Growth State-Dependent Activation of eNOS in Response to DHA: Involvement of p38 MAPK
Abstract
1. Introduction
2. Results
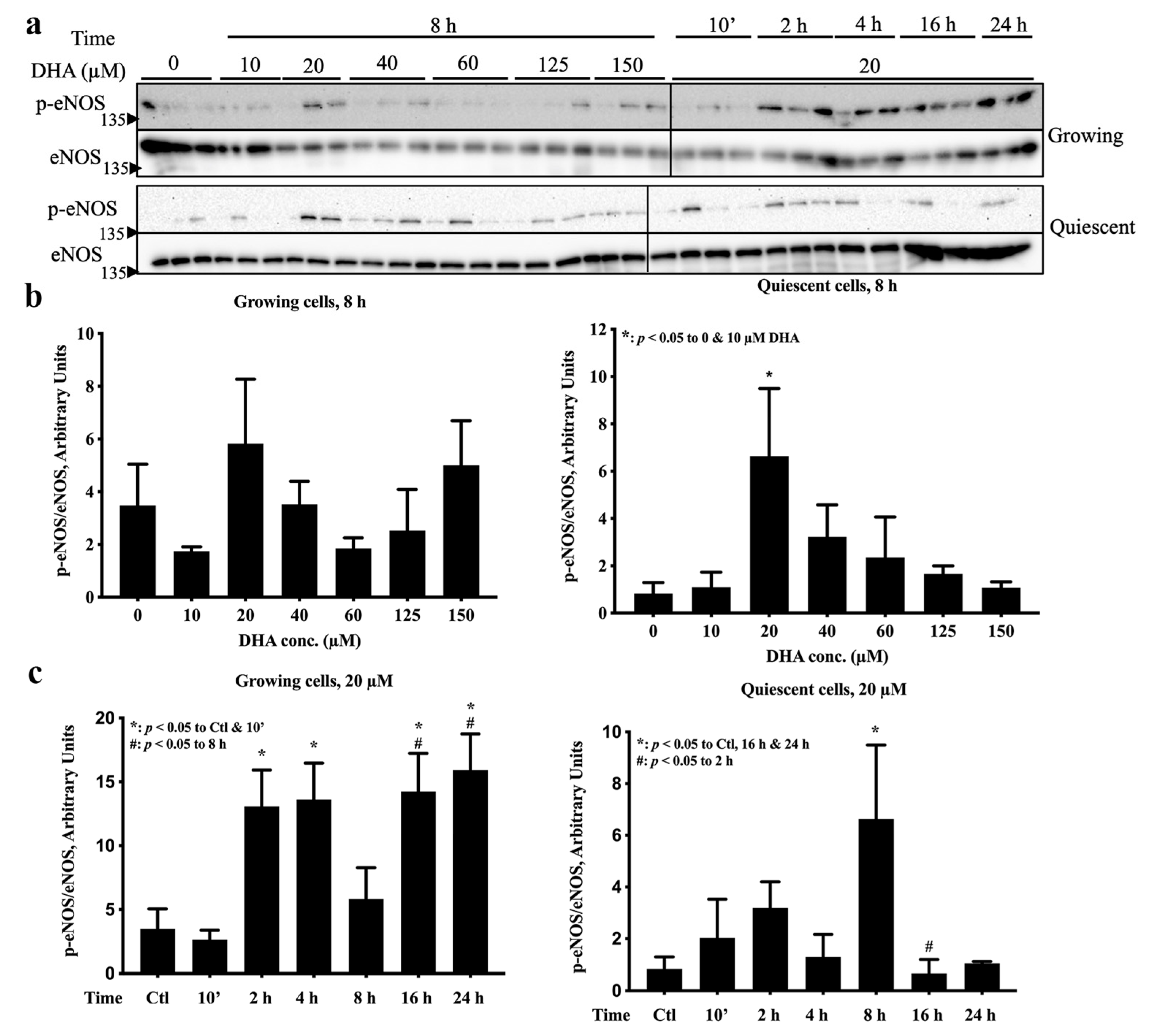
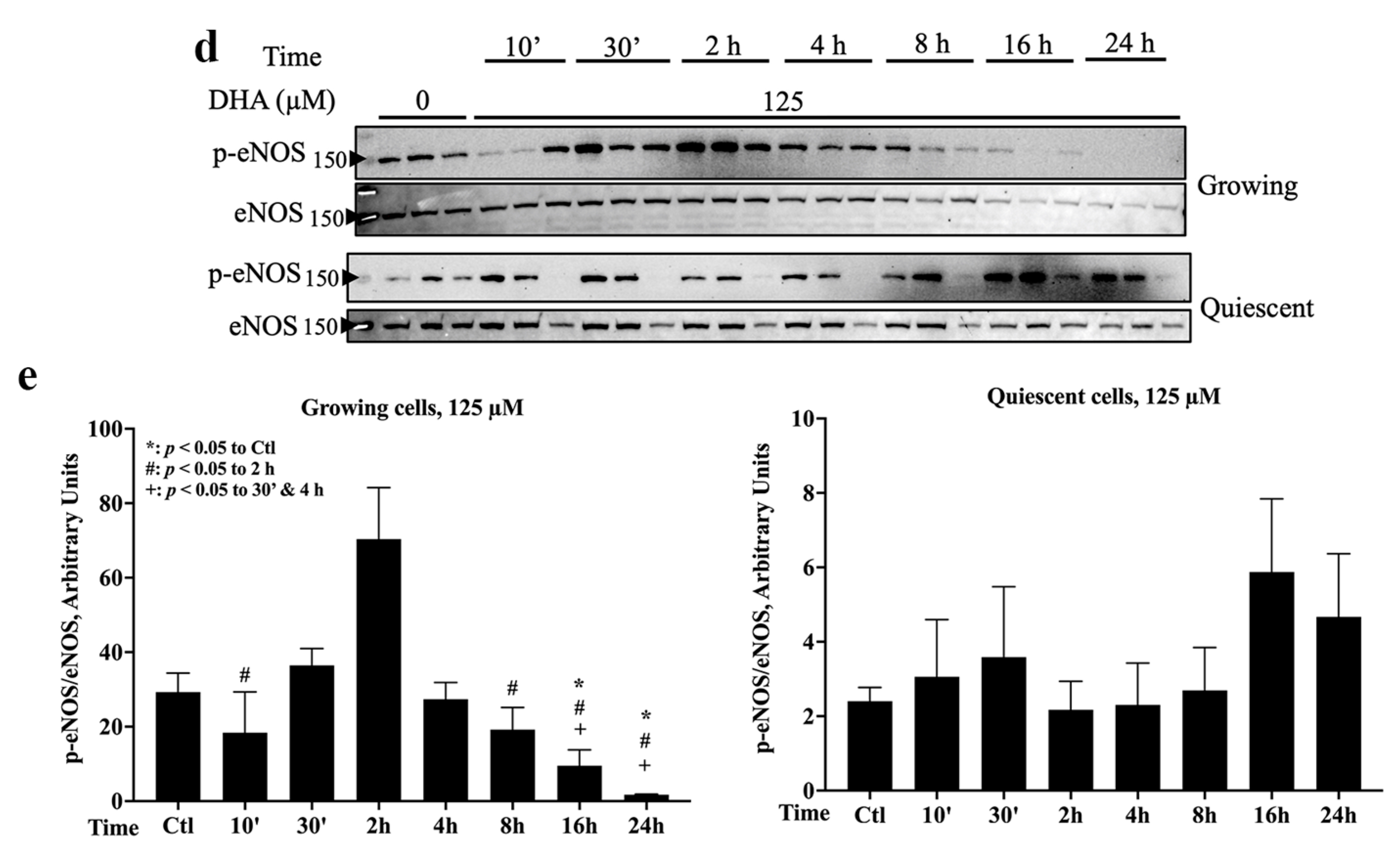
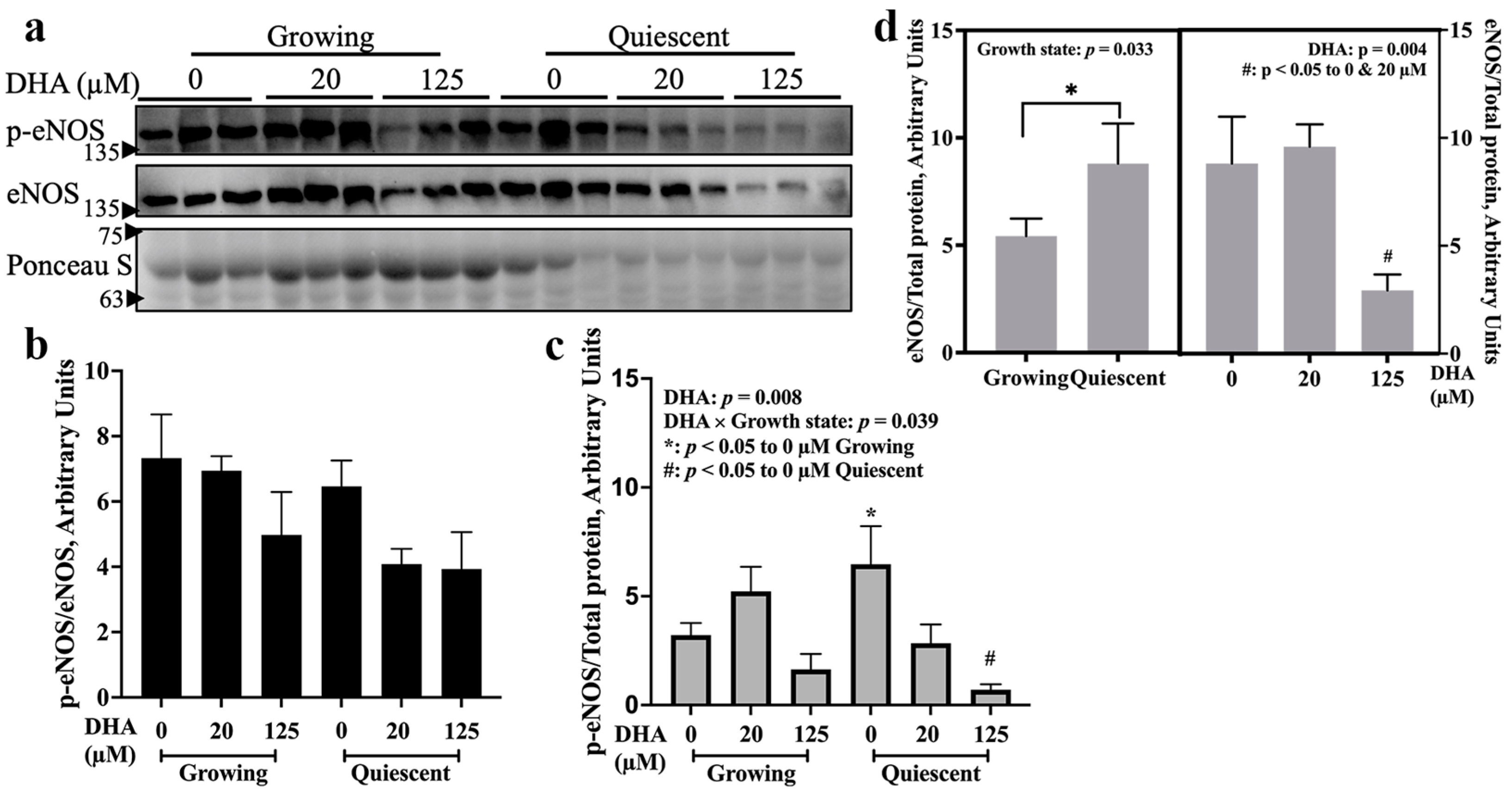
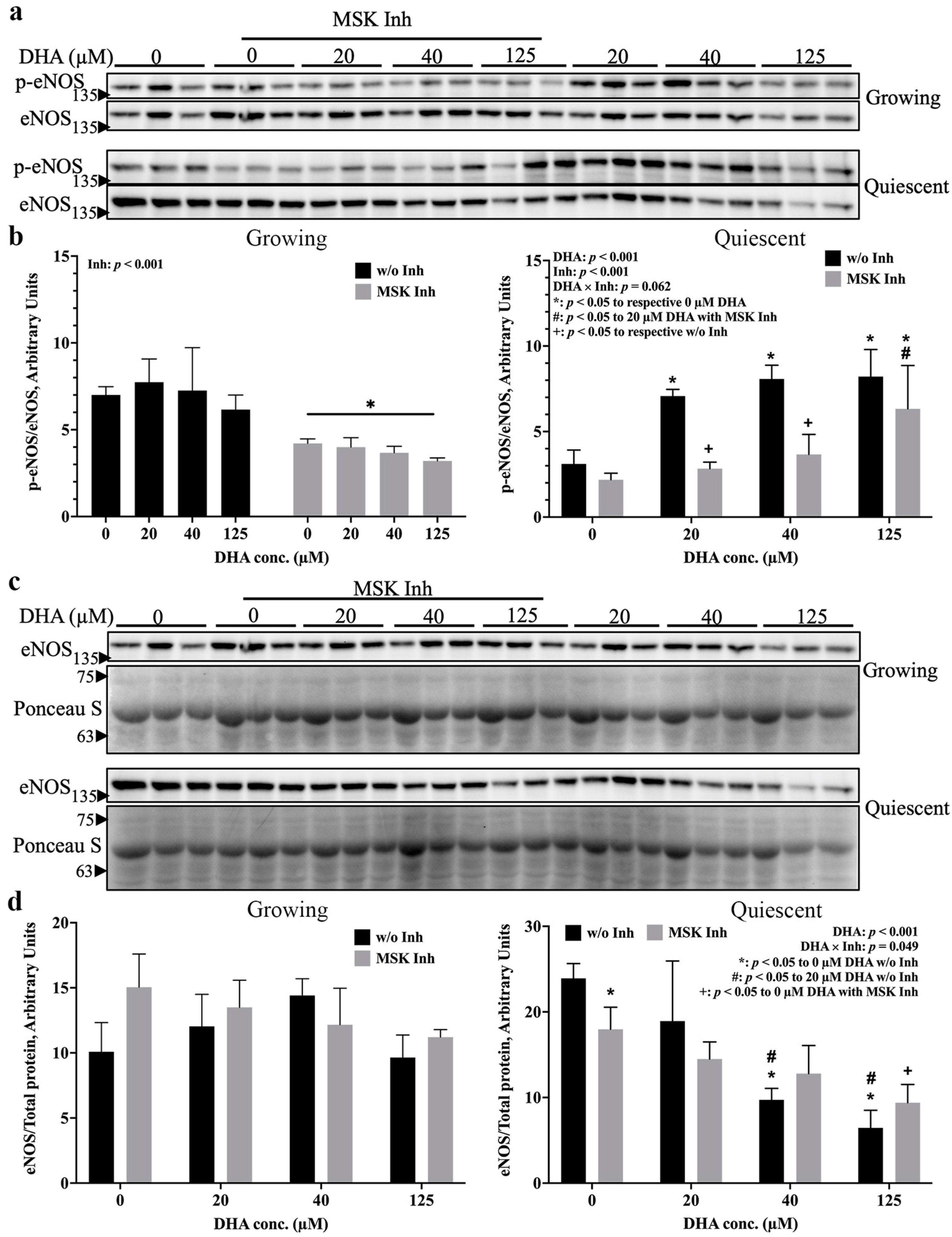
2.1. eNOS Was Activated by 20 µM DHA in Both Growth States, but 125 µM DHA Decreased eNOS Activation in Growing Cells
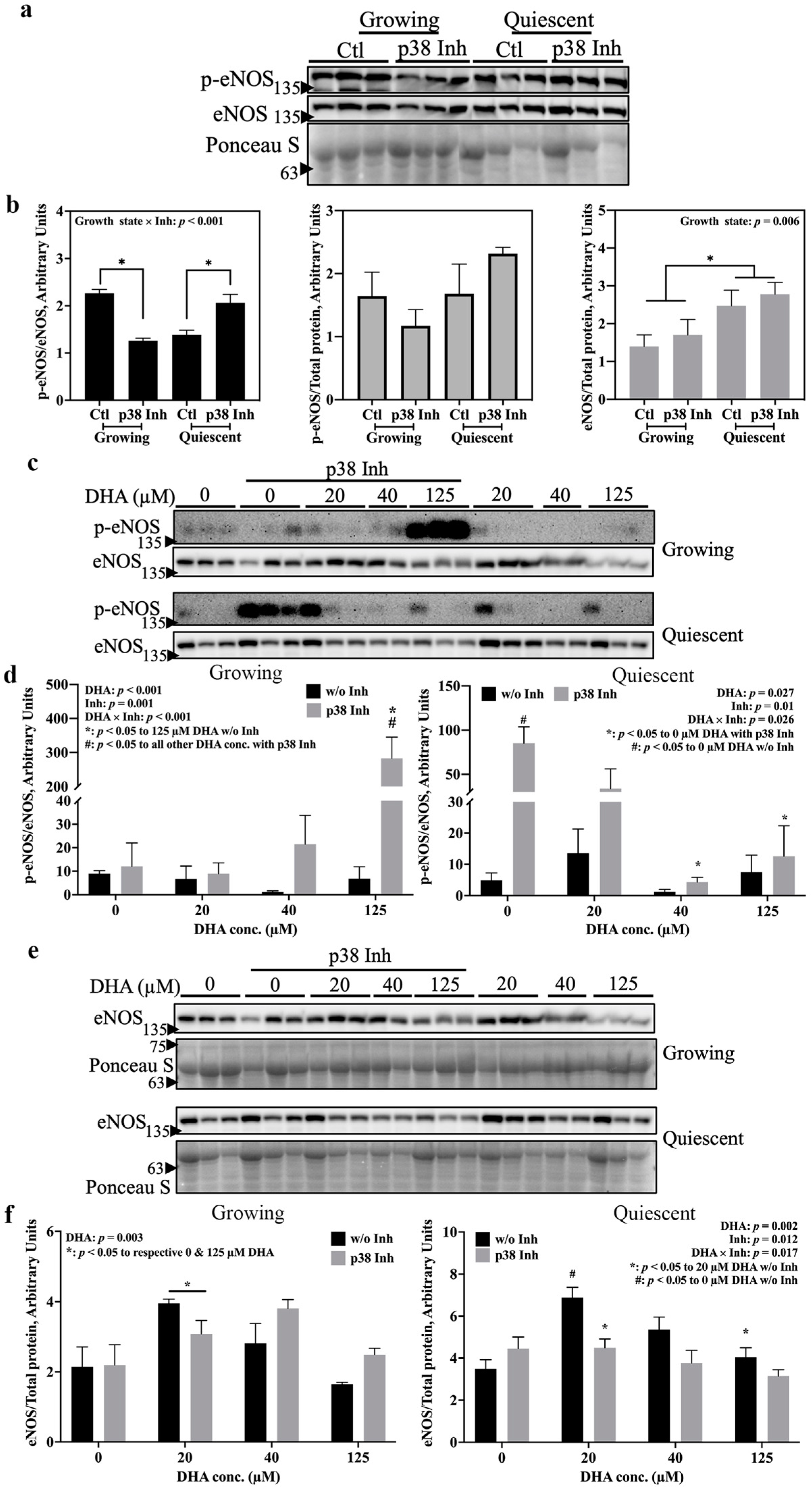
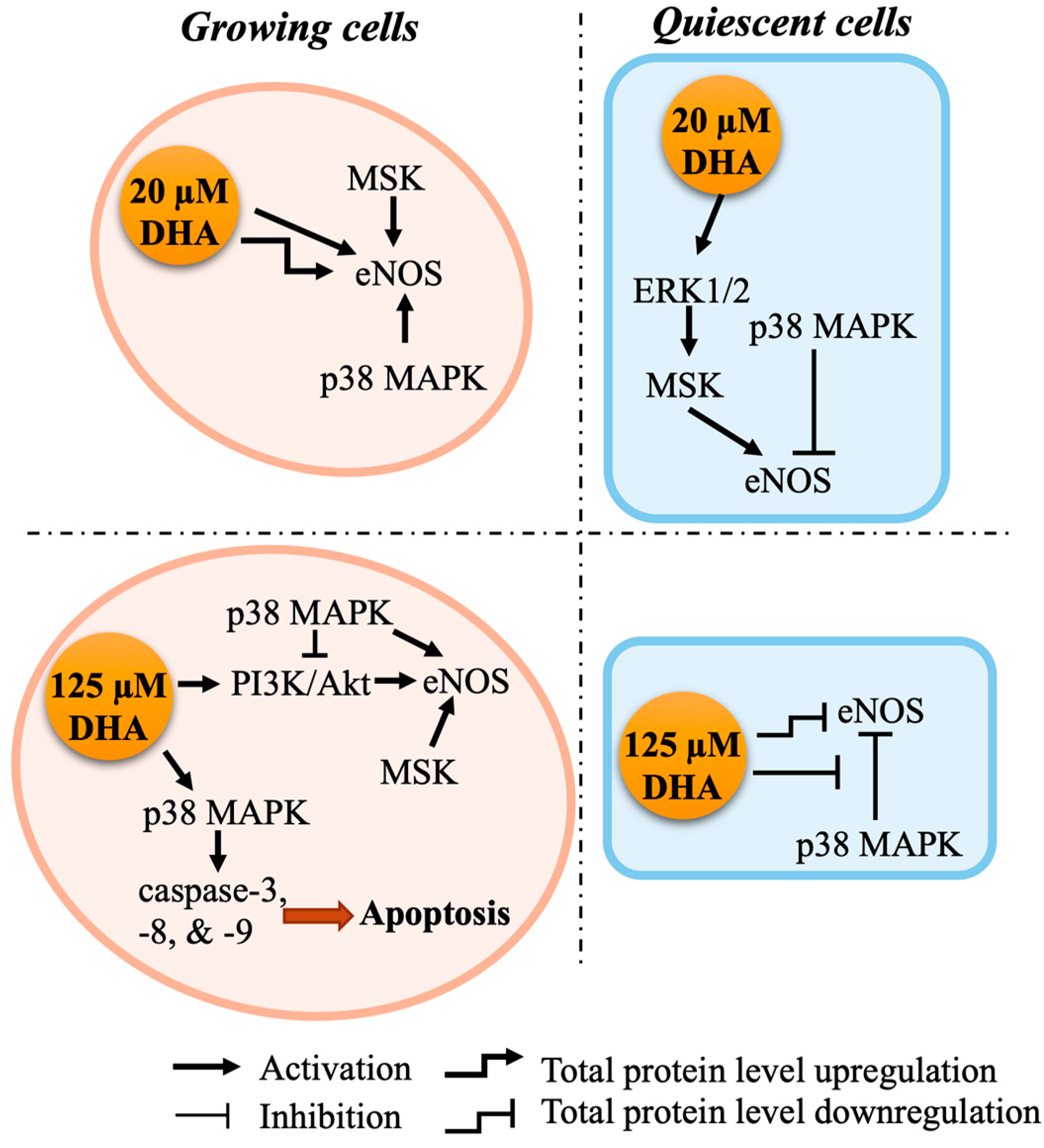
2.2. p38 MAPK and MSK Mediate eNOS Activation by DHA via Distinct Pathways
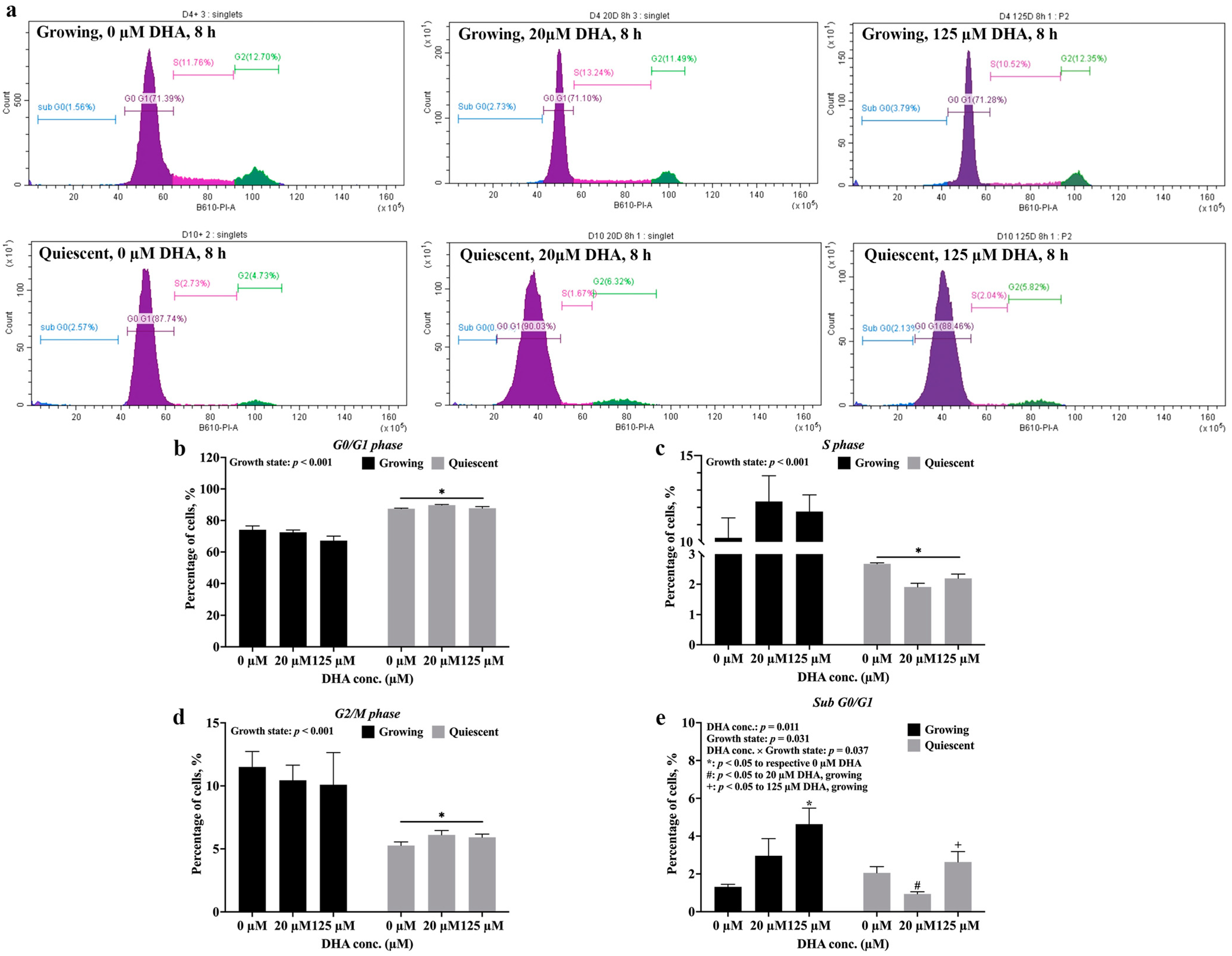
2.3. Growth State Determines Whether DHA Induces Apoptosis of Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Cell Cycle Analysis
