SOCS3 Protein Mediates the Therapeutic Efficacy of Mesenchymal Stem Cells against Acute Lung Injury
Abstract
:1. Introduction
2. Results
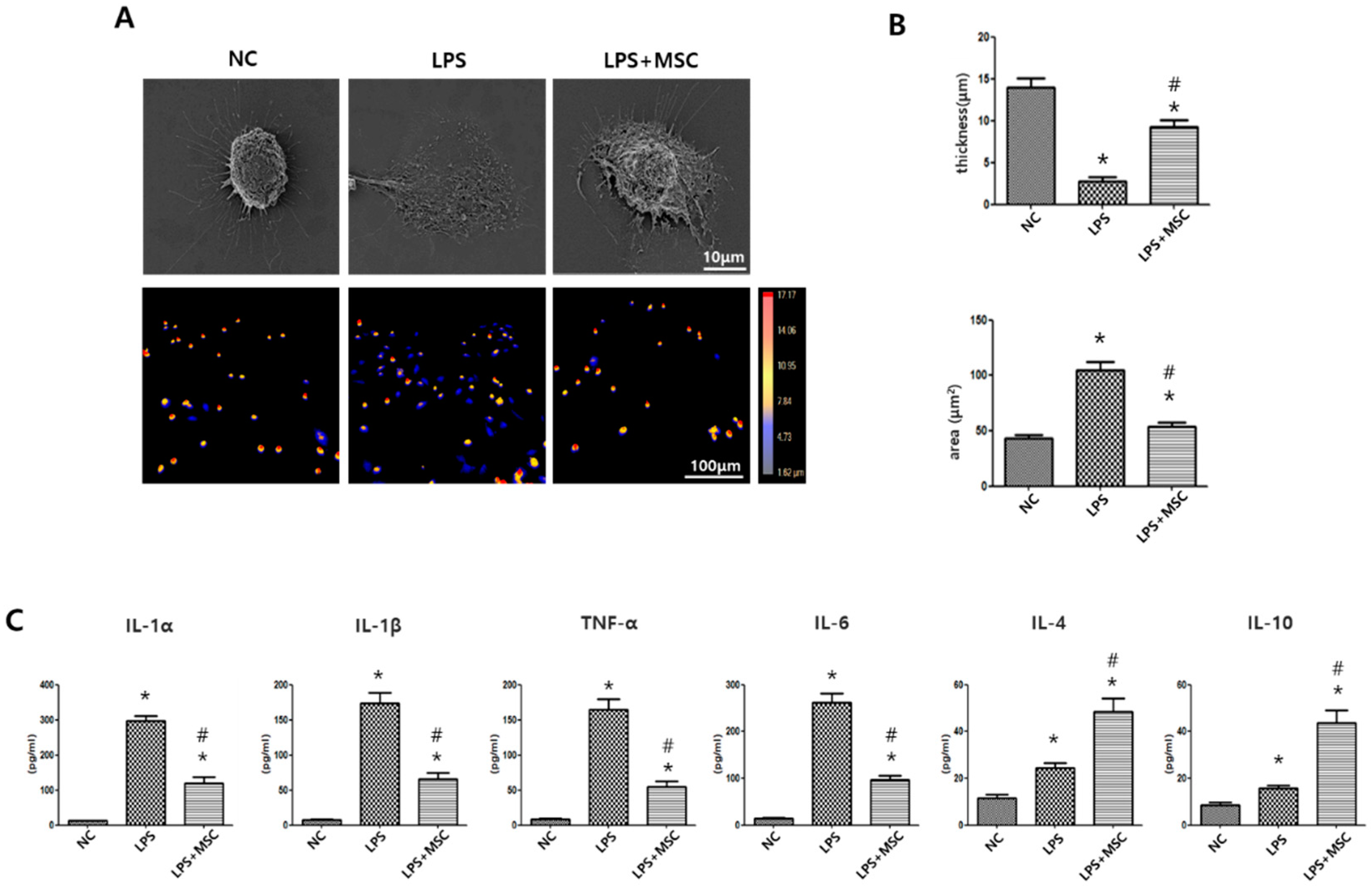
2.1. Effect of MSCs on Morphometric Changes of LPS-Stimulated Alveolar Macrophages
2.2. Effect of MSCs on Regulation of Cytokine Production of LPS-Stimulated Alveolar Macrophages
2.3. MSC Transcriptome Analyses after LPS Induction
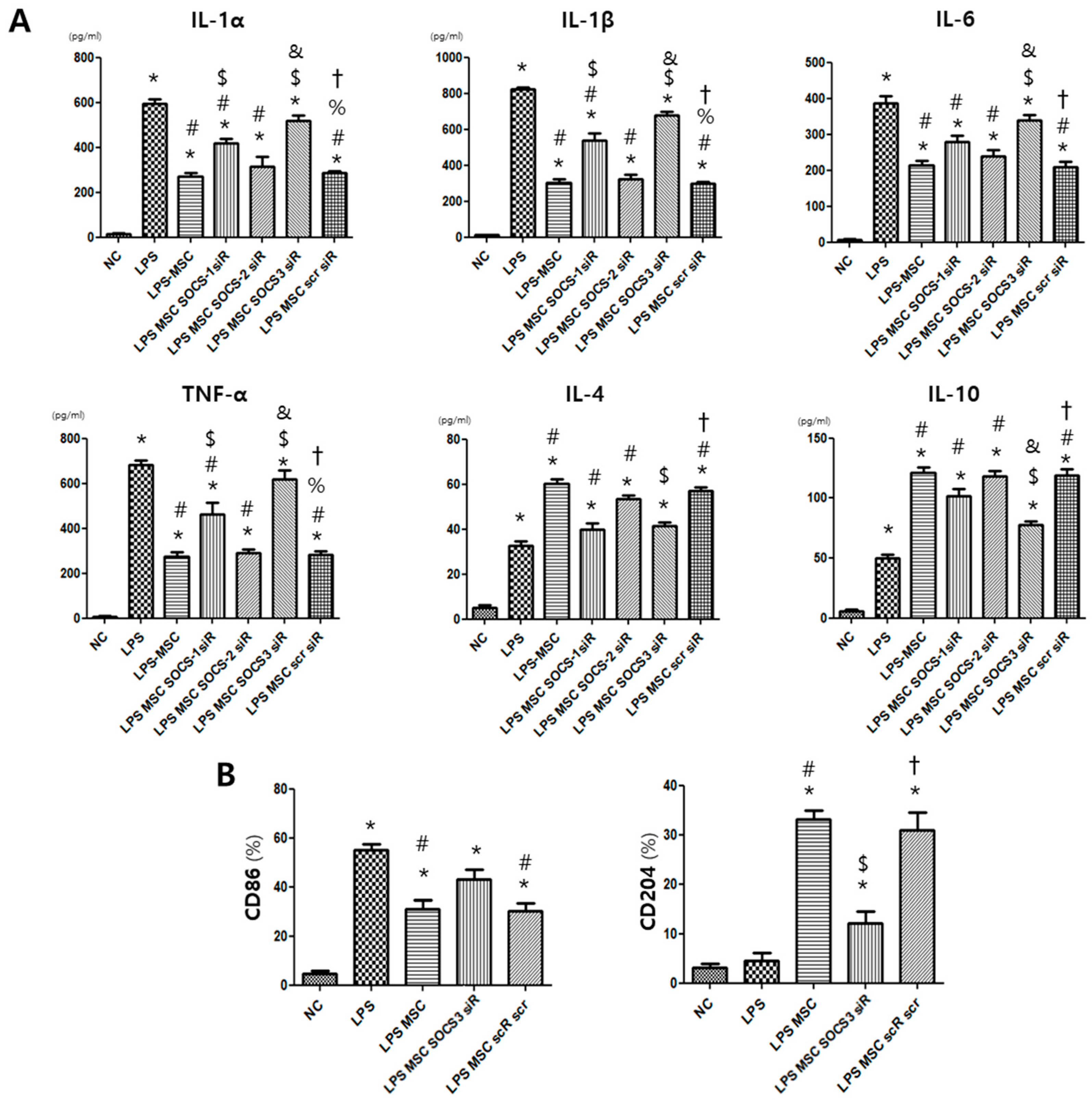
2.4. MSC Effect on Alveolar Macrophage Modulation
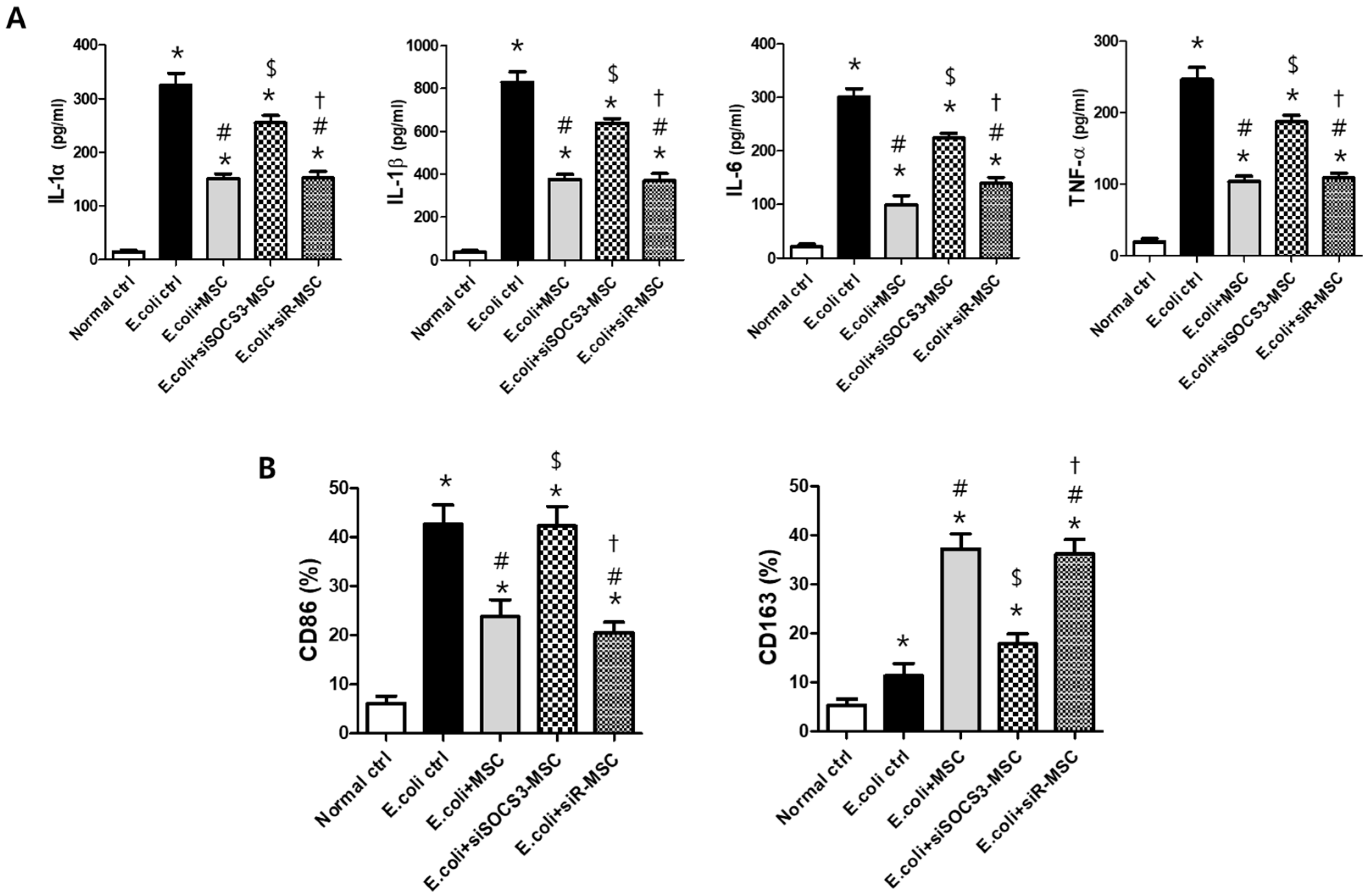
2.5. Histological Examination in the E. Coli-Induced ALI Mouse Model
2.6. Macrophage Modulating Function of MSCs
3. Discussion
4. Materials and Methods
4.1. Preparation of MSCs
4.2. SOCSs Suppression in MSCs
4.3. In Vitro Models of LPS-Induced Inflammation in Alveolar Macrophages
4.4. Quantification of Morphometric Changes in Alveolar Macrophages
4.5. Microarray Analysis
4.6. Preparation of Bacteria
4.7. In Vivo Model of Bacterial-Induced ALI
4.8. Tissue Preparation
4.9. Lung Injury Scores
4.10. Western Blots
4.11. Enzyme-Linked Immunosorbent Assay
4.12. Fluorescence Activated Cell Sorting (FACS)
4.13. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhadade, R.R.; de Souza, R.A.; Harde, M.J.; Khot, A. Clinical characteristics and outcomes of patients with acute lung injury and ARDS. J. Postgrad. Med. 2011, 57, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Allard, B.; Panariti, A.; Martin, J.G. Alveolar Macrophages in the Resolution of Inflammation, Tissue Repair, and Tolerance to Infection. Front. Immunol. 2018, 9, 1777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mosser, D.M. Macrophage activation by endogenous danger signals. J. Pathol. 2008, 214, 161–178. [Google Scholar] [CrossRef]
