Experimental and Clinical Investigation of Cytokines in Migraine: A Narrative Review
Abstract
1. Introduction
2. Summary of Search
3. Experimental Study on Neuroinflammation
3.1. CSD Mouse Model of Migraine
3.2. Spontaneous Migraine-Like Mouse Model by Nitroglycerin
3.3. Inflammatory Soup Model of Migraine
3.4. Sound Stress Migraine Model
4. Clinical Research on Neuroinflammation
4.1. Examination of Serum or Plasma Cytokines during Migraine Attacks in Patients with Migraine
4.2. Examination of Serum or Plasma Cytokines during the Interictal Period in Patients with Migraine
4.3. Cerebrospinal Fluid (CSF) Analysis of Patients with Migraine
4.4. Saliva Study of Patients with Migraine
| Examination of serum or plasma cytokines during migraine attacks in patients with migraine | No changes in proinflammatory cytokine TNF-α levels have been reported in serum or plasma during migraine attacks [63,64,65,66,67,68]; results for IL-6 and IL-1β levels are inconsistent. Lower or unchanged anti-inflammatory cytokine, IL-4, levels, and increased or unchanged IL-10 levels during migraine attacks have been reported, with inconsistent results throughout. However, serial analyses of cytokines in jugular venous blood collected by catheterization during migraine attacks resulted in consistent data, an initial increase in IL-1β, followed by increases in IL-6 and TNF-α, and a decrease in IL-4, which returned to its initial value at the end of the attack [64]. This study revealed no change in cytokine levels in peripheral blood collected simultaneously [64]. |
| Examination of serum or plasma cytokines during the interictal period in patients with migraine | Most studies examining the interictal period of TNF-α [70,82,83] and IL-6 [64,65,67,70,71,72,82,88,89,90,91] have reported higher levels compared to controls and no change with respect to IL-1β levels [69,72,82,83,84,87]. IL-10 levels during attacks in migraine patients have been reduced compared to controls [67,79,83,89,93], but IL-4 levels have been reported less frequently and without certainty. |
| Cerebrospinal fluid (CSF) analysis of patients with migraine | CSF studies are limited and not definitive, and a certain view of inflammatory and non-inflammatory cytokines has not been obtained. However, soluble vascular adhesion molecule-1 (sVCAM-1) in CSF, but not plasma, is higher with more frequent headache frequency, suggesting that sVCAM-1 levels in CSF might be a potential biomarker for frequent migraine and CM [79]. |
| Saliva study of patients with migraine | There are markedly fewer saliva-based tests, and no certain view of cytokines has been obtained. IL-1β might be useful in discriminating against migraine [102]. |
5. Cytokines and Genetics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K. Does inflammation have a role in migraine? Nat. Rev. Neurol. 2019, 15, 483–490. [Google Scholar] [CrossRef]
- Ramachandran, R. Neurogenic inflammation and its role in migraine. Semin. Immunopathol. 2018, 40, 301–314. [Google Scholar] [CrossRef]
- Malhotra, R. Understanding migraine: Potential role of neurogenic inflammation. Ann. Indian Acad. Neurol. 2016, 19, 175–182. [Google Scholar] [CrossRef]
- Gao, Y.J.; Ji, R.R. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef]
- Kraig, R.P.; Mitchell, H.M.; Christie-Pope, B.; Kunkler, P.E.; White, D.M.; Tang, Y.P.; Langan, G. TNF-alpha and microglial hormetic involvement in neurological health & migraine. Dose Response 2010, 8, 389–413. [Google Scholar] [CrossRef]
- Levy, D. Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: The role of immuno-vascular interactions and cortical spreading depression. Curr. Pain Headache Rep. 2012, 16, 270–277. [Google Scholar] [CrossRef]
- Jander, S.; Schroeter, M.; Peters, O.; Witte, O.W.; Stoll, G. Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain. J. Cereb. Blood Flow Metab. 2001, 21, 218–225. [Google Scholar] [CrossRef]
- Ghaemi, A.; Sajadian, A.; Khodaie, B.; Lotfinia, A.A.; Lotfinia, M.; Aghabarari, A.; Khaleghi Ghadiri, M.; Meuth, S.; Gorji, A. Immunomodulatory Effect of Toll-Like Receptor-3 Ligand Poly I:C on Cortical Spreading Depression. Mol. Neurobiol. 2016, 53, 143–154. [Google Scholar] [CrossRef]
- Ghaemi, A.; Alizadeh, L.; Babaei, S.; Jafarian, M.; Khaleghi Ghadiri, M.; Meuth, S.G.; Kovac, S.; Gorji, A. Astrocyte-mediated inflammation in cortical spreading depression. Cephalalgia 2018, 38, 626–638. [Google Scholar] [CrossRef]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Friedman, B.W.; Greenwald, P.; Bania, T.C.; Esses, D.; Hochberg, M.; Solorzano, C.; Corbo, J.; Chu, J.; Chew, E.; Cheung, P.; et al. Randomized trial of IV dexamethasone for acute migraine in the emergency department. Neurology 2007, 69, 2038–2044. [Google Scholar] [CrossRef]
