H.; Yu, X.; Yu, L. Reassortant infectious bursal disease virus isolated in China. Virus Res. 2008, 131, 279–282. [Google Scholar] [CrossRef]
- Lee, H.-J.; Jang, I.; Shin, S.-H.; Lee, H.-S.; Choi, K.-S. Genome Sequence of a Novel Reassortant and Very Virulent Strain of Infectious Bursal Disease Virus. Genome Announc. 2017, 5, e00730-17. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Y.; Li, H.; Fan, L.; Jiang, N.; Gao, L.; Li, K.; Gao, Y.; Liu, C.; Cui, H.; et al. Naturally occurring homologous recombination between novel variant infectious bursal disease virus and intermediate vaccine strain. Vet. Microbiol. 2020, 245, 108700. [Google Scholar] [CrossRef]
- Mundt, E.; Vakharia, V.N. Synthetic transcripts of double-stranded Birnavirus genome are infectious. Proc. Natl. Acad. Sci. USA 1996, 93, 11131–11136. [Google Scholar] [CrossRef]
- Qi, X.; Gao, Y.; Gao, H.; Deng, X.; Bu, Z.; Wang, X.; Fu, C.; Wang, X. An improved method for infectious bursal disease virus rescue using RNA polymerase II system. J. Virol. Methods 2007, 142, 81–88. [Google Scholar] [CrossRef]
- Durairaj, V.; Sellers, H.S.; Linnemann, E.G.; Icard, A.H.; Mundt, E. Investigation of the antigenic evolution of field isolates using the reverse genetics system of infectious bursal disease virus (IBDV). Arch Virol. 2011, 156, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Goodwin, M.A.; Vakharia, V.N. Generation of a Mutant Infectious Bursal Disease Virus That Does Not Cause Bursal Lesions. J. Virol. 1998, 72, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Kurukulsuriya, S.; Ahmed, K.A.; Ojkic, D.; Gunawardana, T.; Gupta, A.; Goonewardene, K.; Karunaratne, R.; Popowich, S.; Willson, P.; Tikoo, S.K.; et al. Circulating strains of variant infectious bursal disease virus may pose a challenge for antibiotic-free chicken farming in Canada. Res. Vet. Sci. 2016, 108, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Elankumaran, S.; Yunus, A.S.; Samal, S.K. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J. Virol. 2004, 78, 10054–10063. [Google Scholar] [CrossRef]
- Shah, A.U.; Wang, Z.; Zheng, Y.; Guo, R.; Chen, S.; Xu, M.; Zhang, C.; Liu, Y.; Wang, J. Construction of a Novel Infectious Clone of Recombinant Herpesvirus of Turkey Fc-126 Expressing VP2 of IBDV. Vaccines 2022, 10, 1391. [Google Scholar] [CrossRef]