4.3. Western Blotting
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sturtzel, C. Endothelial Cells. Adv. Exp. Med. Biol. 2017, 1003, 71–91. [Google Scholar] [PubMed]
- Garcia, V.; Sessa, W.C. Endothelial NOS: Perspective and recent developments. Br. J. Pharmacol. 2018, 176, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, M.; Fleming, I. The eNOS signalosome and its link to endothelial dysfunction. Pflugers Arch. Eur. J. Physiol. 2016, 468, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Gerhard, M.D.; Meredith, I.T.; Charbonneau, F.; Delagrange, D.; Creager, M.A.; Selwyn, A.P.; Ganz, P. Systemic nature of endothelial dysfunction in atherosclerosis. Am. J. Cardiol. 1995, 75, 71B–74B. [Google Scholar] [CrossRef] [PubMed]
- McCabe, T.J.; Fulton, D.; Roman, L.J.; Sessa, W.C. Enhanced Electron Flux and Reduced Calmodulin Dissociation May Explain “Calcium-independent” eNOS Activation by Phosphorylation. J. Biol. Chem. 2000, 275, 6123–6128. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2015, 219, 22–96. [Google Scholar] [CrossRef]
- Anter, E.; Chen, K.; Shapira, O.M.; Karas, R.H.; Keaneyjr, J.F. p38 Mitogen-Activated Protein Kinase Activates eNOS in Endothelial Cells by an Estrogen Receptor α-Dependent Pathway in Response to Black Tea Polyphenols. Circ. Res. 2005, 96, 1072–1078. [Google Scholar] [CrossRef]