- Rowe, B.H.; Colman, I.; Edmonds, M.L.; Blitz, S.; Walker, A.; Wiens, S. Randomized controlled trial of intravenous dexamethasone to prevent relapse in acute migraine headache. Headache 2008, 48, 333–340. [Google Scholar] [CrossRef]
- Donaldson, D.; Sundermann, R.; Jackson, R.; Bastani, A. Intravenous dexamethasone vs. placebo as adjunctive therapy to reduce the recurrence rate of acute migraine headaches: A multicenter, double-blinded, placebo-controlled randomized clinical trial. Am. J. Emerg. Med. 2008, 26, 124–130. [Google Scholar] [CrossRef]
- Eising, E.; Shyti, R.; ‘t Hoen, P.A.C.; Vijfhuizen, L.S.; Huisman, S.M.H.; Broos, L.A.M.; Mahfouz, A.; Reinders, M.J.T.; Ferrari, M.D.; Tolner, E.A.; et al. Cortical Spreading Depression Causes Unique Dysregulation of Inflammatory Pathways in a Transgenic Mouse Model of Migraine. Mol. Neurobiol. 2017, 54, 2986–2996. [Google Scholar] [CrossRef]
- Gong, Q.; Lin, Y.; Lu, Z.; Xiao, Z. Microglia-Astrocyte Cross Talk through IL-18/IL-18R Signaling Modulates Migraine-like Behavior in Experimental Models of Migraine. Neuroscience 2020, 451, 207–215. [Google Scholar] [CrossRef]
- Wang, M.M.; Yu, X.H.; Geng, W.; Cui, H.F.; Wang, C.C.; Han, J.; Yang, D.H. [Effect of Manual Acupuncture Preconditioning on Behavior and Contents of Serum CGRP, SP, IL-1 β and TNF-α Levels in Migraine Rats]. Zhen Ci Yan Jiu 2018, 43, 375–379. [Google Scholar] [CrossRef]
- Viero, F.T.; Rodrigues, P.; Frare, J.M.; Da Silva, N.A.R.; Ferreira, M.A.; Da Silva, A.M.; Pereira, G.C.; Ferreira, J.; Pillat, M.M.; Bocchi, G.V.; et al. Unpredictable Sound Stress Model Causes Migraine-Like Behaviors in Mice With Sexual Dimorphism. Front. Pharmacol. 2022, 13, 911105. [Google Scholar] [CrossRef]
- Somjen, G.G.; Aitken, P.G.; Czéh, G.L.; Herreras, O.; Jing, J.; Young, J.N. Mechanism of spreading depression: A review of recent findings and a hypothesis. Can. J. Physiol. Pharmacol. 1992, 70, S248–S254. [Google Scholar] [CrossRef]
- Somjen, G.G. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev. 2001, 81, 1065–1096. [Google Scholar] [CrossRef]
- Karatas, H.; Erdener, S.E.; Gursoy-Ozdemir, Y.; Lule, S.; Eren-Koçak, E.; Sen, Z.D.; Dalkara, T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013, 339, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Kunkler, P.E.; Hulse, R.E.; Kraig, R.P. Multiplexed cytokine protein expression profiles from spreading depression in hippocampal organotypic cultures. J. Cereb. Blood Flow Metab. 2004, 24, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.S.; Hakim, A.M. Cortical spreading depression modifies components of the inflammatory cascade. Mol. Neurobiol. 2005, 32, 51–57. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Lambris, J.D.; Ward, P.A. Innate immune responses to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
- Mayer, C.L.; Huber, B.R.; Peskind, E. Traumatic brain injury, neuroinflammation, and post-traumatic headaches. Headache 2013, 53, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, R.; Cui, L.; Yong, C.; Bowyer, S.; Klein, R.M.; Welch, K.M.; Berman, N.E. Cortical spreading depression and gene regulation: Relevance to migraine. Ann. Neurol. 2002, 51, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Volobueva, M.N.; Suleymanova, E.M.; Smirnova, M.P.; Bolshakov, A.P.; Vinogradova, L.V. A Single Episode of Cortical Spreading Depolarization Increases mRNA Levels of Proinflammatory Cytokines, Calcitonin Gene-Related Peptide and Pannexin-1 Channels in the Cerebral Cortex. Int. J. Mol. Sci. 2022, 24, 85. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Qin, T.; Lopes de Morais, A.; Sugimoto, K.; Chung, J.Y.; Morsett, L.; Mulder, I.; Fischer, P.; Suzuki, T.; Anzabi, M.; et al. Non-invasively triggered spreading depolarizations induce a rapid pro-inflammatory response in cerebral cortex. J. Cereb. Blood Flow Metab. 2020, 40, 1117–1131. [Google Scholar] [CrossRef]
- Urbach, A.; Bruehl, C.; Witte, O.W. Microarray-based long-term detection of genes differentially expressed after cortical spreading depression. Eur. J. Neurosci. 2006, 24, 841–856. [Google Scholar] [CrossRef]
- Hulse, R.E.; Kunkler, P.E.; Fedynyshyn, J.P.; Kraig, R.P. Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J. Neurosci. Methods 2004, 136, 87–98. [Google Scholar] [CrossRef]
