Effect of Melittin Complexes with Graphene and Graphene Oxide on Triple-Negative Breast Cancer Tumors Grown on Chicken Embryo Chorioallantoic Membrane
Abstract
:1. Introduction
2. Results
2.1. Characterization of Complexes
2.2. Tumor Mass and Volume
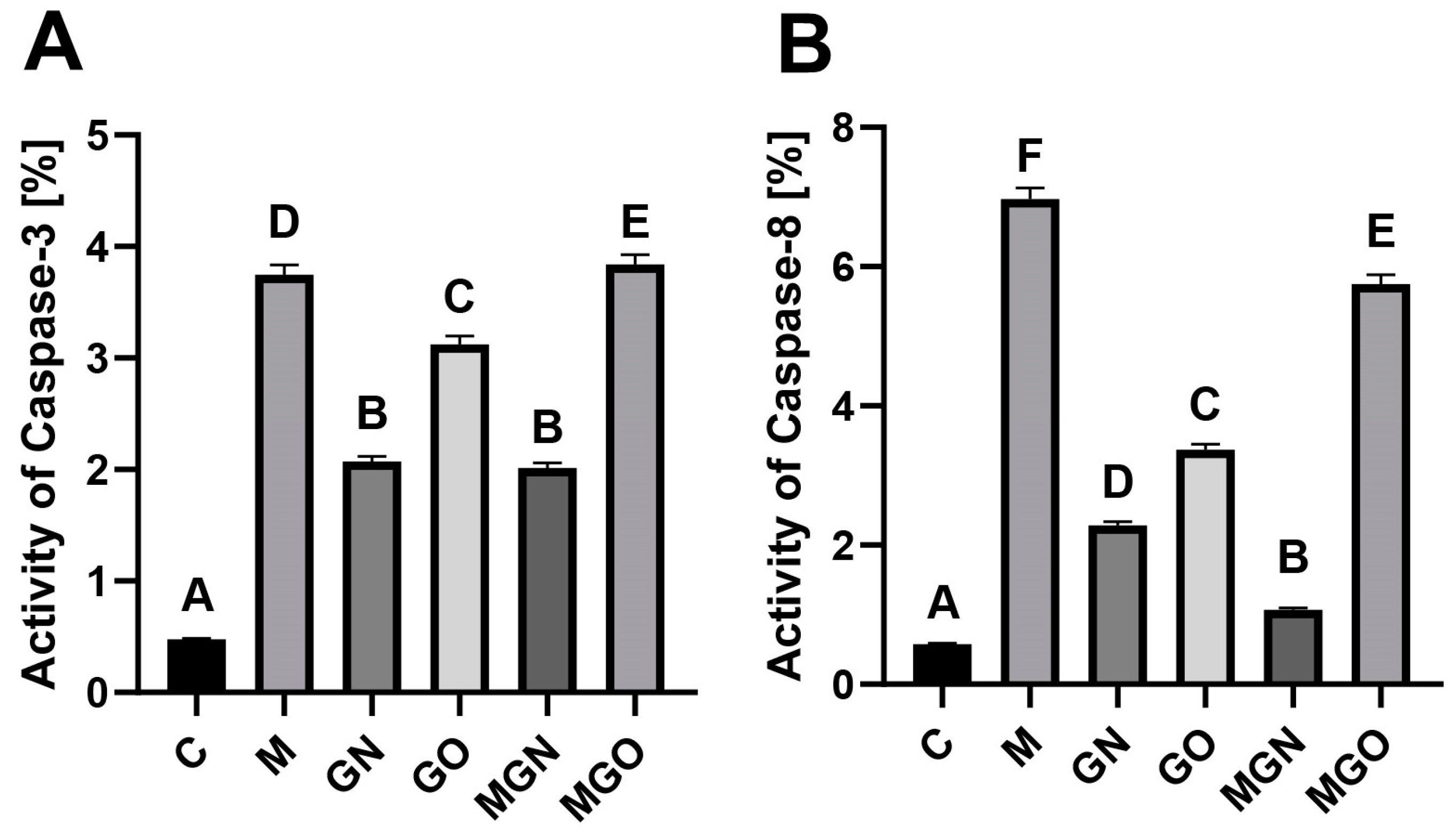
2.3. Activity of Caspases
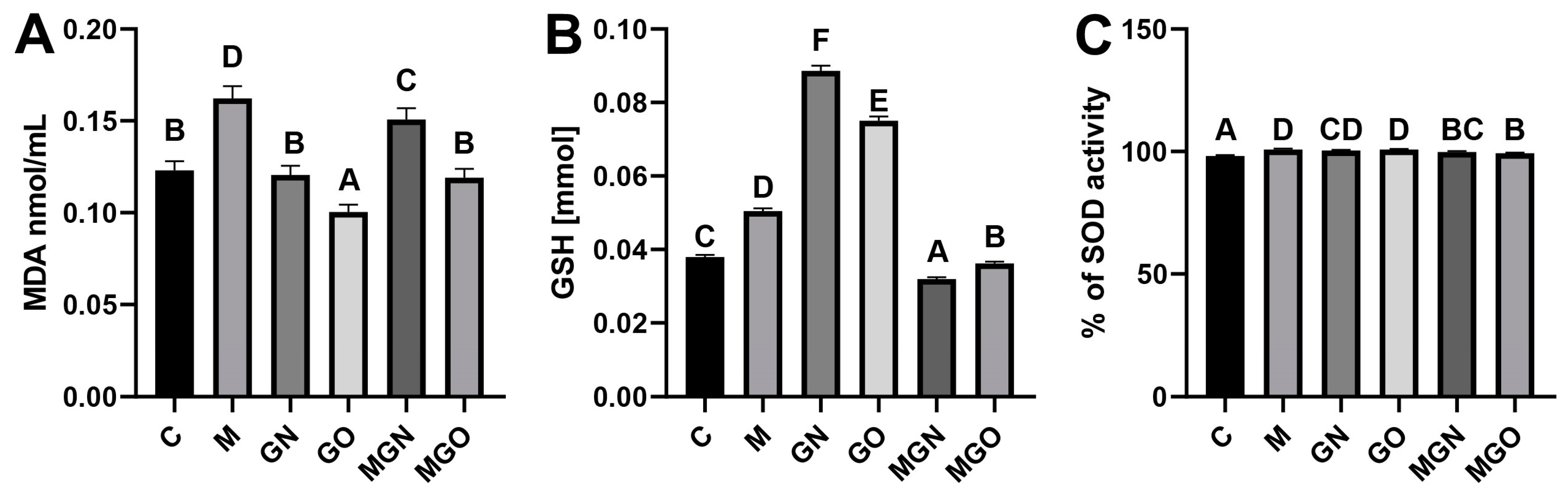
2.4. Analyses of Oxidative Stress Markers
2.5. Measurement of 8-OHdG Concentrations
2.6. Cytokine Protein Level
3. Discussion
4. Materials and Methods
4.1. Preparation of Complexes and Characterization
4.2. Cell Culture and Culture of Tumors on a Chorioallantoic Membrane
4.3. Measurement of Tumor Volume
4.4. Tumor Lysate
4.5. Activity of Caspases
4.6. Lipid Peroxidation Analysis
4.7. SOD Activity
4.8. GSH-Level Analysis
4.9. Measurement of 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) Concentration
4.10. Cytokine Protein Level
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef]
- Arpino, G.; Generali, D.; Sapino, A.; Del Matro, L.; Frassoldati, A.; de Laurentis, M.; Pronzato, P.; Mustacchi, G.; Cazzaniga, M.; De Placido, S.; et al. Gene expression profiling in breast cancer: A clinical perspective. Breast 2013, 22, 109–120. [Google Scholar] [CrossRef]
- Mughal, H.A.; Rahman, M.A. Organochlorine pesticide content of human adipose tissue in Karachi. Arch. Environ. Health Int. J. 1973, 27, 396–398. [Google Scholar] [CrossRef]
- Boyle, P. Triple-negative breast cancer: Epidemiological considerations and recommendations. Ann. Oncol. 2012, 23, vi7–vi12. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.L.; Cardoso, N.N.C.; Izetti, P.; de Mesquita, G.G.; de Mo, A.C. Triple negative breast cancer: A thorough review of biomarkers. Crit. Rev. Oncol. Hematol. 2020, 145, 102855. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/treatment-of-triple-negative.html (accessed on 4 April 2023).
