InDel and SCoT Markers for Genetic Diversity Analysis in a Citrus Collection from the Western Caucasus
Abstract
1. Introduction
2. Results
2.1. Efficiency of SCoT and InDel Primers for Genetic Diversity Analysis of the Citrus Germplasm Collection
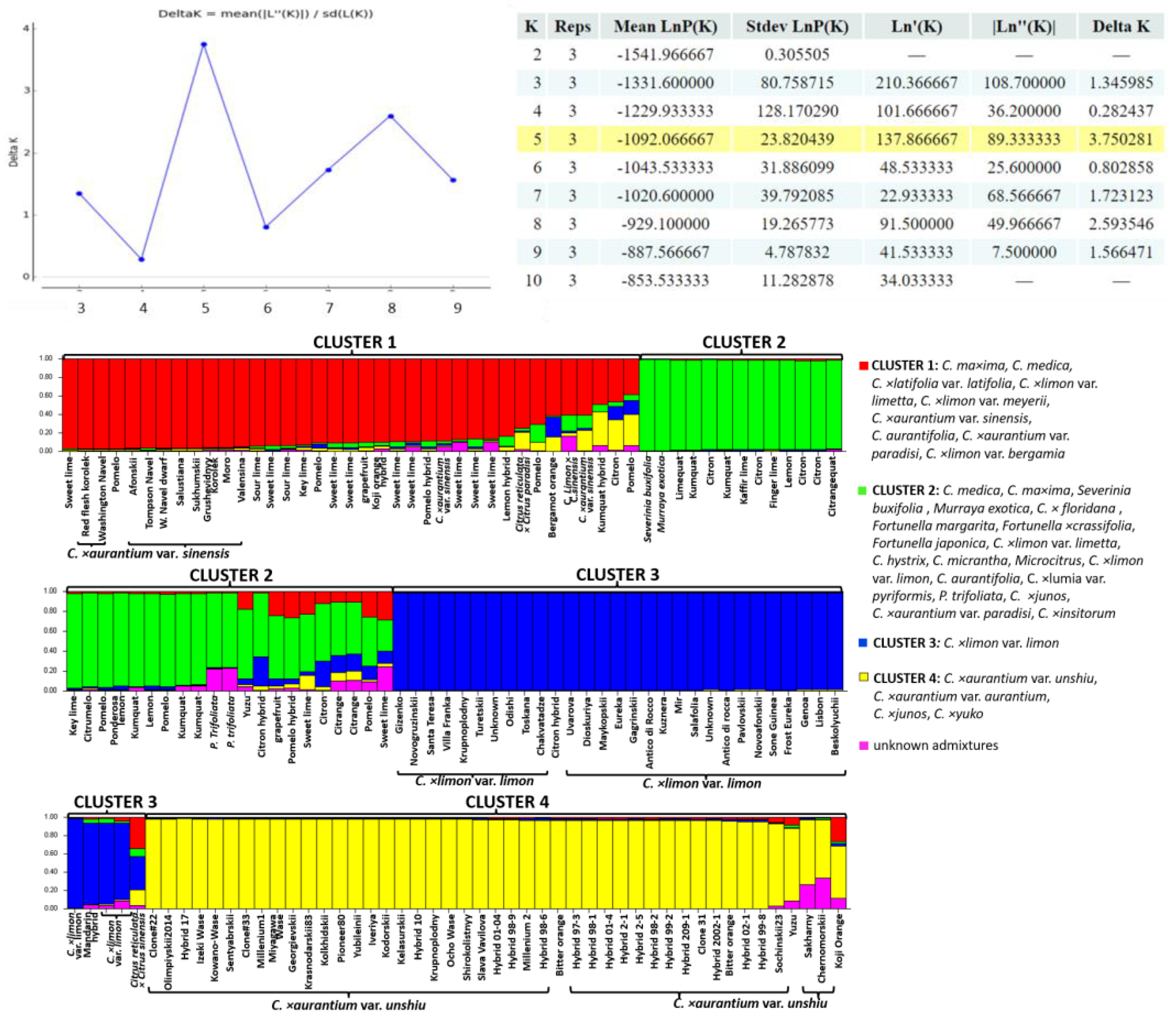
2.2. Genetic Structure of the Citrus Germplasm Collection Based on SCoT and InDel Polymorphisms
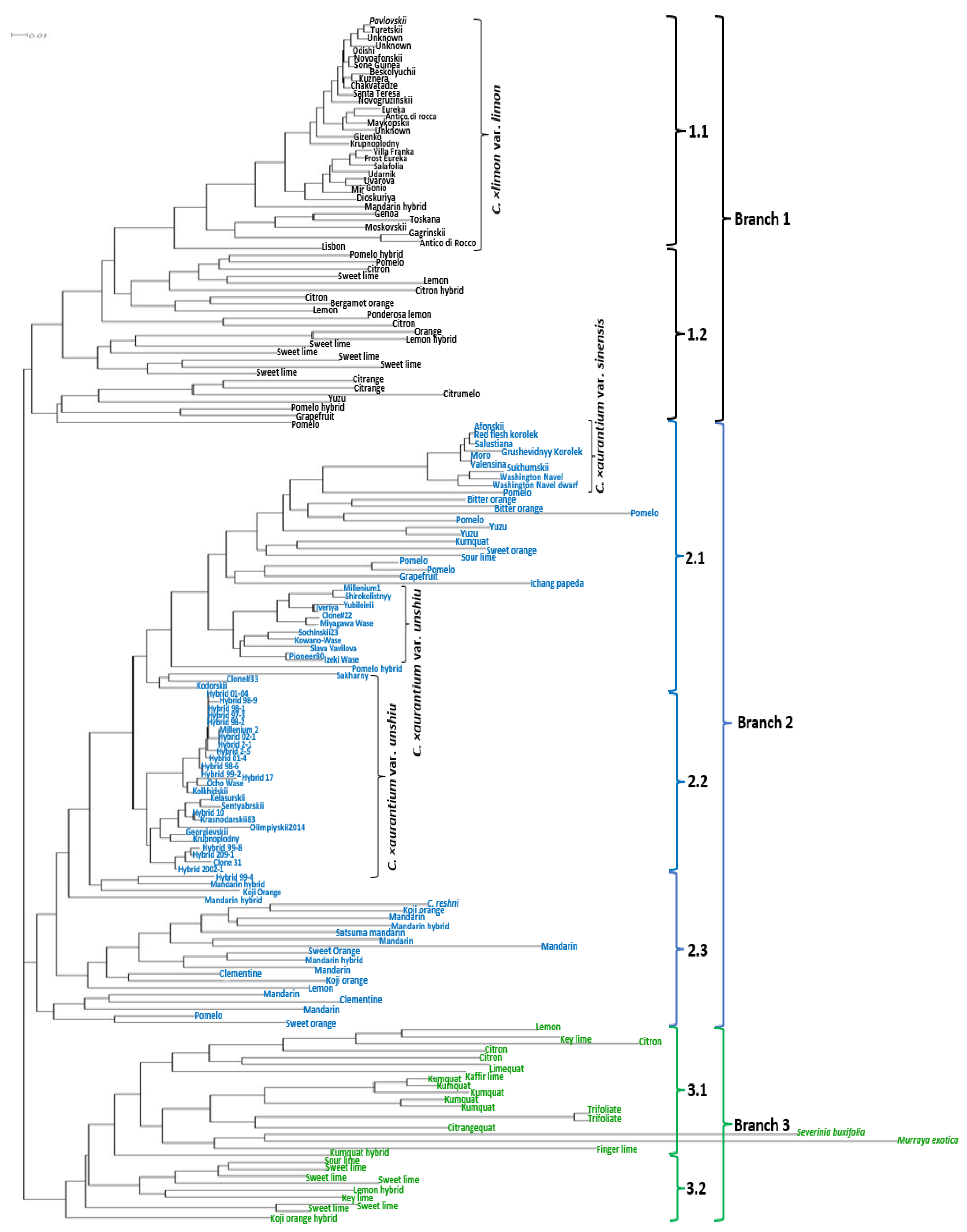
2.3. Phylogenetic Analysis of the Citrus Germplasm Collection Based on SCoT and InDel Polymorphisms
3. Discussion
3.1. Efficiency of SCoT and InDel Markers for Genetic Diversity Analysis of the Citrus Germplasm Collection
3.2. Genetic Structure of the Citrus Germplasm Collection Based on SCoT and InDel Polymorphisms
3.3. Phylogenetic Analysis of the Citrus Germplasm Collection Based on SCoT and InDel Polymorphisms
4. Materials and Methods
4.1. The Plant Material and DNA Extraction
4.2. PCR Analysis and Visualization
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. Business Data Platform. Available online: https://www.statista.com (accessed on 5 April 2023).