- Sureda-Gibert, P.; Romero-Reyes, M.; Akerman, S. Nitroglycerin as a model of migraine: Clinical and preclinical review. Neurobiol. Pain 2022, 12, 100105. [Google Scholar] [CrossRef] [PubMed]
- Reuter, U.; Bolay, H.; Jansen-Olesen, I.; Chiarugi, A.; Sanchez del Rio, M.; Letourneau, R.; Theoharides, T.C.; Waeber, C.; Moskowitz, M.A. Delayed inflammation in rat meninges: Implications for migraine pathophysiology. Brain 2001, 124 Pt 12, 2490–2502. [Google Scholar] [CrossRef] [PubMed]
- Reuter, U.; Chiarugi, A.; Bolay, H.; Moskowitz, M.A. Nuclear factor-kappaB as a molecular target for migraine therapy. Ann. Neurol. 2002, 51, 507–516. [Google Scholar] [CrossRef]
- He, W.; Long, T.; Pan, Q.; Zhang, S.; Zhang, Y.; Zhang, D.; Qin, G.; Chen, L.; Zhou, J. Microglial NLRP3 inflammasome activation mediates IL-1β release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J. Neuroinflamm. 2019, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, Y.; Jing, F.; Long, T.; Qin, G.; Zhang, D.; Chen, L.; Zhou, J. P2X7R-mediated autophagic impairment contributes to central sensitization in a chronic migraine model with recurrent nitroglycerin stimulation in mice. J. Neuroinflamm. 2021, 18, 5. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, Y.; Pan, Q.; Tian, R.; Zhang, D.; Qin, G.; Zhou, J.; Chen, L. MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J. Neuroinflamm. 2021, 18, 287. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Li, J.; Hu, B.; Yang, W.; Zhan, M.; Ma, T.; Xu, S. IL-17 crosses the blood-brain barrier to trigger neuroinflammation: A novel mechanism in nitroglycerin-induced chronic migraine. J. Headache Pain 2022, 23, 1. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Zanaboni, A.; Casini, I.; De Icco, R.; Reggiani, A.; Misto, A.; Piomelli, D.; Tassorelli, C. Characterization of the peripheral FAAH inhibitor, URB937, in animal models of acute and chronic migraine. Neurobiol. Dis. 2021, 147, 105157. [Google Scholar] [CrossRef]
- Demartini, C.; Greco, R.; Magni, G.; Zanaboni, A.M.; Riboldi, B.; Francavilla, M.; Nativi, C.; Ceruti, S.; Tassorelli, C. Modulation of Glia Activation by TRPA1 Antagonism in Preclinical Models of Migraine. Int. J. Mol. Sci. 2022, 23, 14085. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, Y.; Tian, R.; Wen, Q.; Qin, G.; Zhang, D.; Chen, L.; Zhang, Y.; Zhou, J. Sphingosine-1 phosphate receptor 1 contributes to central sensitization in recurrent nitroglycerin-induced chronic migraine model. J. Headache Pain 2022, 23, 25. [Google Scholar] [CrossRef]
- Liu, Y.U.; Ying, Y.; Li, Y.; Eyo, U.B.; Chen, T.; Zheng, J.; Umpierre, A.D.; Zhu, J.; Bosco, D.B.; Dong, H.; et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 2019, 22, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Marinelli, A.; Riccio, D.; Lecca, D.; Tonelli, C.; Abbracchio, M.P.; Petroni, K.; Ceruti, S. Purple Corn Extract as Anti-allodynic Treatment for Trigeminal Pain: Role of Microglia. Front. Cell. Neurosci. 2018, 12, 378. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Yamamura, H.; Malick, A.; Strassman, A.M. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J. Neurophysiol. 1998, 79, 964–982. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Xiao, Z.; Zhu, F.; He, X.; Lu, Z. A new comorbidity model and the common pathological mechanisms of migraine and epilepsy. Am. J. Transl. Res. 2017, 9, 2286–2295. [Google Scholar] [PubMed]
- Wang, Y.; Shan, Z.; Zhang, L.; Fan, S.; Zhou, Y.; Hu, L.; Wang, Y.; Li, W.; Xiao, Z. P2X7R/NLRP3 signaling pathway-mediated pyroptosis and neuroinflammation contributed to cognitive impairment in a mouse model of migraine. J. Headache Pain 2022, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Kursun, O.; Yemisci, M.; van den Maagdenberg, A.; Karatas, H. Migraine and neuroinflammation: The inflammasome perspective. J. Headache Pain 2021, 22, 55. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.; Ghanizada, H.; Nielsen, C.A.W.; Hougaard, A.; Ashina, M. Opening of ATP sensitive potassium channels causes migraine attacks with aura. Brain 2021, 144, 2322–2332. [Google Scholar] [CrossRef]
- Christensen, S.L.; Munro, G.; Petersen, S.; Shabir, A.; Jansen-Olesen, I.; Kristensen, D.M.; Olesen, J. ATP sensitive potassium (K(ATP)) channel inhibition: A promising new drug target for migraine. Cephalalgia 2020, 40, 650–664. [Google Scholar] [CrossRef]
- Chen, S.P.; Qin, T.; Seidel, J.L.; Zheng, Y.; Eikermann, M.; Ferrari, M.D.; van den Maagdenberg, A.; Moskowitz, M.A.; Ayata, C.; Eikermann-Haerter, K. Inhibition of the P2X7-PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain 2017, 140, 1643–1656. [Google Scholar] [CrossRef]