- Abdela, N.; Jilo, K. Bee venom and its therapeutic values: A review. Adv. Life Sci. Technol. 2016, 44, 18–22. [Google Scholar]
- Azam, M.N.K.; Ahmed, M.N.; Biswas, S.; Ara, N.; Rahman, M.M.; Hirashima, A.; Hasan, M.N. A review on bioactivities of honey bee venom. Annu. Res. Rev. Biol. 2019, 30, 1–13. [Google Scholar] [CrossRef]
- Šuran, J.; Cepanec, I.; Mašek, T.; Starčević, K.; Tlak, G.I.; Vranješ, M.; Radić, B.; Radić, S.; Kosalec, I.; Vlainić, J. Nonaqueous polyethylene glycol as a safer alternative to ethanolic propolis extracts with comparable antioxidant and antimicrobial activity. Antioxidants 2021, 10, 978. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Mittin: A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Bee venom composition: From chemistry to biological activity. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 459–484. [Google Scholar]
- Raghuraman, H.; Chattopadhyay, A. Mittin: A membrane-active peptide with diverse functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef]
- Jamasbi, E.; Mularski, A.; Separovic, F. Model membrane and cell studies of antimicrobial activity of Mittin analogues. Curr. Top. Med. Chem. 2015, 16, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, C.; Li, M.; Zong, X.; Han, D.; Chen, Y. Antimicrobial peptide Mittin against Xanthomonas Oryzae Pv. Oryzae, the bacterial leaf blight pathogen in rice. Appl. Microbiol. Biotechnol. 2016, 100, 5059–5067. [Google Scholar] [CrossRef]
- Alizadehnohi, M.; Nabiuni, M.; Nazari, Z.; Safaeinejad, Z.; Irian, S. The synergistic cytotoxic effect of cisplatin and honey bee venom on human ovarian cancer cell line A2780cp. J. Venom Res. 2012, 6, 22–27. [Google Scholar]
- Choi, K.; Hwang, C.; Gu, S.; Park, M.; Kim, J.; Park, J.; Ahn, Y.; Kim, J.; Song, M.; Song, H.; et al. Cancer cell growth inhibitory effect of bee venom via increase of death receptor 3 expression and inactivation of NF-Kappa B in NSCLC cells. Toxins 2014, 6, 2210–2228. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Park, M.H.; Kollipara, P.S.; An, B.J.; Song, H.S.; Han, S.B.; Kim, J.H.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom toxin and Mittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol. Appl. Pharmacol. 2012, 258, 72–81. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Hammerschmid, D.; Privat-Maldonado, A.; Dewilde, S.; Bogaerts, A. Synergistic effects of Mittin and plasma treatment: A promising approach for cancer therapy. Cancers 2019, 11, 1109. [Google Scholar] [CrossRef]
- Jung, G.B.; Huh, J.-E.; Lee, H.-J.; Kim, D.; Lee, G.-J.; Park, H.-K.; Lee, J.-D. Anti-Cancer Effect of Bee Venom on Human MDA-MB-231 Breast Cancer Cells Using Raman Spectroscopy. Biomed. Opt. Express 2018, 9, 5703. [Google Scholar] [CrossRef]
- Daniluk, K.; Kutwin, M.; Grodzik, M.; Wierzbicki, M.; Strojny, B.; Szczepaniak, J.; Balaban, J.; Sosnowska, M.; Chwalibog, A.; Sawosz, E.; et al. Use of selected carbon nanoparticles as Mittin carriers for MCF-7 and MDA-MB-231 human breast cancer cells. Materials 2020, 13, 90. [Google Scholar] [CrossRef]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K.; Ho, D.; Johnstone, E.; Pfleger, K.; Redfern, A.; et al. Honeybee venom and Mittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. NPJ Precis. Oncol. 2020, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, M.F.; Akbarzadeh, I.; Marzbankia, E.; Farid, M.; Khaledi, L.; Reihani, A.H.; Javidfar, M.; Mortazavi, P. Delivery of Mittin-loaded niosomes for breast cancer treatment: An in vitro and in vivo evaluation of anti-cancer effect. Cancer Nanotechnol. 2021, 12, 14. [Google Scholar] [CrossRef]