- Jin, J.; Liu, J.; Luo, Y.; He, H.; Zheng, X.; Zheng, C.; Huang, Y.; Chen, Y. High fructose induces dysfunctional vasodilatation via PP2A-mediated eNOS Ser1177 dephosphorylation. Nutr. Metab. 2022, 19, 24. [Google Scholar] [CrossRef]
- Sherratt, S.C.; Dawoud, H.; Bhatt, D.L.; Malinski, T.; Mason, R.P. Omega-3 and omega-6 fatty acids have distinct effects on endothelial fatty acid content and nitric oxide bioavailability. Prostaglandins Leukot. Essent. Fat. Acids 2021, 173, 102337. [Google Scholar] [CrossRef]
- Saravanan, P.; Davidson, N.C.; Schmidt, E.B.; Calder, P.C. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010, 376, 540–550. [Google Scholar] [CrossRef]
- Du, Y.; Taylor, C.G.; Aukema, H.; Zahradka, P. Importance of extracellular matrix and growth state for the EA.hy926 endothelial cell response to polyunsaturated fatty acids. PLoS ONE 2018, 13, e0197613. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Taylor, C.G.; Aukema, H.M.; Zahradka, P. Regulation of docosahexaenoic acid-induced apoptosis of confluent endothelial cells: Contributions of MAPKs and caspases. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2021, 1866, 158902. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef]
- Fabian, M.A.; Biggs, W.H., III; Treiber, D.K.; Atteridge, C.E.; Azimioara, M.D.; Benedetti, M.G.; Carter, T.A.; Ciceri, P.; Edeen, P.T.; Floyd, M.; et al. A small molecule–kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 2005, 23, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Macdonald, A.; McCoy, C.E.; Darragh, J.; Reith, A.D.; Arthur, J.S.C. Characterization of the cellular action of the MSK inhibitor SB-747651A. Biochem. J. 2011, 441, 347–357. [Google Scholar] [CrossRef]
- Warnes, G.; Blizard Institute of Cell and Molecular Science. DNA Fragmentation and Apoptosis [Internet]. Uses of Flow Cytometry. 2019. Available online: http://www.icms.qmul.ac.uk/flowcytometry/uses/apoptosis/dnafragmentation/ (accessed on 20 November 2021).