- Qin, L.; Qi, X.; Gao, Y.; Gao, H.; Lu, X.; Wang, Y.; Bu, Z.; Wang, X. VP5-deficient mutant virus induced protection against challenge with very virulent infectious bursal disease virus of chickens. Vaccine 2010, 28, 3735–3740. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Ye, C. Rapid Generation of Attenuated Infectious Bursal Disease Virus from Dual-Promoter Plasmids by Reduction of Viral Ribonucleoprotein Activity. J. Virol. 2020, 94, e01569-19. [Google Scholar] [CrossRef]
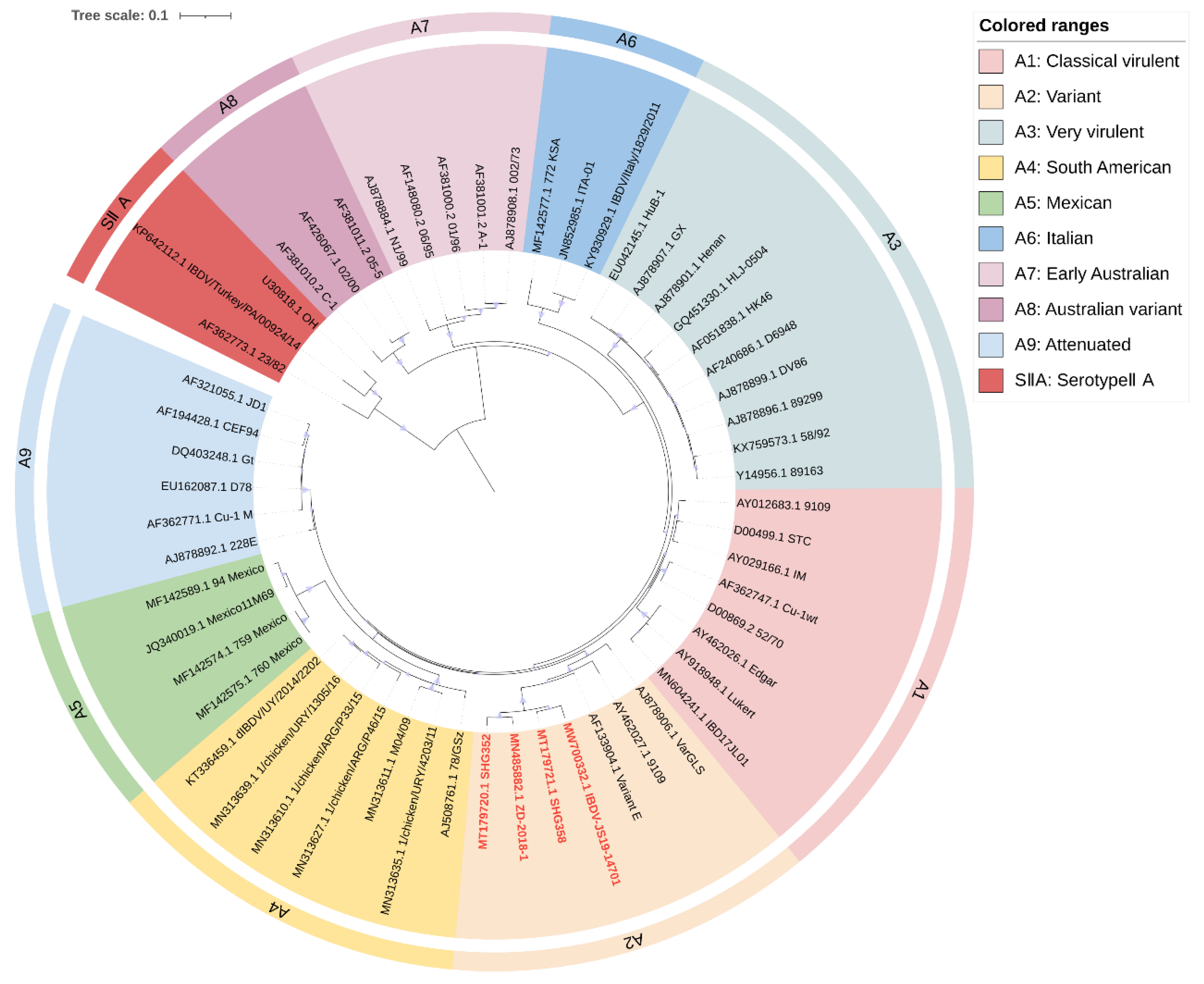


| Genetic Characteristics | Genogroups by the Following Authors | |||
|---|---|---|---|---|
| Michel and Jackwood [21] | Islam et al. [23] | Wang et al. [24] | Gao et al. | |
| Classical (Virulent) | G1 | A1a | A1 | A1 |
| Variant | G2 | A2 | A2 | A2 |
| Very virulent | G3 | A3 | A3 | A3 |
| South American | G4 | A4 | A4 | A4 |
| Mexican | G5 | A5 | A5 | A5 |
| Italian | G6 | A6 | A6 | A6 |
| Early Australian | G7 | A7 | A7 | A7 |
| Australian variant | A8 | A8 | ||
| Attenuated | N/A a | A1b | A8 | A9 |
| SerotypeIIA | N/A | A0 | AII | SIIA |
| Genetic Characteristics | Genogroups by the Following Authors | |||
|---|---|---|---|---|
| Michel and Jackwood [21] | Islam et al. [23] | Wang et al. [24] | Gao et al. | |
| Classical-like | N/A a | B1 | B1 | B1 |
| Very virulent-like | N/A | B2 | B2 | B2 |
| Early Australian-like | N/A | B3 | B3 | B3 |
| Polish and Tanzanian | N/A | B4 | B4 | B4 |
| Nigerian | N/A | B5 | N/A | B5 |
| SerotypeIIB | N/A | B1 | BII | SIIB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Wang, Y.; Gao, L.; Zheng, S.J. Genetic Insight into the Interaction of IBDV with Host—A Clue to the Development of Novel IBDV Vaccines. Int. J. Mol. Sci. 2023, 24, 8255. https://doi.org/10.3390/ijms24098255
Gao H, Wang Y, Gao L, Zheng SJ. Genetic Insight into the Interaction of IBDV with Host—A Clue to the Development of Novel IBDV Vaccines. International Journal of Molecular Sciences. 2023; 24(9):8255. https://doi.org/10.3390/ijms24098255
Chicago/Turabian StyleGao, Hui, Yongqiang Wang, Li Gao, and Shijun J. Zheng. 2023. "Genetic Insight into the Interaction of IBDV with Host—A Clue to the Development of Novel IBDV Vaccines" International Journal of Molecular Sciences 24, no. 9: 8255. https://doi.org/10.3390/ijms24098255