- Soman, N.R.; Baldwin, S.L.; Hu, G.; Marsh, J.N.; Lanza, G.M.; Heuser, J.E.; Arbeit, J.M.; Wickline, S.A.; Schlesinger, P.H. Molecularly targeted nanocarriers deliver the cytolytic peptide Mittin specifically to tumor cells in mice, reducing tumor growth. J. Clin. Investig. 2009, 119, 2830–2842. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Shon, Y.; Kim, J.; Oh, Y.-K. Selective activation of anticancer chemotherapy by cancer-associated fibroblasts in the tumor microenvironment. J. Natl. Cancer Inst. 2017, 109, djw186. [Google Scholar] [CrossRef]
- Rahimi, S.; Chen, Y.; Zareian, M.; Pandit, S.; Mijakovic, I. Cellular and subcellular interactions of graphene-based materials with cancerous and non-cancerous cells. Adv. Drug Deliv. Rev. 2022, 189, 114467. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Akbarzadeh, A.; Kouhi, M.; Milani, M. Graphene: Synthesis, Bio-Applications, and Properties. Artif. Cells Nanomed. Biotechnol. 2016, 44, 150–156. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, M.; Gao, M.; Zhang, Z.; Xu, Y.; Xia, T.; Liu, S. Graphene Oxide Induced Perturbation to Plasma Membrane and Cytoskeletal Meshwork Sensitize Cancer Cells to Chemotherapeutic Agents. ACS Nano 2017, 11, 2637–2651. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Zhang, L.; Fan, Z.; Liu, S. Cytotoxicity Effect of Graphene Oxide on Human MDA-MB-231 Cells. Toxicol. Mech. Methods 2015, 25, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Min, K.; Jeon, J.; Yang, H.S.; Tae, G. Catalytic Nanographene Oxide with Hemin for Enhanced Photodynamic Therapy. J. Control Release 2020, 326, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, B.; Wei, P.; Du, Y.; Zhou, H.; Yu, M.; Yan, L.; Zhang, W.; Nie, G.; Chen, C.; et al. Energy Metabolism Analysis Reveals the Mechanism of Inhibition of Breast Cancer Cell Metastasis by PEG-Modified Graphene Oxide Nanosheets. Biomaterials 2014, 35, 9833–9843. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, B.; Zheng, J.; Yu, M.; Zhou, T.; Zhao, K.; Jia, Y.; Gao, X.; Chen, C.; Wei, T. The Inhibition of Migration and Invasion of Cancer Cells by Graphene via the Impairment of Mitochondrial Respiration. Biomaterials 2014, 35, 1597–1607. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, L.; Yang, Z.; Liu, Z.; Gu, J.; Bai, B.; Liu, J.; Xu, J.; Yang, H. Mechanisms of Oxidative Stress, Apoptosis, and Autophagy Involved in Graphene Oxide Nanomaterial Anti-Osteosarcoma Effect. Int. J. Nanomed. 2018, 13, 2907–2919. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In Vitro Toxicity Evaluation of Graphene Oxide on A549 Cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Krętowski, R.; Jabłońska-Trypuć, A.; Cechowska-Pasko, M. The Preliminary Study on the Proapoptotic Effect of Reduced Graphene Oxide in Breast Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 12593. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, C.; Li, Y.; Li, Y.; Chen, G.; He, Y.; Yi, C.; Wang, C.; Yu, D. Dose-dependent Cytotoxicity Induced by Pristine Graphene Oxide Nanosheets for Potential Bone Tissue Regeneration. J. Biomed. Mater. Res. A 2020, 108, 614–624. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Fu, Y.; Wei, T.; Le Guyader, L.; Gao, G.; Liu, R.-S.; Chang, Y.-Z.; Chen, C. The Triggering of Apoptosis in Macrophages by Pristine Graphene through the MAPK and TGF-Beta Signaling Pathways. Biomaterials 2012, 33, 402–411. [Google Scholar] [CrossRef]