- Nie, L.; Ma, D.; Quinn, J.P.; Wang, M. Src family kinases activity is required for transmitting purinergic P2X7 receptor signaling in cortical spreading depression and neuroinflammation. J. Headache Pain 2021, 22, 146. [Google Scholar] [CrossRef]
- Romano, G.L.; Amato, R.; Lazzara, F.; Porciatti, V.; Chou, T.H.; Drago, F.; Bucolo, C. P2X7 receptor antagonism preserves retinal ganglion cells in glaucomatous mice. Biochem. Pharmacol. 2020, 180, 114199. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, D.; Brennan, K.C. The Effects of Chronic Stress on Migraine Relevant Phenotypes in Male Mice. Front. Cell. Neurosci. 2018, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Avona, A.; Mason, B.N.; Lackovic, J.; Wajahat, N.; Motina, M.; Quigley, L.; Burgos-Vega, C.; Moldovan Loomis, C.; Garcia-Martinez, L.F.; Akopian, A.N.; et al. Repetitive stress in mice causes migraine-like behaviors and calcitonin gene-related peptide-dependent hyperalgesic priming to a migraine trigger. Pain 2020, 161, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Eller, O.C.; Yang, X.; Fuentes, I.M.; Pierce, A.N.; Jones, B.M.; Brake, A.D.; Wang, R.; Dussor, G.; Christianson, J.A. Voluntary Wheel Running Partially Attenuates Early Life Stress-Induced Neuroimmune Measures in the Dura and Evoked Migraine-Like Behaviors in Female Mice. Front. Physiol. 2021, 12, 665732. [Google Scholar] [CrossRef]
- Ishikawa, T.; Tatsumoto, M.; Maki, K.; Mitsui, M.; Hasegawa, H.; Hirata, K. Identification of Everyday Sounds Perceived as Noise by Migraine Patients. Intern. Med. 2019, 58, 1565–1572. [Google Scholar] [CrossRef]
- Zhang, X.C.; Kainz, V.; Burstein, R.; Levy, D. Tumor necrosis factor-α induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain 2011, 152, 140–149. [Google Scholar] [CrossRef]
- Avona, A.; Price, T.J.; Dussor, G. Interleukin-6 induces spatially dependent whole-body hypersensitivity in rats: Implications for extracephalic hypersensitivity in migraine. J. Headache Pain 2021, 22, 70. [Google Scholar] [CrossRef]
- Alarcón-Alarcón, D.; Cabañero, D.; de Andrés-López, J.; Nikolaeva-Koleva, M.; Giorgi, S.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine. Nat. Commun. 2022, 13, 6304. [Google Scholar] [CrossRef]
- Watanabe, M.; Kopruszinski, C.M.; Moutal, A.; Ikegami, D.; Khanna, R.; Chen, Y.; Ross, S.; Mackenzie, K.; Stratton, J.; Dodick, D.W.; et al. Dysregulation of serum prolactin links the hypothalamus with female nociceptors to promote migraine. Brain 2022, 145, 2894–2909. [Google Scholar] [CrossRef]
- Al-Hassany, L.; Haas, J.; Piccininni, M.; Kurth, T.; Maassen Van Den Brink, A.; Rohmann, J.L. Giving Researchers a Headache—Sex and Gender Differences in Migraine. Front. Neurol. 2020, 11, 549038. [Google Scholar] [CrossRef]
- Jing, F.; Zou, Q.; Wang, Y.; Cai, Z.; Tang, Y. Activation of microglial GLP-1R in the trigeminal nucleus caudalis suppresses central sensitization of chronic migraine after recurrent nitroglycerin stimulation. J. Headache Pain 2021, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Thuraiaiyah, J.; Erritzøe-Jervild, M.; Al-Khazali, H.M.; Schytz, H.W.; Younis, S. The role of cytokines in migraine: A systematic review. Cephalalgia 2022, 42, 1565–1588. [Google Scholar] [CrossRef] [PubMed]
- van Hilten, J.J.; Ferrari, M.D.; Van der Meer, J.W.; Gijsman, H.J.; Looij, B.J., Jr. Plasma interleukin-1, tumour necrosis factor and hypothalamic-pituitary-adrenal axis responses during migraine attacks. Cephalalgia 1991, 11, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Alberti, A.; Baldi, A.; Coppola, F.; Rossi, C.; Pierguidi, L.; Floridi, A.; Calabresi, P. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache 2006, 46, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Fidan, I.; Yüksel, S.; Ymir, T.; Irkeç, C.; Aksakal, F.N. The importance of cytokines, chemokines and nitric oxide in pathophysiology of migraine. J. Neuroimmunol. 2006, 171, 184–188. [Google Scholar] [CrossRef]
- Tanure, M.T.; Gomez, R.S.; Hurtado, R.C.; Teixeira, A.L.; Domingues, R.B. Increased serum levels of brain-derived neurotropic factor during migraine attacks: A pilot study. J. Headache Pain 2010, 11, 427–430. [Google Scholar] [CrossRef]
- Uzar, E.; Evliyaoglu, O.; Yucel, Y.; Ugur Cevik, M.; Acar, A.; Guzel, I.; Islamoglu, Y.; Colpan, L.; Tasdemir, N. Serum cytokine and pro-brain natriuretic peptide (BNP) levels in patients with migraine. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1111–1116. [Google Scholar]