- Labonte, A.C.; Tosello-Trampont, A.C.; Hahn, Y.S. The role of macrophage polarization in infectious and inflammatory diseases. Mol. Cells 2014, 37, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Yu, C.R.; Mahdi, R.R.; Oh, H.M.; Amadi-Obi, A.; Levy-Clarke, G.; Burton, J.; Eseonu, A.; Lee, Y.; Chan, C.C.; Egwuagu, C.E. Suppressor of cytokine signaling-1 (SOCS1) inhibits lymphocyte recruitment into the retina and protects SOCS1 transgenic rats and mice from ocular inflammation. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6978–6986. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Chen, X.; Shi, Q.; Su, W.; Zhao, H. SOCS-1 Suppresses Inflammation Through Inhibition of NALP3 Inflammasome Formation in Smoke Inhalation-Induced Acute Lung Injury. Inflammation 2018, 41, 1557–1567. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Su, J.; Chen, W.S.; Wang, X.W.; Bai, S.X.; Zhang, J.Z.; Yu, S.Q. Macrophage micro-RNA-155 promotes lipopolysaccharide-induced acute lung injury in mice and rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L494–L506. [Google Scholar] [CrossRef]
- Wong, P.K.; Egan, P.J.; Croker, B.A.; O’Donnell, K.; Sims, N.A.; Drake, S.; Kiu, H.; McManus, E.J.; Alexander, W.S.; Roberts, A.W.; et al. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J. Clin. Investig. 2006, 116, 1571–1581. [Google Scholar] [CrossRef]
- Kode, J.A.; Mukherjee, S.; Joglekar, M.V.; Hardikar, A.A. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009, 11, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, C.; Chen, L.; Tang, L.; Zhu, Y.; Xu, X.; Chen, L.; Gao, H.; Lu, X.; Yu, L.; et al. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering 2020, 6, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Feng, Y.; Li, D.; Li, T.; Gao, P.; Xu, T. Activation of aryl hydrocarbon receptor (AhR) in mesenchymal stem cells modulates macrophage polarization in asthma. J. Immunotoxicol. 2020, 17, 21–30. [Google Scholar] [CrossRef]