- Food and Agriculture Organization of the United Nation. Available online: http://www.fao.org (accessed on 5 April 2023).
- Kulyan, R.V.; Samarina, L.S.; Rakhmangulov, R.S.; Kikavskii, I.V.; Alehna, A.I. Citrus genetic resources in Russia, Ukraine, Belarus: Conservation and management. Vavilov J. Genet. Breed. 2017, 21, 506–514. [Google Scholar] [CrossRef]
- Volk, G.; Samarina, L.; Kulyan, R.; Gorshkov, V.; Malyarovskaya, V.; Ryndin, A.; Polek, M.; Krueger, R.; Stover, E. Citrus genebank collections: International collaboration opportunities between the US and Russia. Genet. Resour. Crop. Evol. 2018, 65, 433–447. [Google Scholar] [CrossRef]
- Jeong, H.; Yun, Y.B.; Jeong, S.Y.; Cho, Y.; Kim, S. Characterization of miniature inverted repeat transposable elements inserted in the CitRWP gene controlling nucellar embryony and development of molecular markers for reliable genotyping of CitRWP in Citrus species. Sci. Hortic. 2023, 315, 112003. [Google Scholar] [CrossRef]
- Distefano, G.; Las Casas, G.; Deng, X.; Chai, L. Citrus Reproductive Biology from Flowering to Fruiting. In The Citrus Genome. Compendium of Plant Genomes; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer: Cham, Switzerland, 2020; pp. 167–176. [Google Scholar] [CrossRef]
- Abouzari, A.; Solouki, M.; Golein, B.; Fakheri, B.A.; Sabouri, A.; Dadras, A.R. Screening of molecular markers associated to cold tolerance-related traits in Citrus. Sci. Hortic. 2020, 263, 109145. [Google Scholar] [CrossRef]
- Nonaka, K.; Fujii, H.; Kita, M.; Shimada, T.; Endo, T.; Yoshioka, T.; Omura, M. Identification and Parentage Analysis of Citrus Cultivars Developed in Japan by CAPS Markers. Hortic. J. 2017, 86, 208–221. [Google Scholar] [CrossRef]
- Noda, T.; Daiou, K.; Mihara, T.; Nagano, Y. Development of Indel markers for the selection of Satsuma mandarin (Citrus unshiu Marc.) hybrids that can be used for low-cost genotyping with agarose gels. Euphytica 2020, 216, 179–187. [Google Scholar] [CrossRef]
- Noda, T.; Daiou, K.; Mihara, T.; Nagano, Y. Potential application of simple easy-to-use insertion-deletion (InDel) markers in citrus cultivar identification. Breed. Sci. 2021, 71, 601–608. [Google Scholar] [CrossRef]
- Samarina, L.S.; Malyarovskaya, V.I.; Reim, S.; Yakushina, L.G.; Koninskaya, N.G.; Klemeshova, K.V.; Shkhalakhova, R.M.; Matskiv, A.O.; Shurkina, E.A.; Gabueva, T.Y.; et al. Transferability of ISSR, SCoT and SSR Markers for Chrysanthemum × Morifolium Ramat and Genetic Relationships Among Commercial Russian Cultivars. Plants 2021, 10, 1302. [Google Scholar] [CrossRef]
- Juibary, P.L.; Seyedmehdi, F.S.; Sheidai, M.; Noormohammadi, Z.; Koohdar, F. Genetic structure analysis and genetic finger printing of sweet orange cultivars (Citrus sinensis (L.) Osbeck) by using SCoT molecular markers. Genet. Resour. Crop Evol. 2021, 68, 1645–1654. [Google Scholar] [CrossRef]