- Guo, S.; Vollesen, A.L.; Hansen, Y.B.; Frandsen, E.; Andersen, M.R.; Amin, F.M.; Fahrenkrug, J.; Olesen, J.; Ashina, M. Part II: Biochemical changes after pituitary adenylate cyclase-activating polypeptide-38 infusion in migraine patients. Cephalalgia 2017, 37, 136–147. [Google Scholar] [CrossRef]
- Perini, F.; D’Andrea, G.; Galloni, E.; Pignatelli, F.; Billo, G.; Alba, S.; Bussone, G.; Toso, V. Plasma Cytokine Levels in Migraineurs and Controls. Headache 2005, 45, 926–931. [Google Scholar] [CrossRef]
- Yücel, M.; Kotan, D.; Gurol Çiftçi, G.; Çiftçi, I.H.; Cikriklar, H.I. Serum levels of endocan, claudin-5 and cytokines in migraine. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 930–936. [Google Scholar]
- Wang, Y.; Wang, D.; Guo, D. Interictal cytokine levels were correlated to seizure severity of epileptic patients: A retrospective study on 1218 epileptic patients. J. Transl. Med. 2015, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, Z.; Özçelik, P.; Ulukan, Ç.; Ulusoy, C.; Orhan, K.S.; Orhan, E.K.; Küçükali, C.; Tüzün, E.; Baykan, B.; Akdal, G. Plasma levels of inflammatory mediators in vestibular migraine. Int. J. Neurosci. 2020, 130, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Kacinski, M.; Gergont, A.; Kubik, A.; Steczkowska-Klucznik, M. [Proinflammatory cytokines in children with migraine with or without aura]. Przegl Lek. 2005, 62, 1276–1280. [Google Scholar]
- Sarchielli, P.; Floridi, A.; Mancini, M.L.; Rossi, C.; Coppola, F.; Baldi, A.; Pini, L.A.; Calabresi, P. NF-kappaB activity and iNOS expression in monocytes from internal jugular blood of migraine without aura patients during attacks. Cephalalgia 2006, 26, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Martelletti, P.; Stirparo, G.; Rinaldi, C.; Frati, L.; Giacovazzo, M. Disruption of the immunopeptidergic network in dietary migraine. Headache 1993, 33, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Martelletti, P.; Stirparo, G.; Morrone, S.; Rinaldi, C.; Giacovazzo, M. Inhibition of intercellular adhesion molecule-1 (ICAM-1), soluble ICAM-1 and interleukin-4 by nitric oxide expression in migraine patients. J. Mol. Med. 1997, 75, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Rozen, T.; Swidan, S.Z. Elevation of CSF Tumor Necrosis Factor α Levels in New Daily Persistent Headache and Treatment Refractory Chronic Migraine. Headache 2007, 47, 1050–1055. [Google Scholar] [CrossRef]
- Bø, S.H.; Davidsen, E.M.; Gulbrandsen, P.; Dietrichs, E.; Bovim, G.; Stovner, L.J.; White, L.R. Cerebrospinal fluid cytokine levels in migraine, tension-type headache and cervicogenic headache. Cephalalgia 2009, 29, 365–372. [Google Scholar] [CrossRef]
- Cowan, R.P.; Gross, N.B.; Sweeney, M.D.; Sagare, A.P.; Montagne, A.; Arakaki, X.; Fonteh, A.N.; Zlokovic, B.V.; Pogoda, J.M.; Harrington, M.G. Evidence that blood-CSF barrier transport, but not inflammatory biomarkers, change in migraine, while CSF sVCAM1 associates with migraine frequency and CSF fibrinogen. Headache 2021, 61, 536–545. [Google Scholar] [CrossRef]
- Sarchielli, P.; Alberti, A.; Vaianella, L.; Pierguidi, L.; Floridi, A.; Mazzotta, G.; Floridi, A.; Gallai, V. Chemokine levels in the jugular venous blood of migraine without aura patients during attacks. Headache 2004, 44, 961–968. [Google Scholar] [CrossRef]
- Erdener, S.E.; Dalkara, T. Modelling headache and migraine and its pharmacological manipulation. Br. J. Pharmacol. 2014, 171, 4575–4594. [Google Scholar] [CrossRef]
- Hirfanoglu, T.; Serdaroglu, A.; Gulbahar, O.; Cansu, A. Prophylactic drugs and cytokine and leptin levels in children with migraine. Pediatr. Neurol. 2009, 41, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.B.; Bachi, A.L.L.; Ribeiro, R.T.; Mello, M.T.; Tufik, S.; Peres, M.F.P. Unbalanced plasma TNF-α and IL-12/IL-10 profile in women with migraine is associated with psychological and physiological outcomes. J. Neuroimmunol. 2017, 313, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.; Gupta, A.K.; Stein, T.P. Deficiency of tumor necrosis factor alpha in a subclass of menstrual migraineurs. Headache 2001, 41, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Empl, M.; Sostak, P.; Riedel, M.; Schwarz, M.; Müller, N.; Förderreuther, S.; Straube, A. Decreased sTNF-RI in migraine patients? Cephalalgia 2003, 23, 55–58. [Google Scholar] [CrossRef]
- Boćkowski, L.; Sobaniec, W.; Zelazowska-Rutkowska, B. Proinflammatory plasma cytokines in children with migraine. Pediatr. Neurol. 2009, 41, 17–21. [Google Scholar] [CrossRef]