- Naeem, A.; Rai, S.N.; Pierre, L. Histology, Alveolar Macrophages; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kim, E.S.; Chang, Y.S.; Choi, S.J.; Kim, J.K.; Yoo, H.S.; Ahn, S.Y.; Sung, D.K.; Kim, S.Y.; Park, Y.R.; Park, W.S. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir. Res. 2011, 12, 108. [Google Scholar] [CrossRef]
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Yoo, H.S.; Ahn, S.Y.; Park, W.S. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta-defensin-2 via toll-like receptor 4 signalling. Cell Microbiol. 2016, 18, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Kim, Y.E.; Sung, S.I.; Sung, D.K.; Park, W.S. Mesenchymal stem cells transplantation attenuates brain injury and enhances bacterial clearance in Escherichia coli meningitis in newborn rats. Pediatr. Res. 2018, 84, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Beasley, M.B. The pathologist’s approach to acute lung injury. Arch. Pathol. Lab. Med. 2010, 134, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, Through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef]
- Chernov, A.S.; Minakov, A.A.; Kazakov, V.A.; Rodionov, M.V.; Rybalkin, I.N.; Vlasik, T.N.; Yashin, D.V.; Saschenko, L.P.; Kudriaeva, A.A.; Belogurov, A.A.; et al. A new mouse unilateral model of diffuse alveolar damage of the lung. Inflamm. Res. 2022, 71, 627–639. [Google Scholar] [CrossRef]
- Hwang, D.J.; Song, H.K.; Kim, K.S.; Jung, Y.S.; Hwang, D.Y.; Cho, J.Y. Comparative analysis of basal locomotor activity-related metabolic phenotypes between C57BL/6 mice and ICR mice substrains derived from three different sources. Lab. Anim. Res. 2017, 33, 140–149. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Kim, Y.E.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef]
- Hu, G.; Christman, J.W. Editorial: Alveolar Macrophages in Lung Inflammation and Resolution. Front. Immunol. 2019, 10, 2275. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, M.; Zhao, J.; Zheng, M.; Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 5009–5014. [Google Scholar] [CrossRef]