- Zanganeh, F.; Sheidai, M. Population genetic diversity and genetic affinity analyses of sweet orange cultivars (Citrus sinensis (L.) Osbeck) by using IRAP molecular markers. Genet. Resour. Crop Evol. 2022, 69, 2437–2446. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) Polymorphism: A simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Etminan, A.; Pour-Aboughadareh, A.; Mohammadi, R.; Ahmadi-Rad, A.; Noori, A.; Mahdavian, Z.; Moradi, Z. Applicability of start codon targeted (SCoT) and inter-simple sequence repeat (ISSR) markers for genetic diversity analysis in durum wheat genotypes. Biotechnol. Biotechnol. Equip. 2016, 30, 1075–1081. [Google Scholar] [CrossRef]
- Volk, G.M. Widespread applications of citrus cryopreservation. Citrograph 2015, 6, 42–44. [Google Scholar]
- Wang, Y.; Zhou, L.; Yu, X.; Stover, E.; Luo, F.; Duan, Y. Transcriptome Profiling of Huanglongbing (HLB) Tolerant and Susceptible Citrus Plants Reveals the Role of Basal Resistance in HLB Tolerance. Front. Plant Sci. 2016, 7, 933. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhang, S.; Cao, L.; Huang, Y.; Cheng, J.; Wu, G.; Tian, S.; Chen, C.; Liu, Y.; et al. Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction. Nat. Genet. 2017, 49, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borreda´, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Liu, T.J.; Li, Y.P.; Zhou, J.J.; Hu, C.G.; Zhang, J.Z. Genome-wide genetic variation and comparison of fruit-associated traits between kumquat (Citrus japonica) and Clementine mandarin (Citrus clementina). Plant Mol. Biol. 2018, 96, 493–507. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, F.; Xu, C.; Tang, W. Genetic diversity analysis of Shatangju mandarin (Citrus reticulata) by SCoT-PCR. Agric. Sci. Technol. 2016, 17, 34. [Google Scholar]
- Etminan, A.; Pour-Aboughadareh, A.; Noori, A.; Ahmadi-Rad, A.; Shooshtari, L.; Mahdavian, Z.; Yousefiazar-Khanian, M. Genetic relationships and diversity among wild Salvia accessions revealed by ISSR and SCoT markers. Biotechnol. Biotechnol. Equip. 2018, 32, 610–617. [Google Scholar] [CrossRef]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; Rabaiolli, S.M.D.S.; Stefanel, C.M.; Lemos, S.C.M.; Silveira, R.L.R.; Buuron, S.K.; Dos Santos, R.S.M.; Moro, S.C. Determining the Polymorphism Information Content of a molecular marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef]
- Giachino, R.R.A. Investigation of the genetic variation of anise (Pimpinella anisum L.) using RAPD and ISSR markers. Genet. Resour. Crop. Evol. 2019, 67, 763–780. [Google Scholar] [CrossRef]
- Ismail, N.A.; Rafii, M.Y.; Mahmud, T.M.M.; Hanafi, M.M.; Miah, G. Genetic diversity of torch ginger (Etlingera elatior) Germplasm Revealed by ISSR and SSR Markers. BioMed Res. Int. 2019, 2019, 5904804. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, S.M.; Medraoui, L.; Alami, M.; Pakhrou, O.; Makkaoui, M.; Boukhary, A.O.M.S.; Filali-Maltouf, A. Inter simple sequence repeat markers to assess genetic diversity of the desert date (Balanites aegyptiaca Del.) for Sahelian ecosystem restoration. Sci. Rep. 2020, 10, 14948. [Google Scholar] [CrossRef] [PubMed]