- Chaudhry, S.R.; Lendvai, I.S.; Muhammad, S.; Westhofen, P.; Kruppenbacher, J.; Scheef, L.; Boecker, H.; Scheele, D.; Hurlemann, R.; Kinfe, T.M. Inter-ictal assay of peripheral circulating inflammatory mediators in migraine patients under adjunctive cervical non-invasive vagus nerve stimulation (nVNS): A proof-of-concept study. Brain Stimul. 2019, 12, 643–651. [Google Scholar] [CrossRef]
- Koçer, A.; Memişoğullari, R.; Domaç, F.M.; Ilhan, A.; Koçer, E.; Okuyucu, S.; Ozdemir, B.; Yüksel, H. IL-6 levels in migraine patients receiving topiramate. Pain Pract. 2009, 9, 375–379. [Google Scholar] [CrossRef]
- Domínguez, C.; Vieites-Prado, A.; Pérez-Mato, M.; Sobrino, T.; Rodríguez-Osorio, X.; López, A.; Campos, F.; Martínez, F.; Castillo, J.; Leira, R. CGRP and PTX3 as Predictors of Efficacy of Onabotulinumtoxin Type A in Chronic Migraine: An Observational Study. Headache 2018, 58, 78–87. [Google Scholar] [CrossRef]
- Togha, M.; Razeghi Jahromi, S.; Ghorbani, Z.; Ghaemi, A.; Rafiee, P. Evaluation of Inflammatory State in Migraineurs: A Case-control Study. Iran. J. Allergy Asthma Immunol. 2020, 19, 83–90. [Google Scholar] [CrossRef]
- Mosca, A.; Crudele, A.; Smeriglio, A.; Braghini, M.R.; Panera, N.; Comparcola, D.; Alterio, A.; Sartorelli, M.R.; Tozzi, G.; Raponi, M.; et al. Antioxidant activity of Hydroxytyrosol and Vitamin E reduces systemic inflammation in children with paediatric NAFLD. Dig. Liver Dis. 2021, 53, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, A.; Scheele, D.; Stoffel-Wagner, B.; Honig, F.; Chaudhry, S.R.; Muhammad, S.; Hurlemann, R.; Krauss, J.K.; Lendvai, I.S.; Chakravarthy, K.V.; et al. Saliva molecular inflammatory profiling in female migraine patients responsive to adjunctive cervical non-invasive vagus nerve stimulation: The MOXY Study. J. Transl. Med. 2019, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Yang, Y.P.; Huang, P.I.; Li, W.C.; Huang, M.C.; Kao, C.L.; Chen, Y.J.; Chen, M.T. Exercise suppresses COX-2 pro-inflammatory pathway in vestibular migraine. Brain Res. Bull. 2015, 116, 98–105. [Google Scholar] [CrossRef]
- Martami, F.; Razeghi Jahromi, S.; Togha, M.; Ghorbani, Z.; Seifishahpar, M.; Saidpour, A. The serum level of inflammatory markers in chronic and episodic migraine: A case-control study. Neurol. Sci. 2018, 39, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Michalak, S.; Kalinowska-Lyszczarz, A.; Wegrzyn, D.; Niezgoda, A.; Losy, J.; Osztynowicz, K.; Kozubski, W. Increased Serum CD14 Level Is Associated with Depletion of TNF-alpha in Monocytes in Migraine Patients during Interictal Period. Int. J. Mol. Sci. 2017, 18, 398. [Google Scholar] [CrossRef]
- Munno, I.; Centonze, V.; Marinaro, M.; Bassi, A.; Lacedra, G.; Causarano, V.; Nardelli, P.; Cassiano, M.A.; Albano, O. Cytokines and migraine: Increase of IL-5 and IL-4 plasma levels. Headache 1998, 38, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Takeshima, T.; Fusayasu, E.; Nakashima, K. Increased plasma matrix metalloproteinase-9 levels in migraineurs. Headache 2008, 48, 135–139. [Google Scholar] [CrossRef]
- Wang, F.; He, Q.; Ren, Z.; Li, F.; Chen, W.; Lin, X.; Zhang, H.; Tai, G. Association of serum levels of intercellular adhesion molecule-1 and interleukin-6 with migraine. Neurol. Sci. 2015, 36, 535–540. [Google Scholar] [CrossRef]
- Sabri, M.R.; Dehghan, B.; Yaghini, O.; Nasiri, J.; Mansourian, M.; Khalifehsoltani, S. Endothelial dysfunction state in migraine headache and neutrally mediated syncope in children and young adults. J. Res. Med. Sci. 2015, 20, 771–776. [Google Scholar] [CrossRef]
- Hougaard, A.; Amin, F.M.; Christensen, C.E.; Younis, S.; Wolfram, F.; Cramer, S.P.; Larsson, H.B.W.; Ashina, M. Increased brainstem perfusion, but no blood-brain barrier disruption, during attacks of migraine with aura. Brain 2017, 140, 1633–1642. [Google Scholar] [CrossRef]
- Amin, F.M.; Hougaard, A.; Cramer, S.P.; Christensen, C.E.; Wolfram, F.; Larsson, H.B.W.; Ashina, M. Intact blood-brain barrier during spontaneous attacks of migraine without aura: A 3T DCE-MRI study. Eur. J. Neurol. 2017, 24, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Bougea, A.; Spantideas, N.; Galanis, P.; Katsika, P.; Boufidou, F.; Voskou, P.; Vamvakaris, I.; Anagnostou, E.; Nikolaou, X.; Kararizou, E. Salivary inflammatory markers in tension type headache and migraine: The SalHead cohort study. Neurol. Sci. 2020, 41, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Bouma, G.; Crusius, J.B.; Oudkerk Pool, M.; Kolkman, J.J.; von Blomberg, B.M.; Kostense, P.J.; Giphart, M.J.; Schreuder, G.M.; Meuwissen, S.G.; Peña, A.S. Secretion of tumour necrosis factor alpha and lymphotoxin alpha in relation to polymorphisms in the TNF genes and HLA-DR alleles. Relevance for inflammatory bowel disease. Scand. J. Immunol. 1996, 43, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Yan, Y.; Long, J.; Su, L.; Hu, Y.; Chen, Q.; Xie, J.; Wu, G. The TNF-α-308G/A polymorphism is associated with migraine risk: A meta-analysis. Exp. Ther. Med. 2012, 3, 1082–1086. [Google Scholar] [CrossRef]