- Fadini, G.P.; Simoni, F.; Cappellari, R.; Vitturi, N.; Galasso, S.; Vigili de Kreutzenberg, S.; Previato, L.; Avogaro, A. Pro-inflammatory monocyte-macrophage polarization imbalance in human hypercholesterolemia and atherosclerosis. Atherosclerosis 2014, 237, 805–808. [Google Scholar] [CrossRef]
- Saparov, A.; Ogay, V.; Nurgozhin, T.; Jumabay, M.; Chen, W.C. Preconditioning of Human Mesenchymal Stem Cells to Enhance Their Regulation of the Immune Response. Stem Cells Int. 2016, 2016, 3924858. [Google Scholar] [CrossRef]
- Sung, D.K.; Chang, Y.S.; Sung, S.I.; Ahn, S.Y.; Park, W.S. Thrombin Preconditioning of Extracellular Vesicles Derived from Mesenchymal Stem Cells Accelerates Cutaneous Wound Healing by Boosting Their Biogenesis and Enriching Cargo Content. J. Clin. Med. 2019, 8, 533. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, Y.E.; Park, W.S.; Ahn, S.Y.; Sung, D.K.; Sung, S.I.; Joo, K.M.; Kim, S.G.; Chang, Y.S. Thrombin Preconditioning Improves the Therapeutic Efficacy of Mesenchymal Stem Cells in Severe Intraventricular Hemorrhage Induced Neonatal Rats. Int. J. Mol. Sci. 2022, 23, 4447. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Park, W.S. Cell type-dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy 2015, 17, 1025–1035. [Google Scholar] [CrossRef]
- Yang, S.E.; Ha, C.W.; Jung, M.; Jin, H.J.; Lee, M.; Song, H.; Choi, S.; Oh, W.; Yang, Y.S. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy 2004, 6, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Cordero, L.; Rau, R.; Taylor, D.; Ayers, L.W. Enteric gram-negative bacilli bloodstream infections: 17 years’ experience in a neonatal intensive care unit. Am. J. Infect. Control. 2004, 32, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Chang, Y.S.; Lee, M. N(omega)-nitro-L-arginine methyl ester (L-NAME) attenuates the acute inflammatory responses and brain injury during the early phase of experimental Escherichia coli meningitis in the newborn piglet. Neurol. Res. 2001, 23, 862–868. [Google Scholar] [CrossRef]