- Tilwari, A.; Sharma, R. Random amplified polymorphic DNA and inter simple sequence repeat markers reveals genetic diversity between micro propagated, wild and field cultivated genotypes of Gloriosa superba: An endangered medicinal plant. Mol. Biol. Rep. 2021, 48, 2437–2452. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Thakur, M. Applicability of SCoT markers for detection of variations in Fusarium yellows resistant lines of ginger (Zingiber officinale Rosc.) induced through gamma irradiations. S. Afr. J. Bot. 2021, 140, 454–460. [Google Scholar] [CrossRef]
- Ghobadi, G.; Etminan, A.; Mehrabi, A.M.; Shooshtari, L. Molecular diversity analysis in hexaploid wheat (Triticum aestivum L.) and two Aegilops species (Aegilops crassa and Aegilops cylindrica) using CBDP and SCoT markers. J. Genet. Eng. Biotechnol. 2021, 19, 56. [Google Scholar] [CrossRef]
- Guzmán, F.A.; Moore, S.; De Vicente, M.C.; Jahn, M.M. Microsatellites to enhance characterization, conservation and breeding value of Capsicum germplasm. Genet. Resour. Crop. Evol. 2019, 67, 569–585. [Google Scholar] [CrossRef]
- García-Lor, A.; Luro, F.; Navarro, L.; Ollitrault, P. Comparative use of InDel and SSR markers in deciphering the interspecific structure of cultivated citrus genetic diversity: A perspective for genetic association studies. Mol. Genet. Genomics 2012, 287, 77–94. [Google Scholar] [CrossRef]
- Luro, F.L.; Costantino, G.; Terol, J.; Argout, X.; Allario, T.; Wincker, P.; Talon, M.; Ollitrault, P.; Morillon, R. Transferability of the EST-SSRs developed on Nules clementine (Citrus clementina Hort ex Tan) to other Citrus species and their effectiveness for genetic mapping. BMC Genom. 2008, 9, 287. [Google Scholar] [CrossRef]
- Ramadugu, C.; Pfeil, B.E.; Keremane, M.L.; Lee, R.F.; Maureira-Butler, I.J.; Roose, M.L. A six nuclear gene phylogeny of Citrus (Rutaceae) taking into account hybridization and lineage sorting. PLoS ONE 2013, 8, e68410. [Google Scholar] [CrossRef]
- Samarina, L.S.; Kulyan, R.V.; Koninskaya, N.G.; Gorshkov, V.M.; Ryndin, A.V.; Hanke, M.-V.; Flachowsky, H.; Reim, S. Genetic diversity and phylogenetic relationships among citrus germplasm in the Western Caucasus assessed with SSR and organelle DNA markers. Sci. Hortic. 2021, 288, 110355. [Google Scholar] [CrossRef]
- Ollitrault, P.; Garcia-Lor, A.; Terol, J.; Curk, F.; Ollitrault, F.; Talón, M.; Navarro, L. Comparative values of SSRs, SNPs and InDels for citrus genetic diversity analysis. Acta Hortic. 2015, 1065, 457–466. [Google Scholar] [CrossRef]
- Jannati, M.; Fotouhi, R.; Abad, A.P.; Salehi, Z. Genetic diversity analysis of Iranian citrus varieties using micro satellite (SSR) based markers. J. Hortic. For. 2009, 1, 120–125. [Google Scholar]
- Abedinpour, H.; Babaeian Jelodar, N.A.; Ranjbar, G.A.; Golein, B. Study of genetic diversities and relatedness of Iranian citrus genotypes using morphological and molecular markers. J. Plant Mol. Breed. 2015, 3, 35–49. [Google Scholar] [CrossRef]