- Ghosh, J.; Joshi, G.; Pradhan, S.; Mittal, B. Investigation of TNFA 308G > A and TNFB 252G > A polymorphisms in genetic susceptibility to migraine. J. Neurol. 2010, 257, 898–904. [Google Scholar] [CrossRef]
- Yilmaz, I.A.; Ozge, A.; Erdal, M.E.; Edgünlü, T.G.; Cakmak, S.E.; Yalin, O.O. Cytokine polymorphism in patients with migraine: Some suggestive clues of migraine and inflammation. Pain Med. 2010, 11, 492–497. [Google Scholar] [CrossRef]
- Trabace, S.; Brioli, G.; Lulli, P.; Morellini, M.; Giacovazzo, M.; Cicciarelli, G.; Martelletti, P. Tumor necrosis factor gene polymorphism in migraine. Headache 2002, 42, 341–345. [Google Scholar] [CrossRef]
- Asuni, C.; Stochino, M.E.; Cherchi, A.; Manchia, M.; Congiu, D.; Manconi, F.; Squassina, A.; Piccardi, M.P.; Del Zompo, M. Migraine and tumour necrosis factor gene polymorphism. An association study in a Sardinian sample. J. Neurol. 2009, 256, 194–197. [Google Scholar] [CrossRef]
- Schürks, M.; Rist, P.M.; Zee, R.Y.; Chasman, D.I.; Kurth, T. Tumour necrosis factor gene polymorphisms and migraine: A systematic review and meta-analysis. Cephalalgia 2011, 31, 1381–1404. [Google Scholar] [CrossRef]
- Lee, K.A.; Jang, S.Y.; Sohn, K.M.; Won, H.H.; Kim, M.J.; Kim, J.W.; Chung, C.S. Association between a polymorphism in the lymphotoxin-a promoter region and migraine. Headache 2007, 47, 1056–1062. [Google Scholar] [CrossRef]
- Rubino, E.; Marcinnò, A.; Grassini, A.; Piella, E.M.; Ferrandes, F.; Roveta, F.; Boschi, S.; Cermelli, A.; Gallone, S.; Savi, L.; et al. Polymorphisms of the Proinflammatory Cytokine Genes Modulate the Response to NSAIDs but Not to Triptans in Migraine Attacks. Int. J. Mol. Sci. 2022, 24, 657. [Google Scholar] [CrossRef] [PubMed]
- Rainero, I.; Pinessi, L.; Salani, G.; Valfrè, W.; Rivoiro, C.; Savi, L.; Gentile, S.; Giudice, R.L.; Grimaldi, L.M. A polymorphism in the interleukin-1alpha gene influences the clinical features of migraine. Headache 2002, 42, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Rainero, I.; Salani, G.; Valfrè, W.; Savi, L.; Rivoiro, C.; Ferrero, M.; Pinessi, L.; Grimaldi, L.M. Absence of linkage between the interleukin-6 gene (−174 G/C) polymorphism and migraine. Neurosci. Lett. 2003, 343, 155–158. [Google Scholar] [CrossRef]
- Ates, O.; Kurt, S.; Altinisik, J.; Karaer, H.; Sezer, S. Genetic variations in tumor necrosis factor alpha, interleukin-10 genes, and migraine susceptibility. Pain Med. 2011, 12, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Gormley, P.; Anttila, V.; Winsvold, B.S.; Palta, P.; Esko, T.; Pers, T.H.; Farh, K.H.; Cuenca-Leon, E.; Muona, M.; Furlotte, N.A.; et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat. Genet. 2016, 48, 856–866. [Google Scholar] [CrossRef]
- Hautakangas, H.; Winsvold, B.S.; Ruotsalainen, S.E.; Bjornsdottir, G.; Harder, A.V.E.; Kogelman, L.J.A.; Thomas, L.F.; Noordam, R.; Benner, C.; Gormley, P.; et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat. Genet. 2022, 54, 152–160. [Google Scholar] [CrossRef]
- Soni, S.; Anand, P.; Padwad, Y.S. MAPKAPK2: The master regulator of RNA-binding proteins modulates transcript stability and tumor progression. J. Exp. Clin. Cancer Res. 2019, 38, 121. [Google Scholar] [CrossRef]
- Murphy, J.M.; Jeong, K.; Rodriguez, Y.A.R.; Kim, J.H.; Ahn, E.E.; Lim, S.S. FAK and Pyk2 activity promote TNF-α and IL-1β-mediated pro-inflammatory gene expression and vascular inflammation. Sci. Rep. 2019, 9, 7617. [Google Scholar] [CrossRef]
- Mazaheri, S.; Hajilooi, M.; Rafiei, A. The G-308A promoter variant of the tumor necrosis factor-alpha gene is associated with migraine without aura. J. Neurol. 2006, 253, 1589–1593. [Google Scholar] [CrossRef]
- Miyamae, T. Cryopyrin-associated periodic syndromes: Diagnosis and management. Paediatr. Drugs 2012, 14, 109–117. [Google Scholar] [CrossRef]