- Gupta, N.; Su, X.; Popov, B.; Lee, J.W.; Serikov, V.; Matthay, M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol. 2007, 179, 1855–1863. [Google Scholar] [CrossRef]
- Kaku, Y.; Imaoka, H.; Morimatsu, Y.; Komohara, Y.; Ohnishi, K.; Oda, H.; Takenaka, S.; Matsuoka, M.; Kawayama, T.; Takeya, M.; et al. Overexpression of CD163, CD204 and CD206 on alveolar macrophages in the lungs of patients with severe chronic obstructive pulmonary disease. PLoS ONE 2014, 9, e87400. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.E.; Sung, D.K.; Bang, Y.; Sung, S.I.; Yang, M.; Ahn, S.Y.; Chang, Y.S. SOCS3 Protein Mediates the Therapeutic Efficacy of Mesenchymal Stem Cells against Acute Lung Injury. Int. J. Mol. Sci. 2023, 24, 8256. https://doi.org/10.3390/ijms24098256
Kim YE, Sung DK, Bang Y, Sung SI, Yang M, Ahn SY, Chang YS. SOCS3 Protein Mediates the Therapeutic Efficacy of Mesenchymal Stem Cells against Acute Lung Injury. International Journal of Molecular Sciences. 2023; 24(9):8256. https://doi.org/10.3390/ijms24098256
Chicago/Turabian StyleKim, Young Eun, Dong Kyung Sung, Yuna Bang, Se In Sung, Misun Yang, So Yoon Ahn, and Yun Sil Chang. 2023. "SOCS3 Protein Mediates the Therapeutic Efficacy of Mesenchymal Stem Cells against Acute Lung Injury" International Journal of Molecular Sciences 24, no. 9: 8256. https://doi.org/10.3390/ijms24098256
APA StyleKim, Y. E., Sung, D. K., Bang, Y., Sung, S. I., Yang, M., Ahn, S. Y., & Chang, Y. S. (2023). SOCS3 Protein Mediates the Therapeutic Efficacy of Mesenchymal Stem Cells against Acute Lung Injury. International Journal of Molecular Sciences, 24(9), 8256. https://doi.org/10.3390/ijms24098256