- Kuriki, K.; Nagaya, T.; Tokudome, Y.; Imaeda, N.; Fujiwara, N.; Sato, J.; Goto, C.; Ikeda, M.; Maki, S.; Tajima, K.; et al. Plasma Concentrations of (n-3) Highly Unsaturated Fatty Acids Are Good Biomarkers of Relative Dietary Fatty Acid Intakes: A Cross-Sectional Study. J. Nutr. 2003, 133, 3643–3650. [Google Scholar] [CrossRef] [PubMed]
- Abdelmagid, S.A.; Clarke, S.E.; Nielsen, D.E.; Badawi, A.; El-Sohemy, A.; Mutch, D.M.; Ma, D.W.L. Comprehensive Profiling of Plasma Fatty Acid Concentrations in Young Healthy Canadian Adults. PLoS ONE 2015, 10, e0116195. [Google Scholar] [CrossRef] [PubMed]
- Gousset-Dupont, A.; Robert, V.; Grynberg, A.; Lacour, B.; Tardivel, S. The effect of n-3 PUFA on eNOS activity and expression in Ea hy 926 cells. Prostaglandins, Leukot. Essent. Fat. Acids 2007, 76, 131–139. [Google Scholar] [CrossRef]
- Vu, T.T.; Dieterich, P.; Vu, T.T.; Deussen, A. Docosahexaenoic acid reduces adenosine triphosphate-induced calcium influx via inhibition of store-operated calcium channels and enhances baseline endothelial nitric oxide synthase phosphorylation in human endothelial cells. Korean J. Physiol. Pharmacol. 2019, 23, 345–356. [Google Scholar] [CrossRef]
- Li, Q.; Atochin, D.; Kashiwagi, S.; Earle, J.; Wang, A.; Mandeville, E.; Hayakawa, K.; D’uscio, L.V.; Lo, E.H.; Katusic, Z.; et al. Deficient eNOS Phosphorylation Is a Mechanism for Diabetic Vascular Dysfunction Contributing to Increased Stroke Size. Stroke 2013, 44, 3183–3188. [Google Scholar] [CrossRef]
- Kashiwagi, S.; Atochin, D.N.; Li, Q.; Schleicher, M.; Pong, T.; Sessa, W.C.; Huang, P.L. eNOS phosphorylation on serine 1176 affects insulin sensitivity and adiposity. Biochem. Biophys. Res. Commun. 2013, 431, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Mima, A.; Li, Q.; Rask-Madsen, C.; He, P.; Mizutani, K.; Katagiri, S.; Maeda, Y.; Wu, I.-H.; Khamaisi, M.; et al. Insulin decreases atherosclerosis by inducing endothelin receptor B expression. JCI Insight 2016, 1, e86574. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Wang, M.; Liu, F.; Zhao, S.; Ma, J.; Luo, N.; Li, N.; Li, Y.; Xu, G.; et al. Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch. Biochem. Biophys. 2007, 466, 250–259. [Google Scholar] [CrossRef]
- Jung, S.-B.; Kwon, S.K.; Kwon, M.; Nagar, H.; Jeon, B.H.; Irani, K.; Yoon, S.H.; Kim, C.S. Docosahexaenoic acid improves vascular function via up-regulation of SIRT1 expression in endothelial cells. Biochem. Biophys. Res. Commun. 2013, 437, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, N.; Park, G.; McAllister, H.; Leahey, W.; Devine, A.; McVeigh, G.E.; Gardiner, T.A.; McDonald, D.M. Docosahexaenoic Acid Improves the Nitroso-Redox Balance and Reduces VEGF-Mediated Angiogenic Signaling in Microvascular Endothelial Cells. Investig. Opthalmology Vis. Sci. 2010, 51, 6815–6825. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, C.L.; Stice, J.P.; Hart, C.M.; Mbai, F.N.; Knowlton, A.A. Effects of Dietary Decosahexaenoic Acid (DHA) on eNOS in Human Coronary Artery Endothelial Cells. J. Cardiovasc. Pharmacol. Ther. 2008, 13, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-Y.; Lii, C.-K.; Ye, S.-Y.; Li, C.-C.; Lu, C.-Y.; Lin, A.-H.; Chen, H.W. Docosahexaenoic Acid Inhibits Vascular Endothelial Growth Factor (VEGF)-Induced Cell Migration via the GPR120/PP2A/ERK1/2/eNOS Signaling Pathway in Human Umbilical Vein Endothelial Cells. J. Agric. Food Chem. 2014, 62, 4152–4158. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Suzuki, S.; Tagami, M. Docosahexaenoic acid prevented tumor necrosis factor alpha-induced endothelial dysfunction and senescence. Prostaglandins Leukot. Essent. Fat. Acids 2016, 104, 11–18. [Google Scholar] [CrossRef]
- Novinbahador, T.; Nourazarian, A.; Asgharzadeh, M.; Rahbarghazi, R.; Avci, Ç.B.; Bagca, B.G.; Ozates, N.P.; Karbasforoush, S.; Khaki-Khatibi, F. Docosahexaenoic acid attenuates the detrimental effect of palmitic acid on human endothelial cells by modulating genes from the atherosclerosis signaling pathway. J. Cell. Biochem. 2018, 119, 9752–9763. [Google Scholar] [CrossRef]