- Keddie, S.; Parker, T.; Lachmann, H.J.; Ginsberg, L. Cryopyrin-Associated Periodic Fever Syndrome and the Nervous System. Curr. Treat. Options Neurol. 2018, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, H.; Geng, L. Intractable Epilepsy (IE) and Responses to Anakinra, a Human Recombinant IL-1 Receptor Agonist (IL-1ra): Case Reports. J. Clin. Cell. Immunol. 2016, 7, 1000456. [Google Scholar] [CrossRef]
- Dilena, R.; Mauri, E.; Aronica, E.; Bernasconi, P.; Bana, C.; Cappelletti, C.; Carrabba, G.; Ferrero, S.; Giorda, R.; Guez, S.; et al. Therapeutic effect of Anakinra in the relapsing chronic phase of febrile infection-related epilepsy syndrome. Epilepsia Open 2019, 4, 344–350. [Google Scholar] [CrossRef]
- Yamanaka, G.; Ishida, Y.; Kanou, K.; Suzuki, S.; Watanabe, Y.; Takamatsu, T.; Morichi, S.; Go, S.; Oana, S.; Yamazaki, T.; et al. Towards a Treatment for Neuroinflammation in Epilepsy: Interleukin-1 Receptor Antagonist, Anakinra, as a Potential Treatment in Intractable Epilepsy. Int. J. Mol. Sci. 2021, 22, 6282. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Nye, B.L.; Thadani, V.M. Migraine and epilepsy: Review of the literature. Headache 2015, 55, 359–380. [Google Scholar] [CrossRef]
- Mantegazza, M.; Cestèle, S. Pathophysiological mechanisms of migraine and epilepsy: Similarities and differences. Neurosci. Lett. 2018, 667, 92–102. [Google Scholar] [CrossRef] [PubMed]
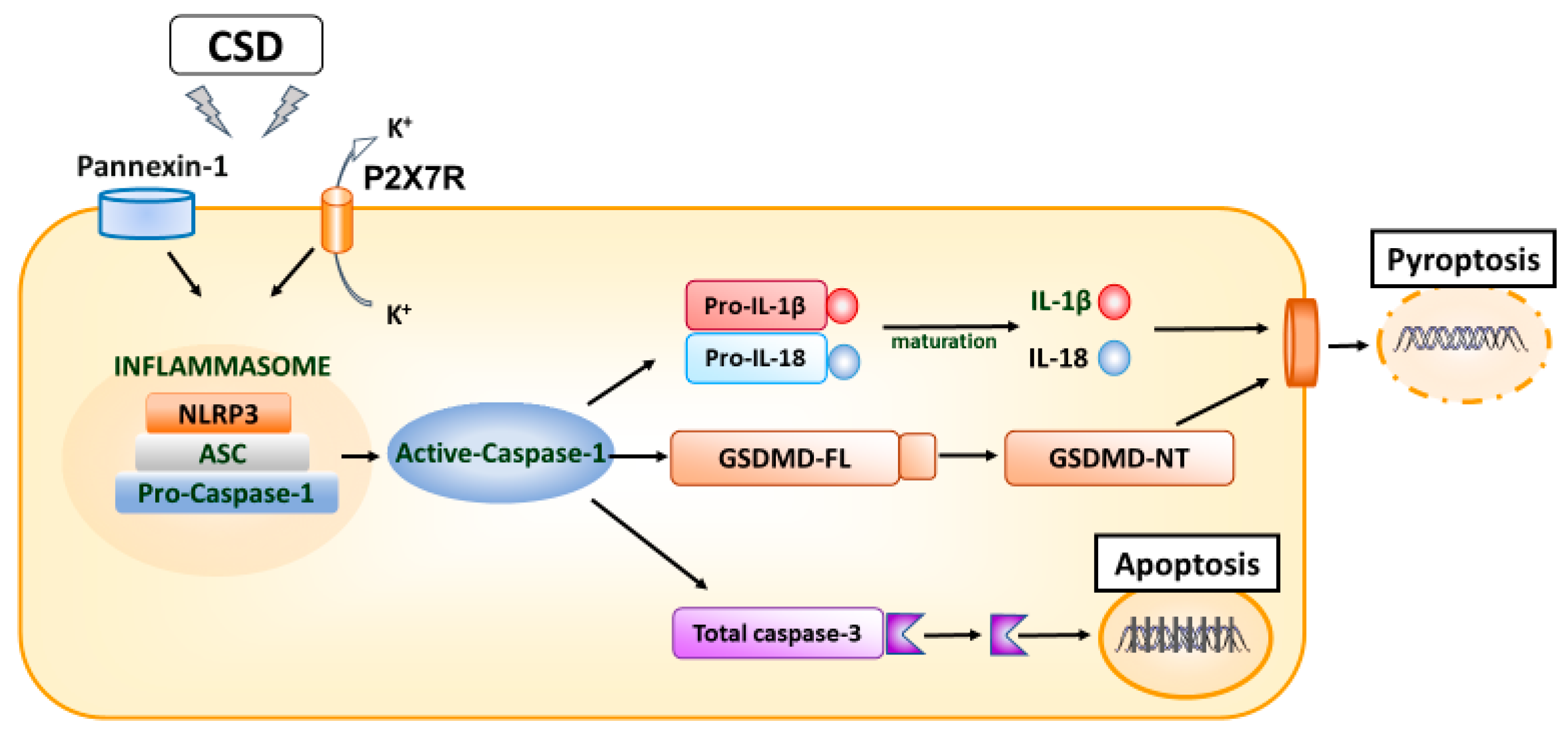
| CSD migraine model | CSD induces temporal alterations in inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [8,10,21,22,23,26,27,28,29]. On the other hand, it is also becoming evident that the invasion considerably influences the inflammatory response when inducing CSD. Some reports suggest an increase in the anti-inflammatory cytokines IL-4 and IL-10 [9,22], but this has not been investigated in recent non-invasive studies, and it is not possible to determine how anti-inflammatory cytokines are affected by CSD. |
| Spontaneous migraine-like mouse model using nitroglycerin | There are studies of an acute migraine model with a single injection of NTG [38,39] and a chronic migraine model with continuous NTG administration [34,36,37,61], and both models have shown increased gene expression of inflammatory cytokines IL-1β, IL-6, and TNF-α and decreased anti-inflammatory IL-10 [34,35,36,37,38,39]. An increase in satellite glial cell reactivity was detected in the chronic phase, suggesting that it may indicate chronic migraine [39]. |
| Inflammatory soup model of migraine | In an inflammatory soup model of migraine, the expression of cytokines IL-1β and IL-18 increases with the upregulation of P2X7R [16,34,45]. Microglial and astrocyte gliosis associated with these inflammatory changes have been implicated in migraine-related cognitive impairment, and P2X7R has been suggested as a potential therapeutic target for migraine headaches [45]. |
| Sound stress migraine model | The sound stress migraine model has been documented to have elevated plasma levels of IL-6, TNF-α, and CGRP but not IFN-γ, IL-2, IL-4, IL-10, or IL-17 levels [18]. Additionally, females expressed higher levels of TNF-α, IL-6, and CGRP than males [18], suggesting that differences in inflammatory response may be involved in the sex differences in migraine. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanaka, G.; Hayashi, K.; Morishita, N.; Takeshita, M.; Ishii, C.; Suzuki, S.; Ishimine, R.; Kasuga, A.; Nakazawa, H.; Takamatsu, T.; et al. Experimental and Clinical Investigation of Cytokines in Migraine: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 8343. https://doi.org/10.3390/ijms24098343
Yamanaka G, Hayashi K, Morishita N, Takeshita M, Ishii C, Suzuki S, Ishimine R, Kasuga A, Nakazawa H, Takamatsu T, et al. Experimental and Clinical Investigation of Cytokines in Migraine: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(9):8343. https://doi.org/10.3390/ijms24098343
Chicago/Turabian StyleYamanaka, Gaku, Kanako Hayashi, Natsumi Morishita, Mika Takeshita, Chiako Ishii, Shinji Suzuki, Rie Ishimine, Akiko Kasuga, Haruka Nakazawa, Tomoko Takamatsu, and et al. 2023. "Experimental and Clinical Investigation of Cytokines in Migraine: A Narrative Review" International Journal of Molecular Sciences 24, no. 9: 8343. https://doi.org/10.3390/ijms24098343
APA StyleYamanaka, G., Hayashi, K., Morishita, N., Takeshita, M., Ishii, C., Suzuki, S., Ishimine, R., Kasuga, A., Nakazawa, H., Takamatsu, T., Watanabe, Y., Morichi, S., Ishida, Y., Yamazaki, T., & Go, S. (2023). Experimental and Clinical Investigation of Cytokines in Migraine: A Narrative Review. International Journal of Molecular Sciences, 24(9), 8343. https://doi.org/10.3390/ijms24098343