- Caruso, M.; Smith, M.W.; Froelicher, Y.; Russo, G.; Gmitter Jr, F.G. Chapter 7–Traditional breeding. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr, Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 129–148. [Google Scholar] [CrossRef]
- Noda, T.; Daiou, K.; Mihara, T.; Murakami, H.; Nagano, Y. Efficient method for generating citrus hybrids with polyembryonic Satsuma mandarin as the female parent. Mol. Breed. 2022, 42, 51. [Google Scholar] [CrossRef]
- Curk, F.; Ollitrault, F.; Garcia-Lor, A.; Luro, F.; Navarro, L.; Ollitrault, P. Phylogenetic origin of limes and lemons revealed by cytoplasmic and nuclear markers. Ann. Bot. 2016, 117, 565–583. [Google Scholar] [CrossRef]
- Froelicher, Y.; Mouhaya, W.; Bassene, J.B.; Costantino, G.; Kamiri, M.; Luro, F.; Morillon, R.; Ollitrault, P. New universal mitochondrial PCR markers reveal new information on maternal citrus phylogeny. Tree Genet. Genomes 2011, 7, 49–61. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.; Huang, M.; Yu, Q.; Du, D.; Mattia, M.R.; Gmitter, F.G. Genetic Diversity and Population Structure Analysis of Citrus Germplasm with Single Nucleotide Polymorphism Markers. J. Amer. Soc. Hort. Sci. 2018, 143, 399–408. [Google Scholar] [CrossRef]
- Fujii, H.; Ohta, S.; Nonaka, K.; Katayose, Y.; Matsumoto, T.; Endo, T.; Yoshioka, T.; Omura, М.; Shimada, T. Parental diagnosis of satsuma mandarin (Citrus unshiu Marc.) revealed by nuclear and cytoplasmic markers. Breed. Sci. 2016, 66, 683–691. [Google Scholar] [CrossRef]
- Ollitrault, P.; Curk, F.; Krueger, R. Chapter 4-Citrus taxonomy. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 57–81. [Google Scholar] [CrossRef]
- Sun, Y.L.; Kang, H.M.; Han, S.H.; Park, Y.C.; Hong, S.K. Taxonomy and phylogeny of the genus Citrus based on the nuclear ribosomal DNA its region sequence. Pak. J. Bot. 2015, 47, 95–101. [Google Scholar]
- Shimizu, T.; Kitajima, A.; Nonaka, K.; Yoshioka, T.; Ohta, S.; Goto, S.; Toyoda, A.; Fujiyama, A.; Mochizuki, T.; Nagasaki, Н.; et al. Hybrid Origins of Citrus Varieties Inferred from DNA Marker Analysis of Nuclear and Organelle Genomes. PLoS ONE 2016, 11, e0166969. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 39–40. [Google Scholar]
- Peakall, R.; Smouse, P.E. genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. iMEC: Online Marker Efficiency Calculator. Appl. Plant Sci. 2018, 6, e1159. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; Vonholdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2011, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- DARwin–Dissimilarity Analysis and Representation for Windows. Available online: https://darwin.cirad.fr/overview (accessed on 5 April 2023).

| SCOT | Primers Sequence 5′-3′ | Na | H | PIC | D |
|---|---|---|---|---|---|
| SCoT2 | CAACAATGGCTACCACCC | 28 | 0.33 | 0.39 | 0.26 |
| SCoT6 | CAACAATGGCTACCACGC | 26 | 0.45 | 0.34 | 0.26 |
| SCoT9 | CAACAATGGCTACCAGCA | 29 | 0.45 | 0.34 | 0.21 |
| SCoT12 | ACGACATGGCGACCAACG | 35 | 0.43 | 0.35 | 0.28 |