- Wang, B.; Xing, F.; Liu, N.; Chen, D.; Li, Z.; Liu, J. p38α subtype is a potential target to inhibit eNOS activity and NO production in human endothelial cells. Microvasc. Res. 2014, 91, 58–65. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lii, C.K.; Wei, Y.L.; Li, C.C.; Lu, C.Y.; Liu, K.L.; Chen, H.W. Docosahexaenoic acid inhibition of inflammation is partially via cross-talk between Nrf2/heme oxygenase 1 and IKK/NF-κB pathways. J. Nutr. Biochem. 2013, 24, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-C.; Chiang, E.-P.I.; Tsai, S.-Y.; Wang, F.-Y.; Pai, M.-H.; Syu, J.-N.; Cheng, C.-C.; Rodriguez, R.L.; Tang, F.-Y. Eicosapentaenoic acid induces neovasculogenesis in human endothelial progenitor cells by modulating c-kit protein and PI3-K/Akt/eNOS signaling pathways. J. Nutr. Biochem. 2014, 25, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Duda, K.; Frödin, M. Stimuli That Activate MSK in Cells and the Molecular Mechanism of Activation. Madame Curie Biosci. Database 2012, 2, 1–29. [Google Scholar]
- Du, Y. Effects of Dietary Polyunsaturated Fatty Acids and Oxylipins on Human EA.hy926 Endothelial Cells. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2018. [Google Scholar]
- Xing, F.; Jiang, Y.; Liu, J.; Zhao, K.; Mo, Y.; Liu, Z.; Zeng, Y. Downregulation of human endothelial nitric oxide synthase promoter activity by p38 mitogen-activated protein kinase activation. Biochem. Cell Biol. 2006, 84, 780–789. [Google Scholar] [CrossRef]
- Illi, B.; Nanni, S.; Scopece, A.; Farsetti, A.; Biglioli, P.; Capogrossi, M.C.; Gaetano, C. Shear stress-mediated chromatin remodeling provides molecular basis for flow-dependent regulation of gene expression. Circ. Res. 2003, 93, 155–161. [Google Scholar] [CrossRef]
- Searles, C.D. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression. Am. J. Physiol. Physiol. 2006, 291, C803–C816. [Google Scholar] [CrossRef]
- Newell, M.; Baker, K.; Postovit, L.M.; Field, C.J. A Critical Review on the Effect of Docosahexaenoic Acid (DHA) on Cancer Cell Cycle Progression. Int. J. Mol. Sci. 2017, 18, 1784. [Google Scholar] [CrossRef]
- Kim, H.J.; Vosseler, C.A.; Weber, P.C.; Erl, W. Docosahexaenoic acid induces apoptosis in proliferating human endothelial cells. J. Cell. Physiol. 2005, 204, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Gabbs, M.; Zahradka, P.; Taylor, C.G.; Aukema, H.M. Time Course and Sex Effects of α-Linolenic Acid-Rich and DHA-Rich Supplements on Human Plasma Oxylipins: A Randomized Double-Blind Crossover Trial. J. Nutr. 2020, 151, 513–522. [Google Scholar] [CrossRef]
- Pauls, S.D.; Rodway, L.R.; Sidhu, K.K.; Winter, T.; Sidhu, N.; Aukema, H.M.; Zahradka, P.; Taylor, C.G. Oils Rich in α-Linolenic Acid or Docosahexaenoic Acid Have Distinct Effects on Plasma Oxylipin and Adiponectin Concentrations and on Monocyte Bioenergetics in Women with Obesity. J. Nutr. 2021, 151, 3053–3066. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.; Lii, C.-K.; Lin, A.-H.; Li, C.-C.; Tsai, C.-H.; Pan, S.-K.; Yang, Y.-C.; Huang, C.-S.; Reshi, L.; Chen, H.-W. Docosahexaenoic acid inhibits TNFα-induced ICAM-1 expression by activating PPARα and autophagy in human endothelial cells. Food Chem. Toxicol. 2019, 134, 110811. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, A.; Fournier, A.; Sergiel, J.-P.; Athias, P. Membrane docosahexaenoic acid vs. eicosapentaenoic acid and the beating function of the cardiomyocyte and its regulation through the adrenergic receptors. Lipids 1996, 31, S250-S210. [Google Scholar] [CrossRef]
- Tate, E.H.; Wilder, M.E.; Cram, L.S.; Wharton, W. A method for staining 3T3 cell nuclei with propidium iodide in hypotonic solution. Cytometry 1983, 4, 211–215. [Google Scholar] [CrossRef]
- Sabbir, M.G.; Taylor, C.G.; Zahradka, P. Hypomorphic CAMKK2 in EA.hy926 endothelial cells causes abnormal transferrin trafficking, iron homeostasis and glucose metabolism. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118763. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Taylor, C.G.; Zahradka, P. Growth State-Dependent Activation of eNOS in Response to DHA: Involvement of p38 MAPK. Int. J. Mol. Sci. 2023, 24, 8346. https://doi.org/10.3390/ijms24098346
Huang S, Taylor CG, Zahradka P. Growth State-Dependent Activation of eNOS in Response to DHA: Involvement of p38 MAPK. International Journal of Molecular Sciences. 2023; 24(9):8346. https://doi.org/10.3390/ijms24098346
Chicago/Turabian StyleHuang, Shiqi, Carla G. Taylor, and Peter Zahradka. 2023. "Growth State-Dependent Activation of eNOS in Response to DHA: Involvement of p38 MAPK" International Journal of Molecular Sciences 24, no. 9: 8346. https://doi.org/10.3390/ijms24098346
APA StyleHuang, S., Taylor, C. G., & Zahradka, P. (2023). Growth State-Dependent Activation of eNOS in Response to DHA: Involvement of p38 MAPK. International Journal of Molecular Sciences, 24(9), 8346. https://doi.org/10.3390/ijms24098346