| SCoT13 | ACGACATGGCGACCATCG | 31 | 0.46 | 0.34 | 0.24 |
| SCoT14 | ACGACATGGCGACCACGC | 34 | 0.43 | 0.35 | 0.27 |
| SCoT18 | ACCATGGCTACCACCGCC | 30 | 0.48 | 0.32 | 0.84 |
| SCoT20 | ACCATGGCTACCACCGCG | 20 | 0.38 | 0.37 | 0.93 |
| SCoT21 | ACGACATGGCGACCCACA | 28 | 0.43 | 0.35 | 0.29 |
| SCoT23 | CACCATGGCTACCACCAG | 20 | 0.46 | 0.33 | 0.87 |
| SCoT31 | CCATGGCTACCACCGCCT | 22 | 0.45 | 0.34 | 0.88 |
| SCoT36 | GCAACAATGGCTACCACC | 19 | 0.46 | 0.33 | 0.87 |
| MEAN | 26.83 | 0.43 | 0.34 | 0.52 | |
| SD | 5.49 | 0.04 | 0.02 | 0.32 |
| InDel | Primers Sequence 5′-3′ | Na | H | PIC | D |
|---|---|---|---|---|---|
| LG 1-4 | F: TACACAGAACCGCCAAATCA R: TCTCCCATGAACCAGCTACC | 7 | 0.36 | 0.42 | 0.94 |
| LG 2-6 | F: CGCGTGTTACTTCTTGACAGA R: CGAGGCATGTGCTTGAATAA | 2 | 0.22 | 0.46 | 0.24 |
| LG3-14 | F: TGCCGGGAGTCTTAAAGATG R: CGAGATGGCCACCTAGAAAT | 2 | 0.37 | 0.42 | 0.44 |
| LG4-2 | F: GGGTTTCTAAGCATTTGGCCA R: ACACTCATCTTCTCGAGCAAAGA | 3 | 0.41 | 0.41 | 0.41 |
| LG 4-3 | F: AAGAGGACATAAGAGGCAAGTTT R: GCCAAGCAAAACTGATAGGG | 4 | 0.47 | 0.38 | 0.86 |
| LG 5-20 | F: GGCATTTGAGCTAGAAATTCGT R: AACACTGTCAAAAGAAAACCACA | 3 | 0.44 | 0.39 | 0.54 |
| LG 7-11 | F: ATTTTGACACGTTCAGCCGC R: TGGATTTTGCACTCACCCTT | 11 | 0.26 | 0.45 | 0.98 |
| LG 8-10 | F: TCTGCTGACCTTGCTTACGA R: CCCTCACAAGACAGTTGAGGA | 4 | 0.48 | 0.37 | 0.83 |
| LG 9-2 | F: GGTGATTTTGAGTATGAGAGGTGG R: AGGGTAGTTTTATGATAGTTATCCACA | 3 | 0.41 | 0.40 | 0.50 |
| MEAN | 4.33 | 0.38 | 0.41 | 0.64 | |
| SD | 2.92 | 0.09 | 0.03 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulyan, R.; Samarina, L.; Shkhalakhova, R.; Kuleshov, A.; Ukhatova, Y.; Antonova, O.; Koninskaya, N.; Matskiv, A.; Malyarovskaya, V.; Ryndin, A. InDel and SCoT Markers for Genetic Diversity Analysis in a Citrus Collection from the Western Caucasus. Int. J. Mol. Sci. 2023, 24, 8276. https://doi.org/10.3390/ijms24098276
Kulyan R, Samarina L, Shkhalakhova R, Kuleshov A, Ukhatova Y, Antonova O, Koninskaya N, Matskiv A, Malyarovskaya V, Ryndin A. InDel and SCoT Markers for Genetic Diversity Analysis in a Citrus Collection from the Western Caucasus. International Journal of Molecular Sciences. 2023; 24(9):8276. https://doi.org/10.3390/ijms24098276
Chicago/Turabian StyleKulyan, Raisa, Lidiia Samarina, Ruset Shkhalakhova, Alexandr Kuleshov, Yulia Ukhatova, Olga Antonova, Natalia Koninskaya, Alexandra Matskiv, Valentina Malyarovskaya, and Alexey Ryndin. 2023. "InDel and SCoT Markers for Genetic Diversity Analysis in a Citrus Collection from the Western Caucasus" International Journal of Molecular Sciences 24, no. 9: 8276. https://doi.org/10.3390/ijms24098276
APA StyleKulyan, R., Samarina, L., Shkhalakhova, R., Kuleshov, A., Ukhatova, Y., Antonova, O., Koninskaya, N., Matskiv, A., Malyarovskaya, V., & Ryndin, A. (2023). InDel and SCoT Markers for Genetic Diversity Analysis in a Citrus Collection from the Western Caucasus. International Journal of Molecular Sciences, 24(9), 8276. https://doi.org/10.3390/ijms24098276