- Daniluk, K.; Lange, A.; Pruchniewski, M.; Małolepszy, A.; Sawosz, E.; Jaworski, S. Delivery of Mittin as a lytic agent via graphene nanoparticles as carriers to breast cancer cells. J. Funct. Biomater. 2022, 13, 278. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 256–273. [Google Scholar] [CrossRef]
- Shen, J.; Dong, J.; Shao, F.; Zhao, J.; Gong, L.; Wang, H.; Chen, W.; Zhang, Y.; Cai, Y. Graphene oxide induces autophagy and apoptosis via the ROS-dependent AMPK/MTOR/ULK-1 pathway in colorectal cancer cells. Nanomedicine 2022, 17, 591–605. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Moldovan, L.; Moldovan, N.I. Oxygen free radicals and redox biology of organelles. Histochem. Cell Biol. 2004, 122, 395–412. [Google Scholar] [CrossRef]
- Gupta, R.K.; Patel, A.K.; Kumari, R.; Chugh, S.; Shrivastav, C.; Mehra, S.; Sharma, A.N. Interactions between oxidative stress, lipid profile and antioxidants in breast cancer: A case control study. Asian Pac. J. Cancer Prev. 2012, 13, 6295–6298. [Google Scholar] [CrossRef]
- Seraj, A.K.; Shankhar, M.; Raju, K.D.; Punam, J.; Anju, P.; Rajat, K.A. Antioxidants and lipid peroxidation status in women with breast cancer. IIUM Med. Malays 2015, 14, 71–75. [Google Scholar] [CrossRef]
- Ataihire, J.U.; Nwangwa, E.K.; Igweh, J.C. Modulations in anti-oxidant activities of selected gastro-intestinal tissues in alloxan-induced, silymarin treated diabetic wistar rats. Open J. Gastroenterol. 2019, 9, 73–90. [Google Scholar] [CrossRef]
- Landis, G.N.; Tower, J. Superoxide dismutase evolution and life span regulation. Mech. Ageing Dev. 2005, 126, 365–379. [Google Scholar] [CrossRef]
- Board, P.G.; Menon, D. Glutathione transferases, regulators of cellular metabolism and physiology. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3267–3288. [Google Scholar] [CrossRef] [PubMed]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.L.; Saha, A.; Liu, J.; Tadi, S.; Tiziani, S.; Yan, W.; Triplett, K.; Lamb, C.; Alters, S.E.; Rowlinson, S.; et al. Systemic depletion of L-Cyst(e)Ine with Cyst(e)Inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 2017, 23, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Tozer, R.G.; Tai, P.; Falconer, W.; Ducruet, T.; Karabadjian, A.; Bounous, G.; Molson, J.H.; Dröge, W. Cysteine-rich protein reverses weight loss in lung cancer patients receiving chemotherapy or radiotherapy. Antioxid. Redox Signal. 2008, 10, 395–402. [Google Scholar] [CrossRef]
- Eroglu, N.; Erduran, E.; Reis, G.P.; Bahadır, A. Therapeutic effect of N-acetylcysteine on chemotherapy-induced liver injury. Ir. J. Med. Sci. 2020, 189, 1189–1194. [Google Scholar] [CrossRef]
- Plasay, M.; Natzir, R.; Cangara, M.H.; Hardjo, M.; Syahrijuita, S.; Soraya, G.V. Mittin-induced cell death through P53 and 8-OHdG in breast cell cancer MCF-7. Biomed. Pharmacol. J. 2022, 15, 979–983. [Google Scholar] [CrossRef]
- Nowsheen, S.; Yang, E.S. The Intersection between DNA Damage Response and Cell Death Pathways. Exp. Oncol. 2012, 34, 243–254. [Google Scholar] [PubMed]
- Singh, S.; Sadanandam, A.; Nannuru, K.C.; Varney, M.L.; Mayer-Ezell, R.; Bond, R.; Singh, R.K. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human Manoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin. Cancer Res. 2009, 15, 2380–2386. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, R.; Chen, L.; Li, S.; Shi, Q.; Jordan, C.; Huang, R.-P. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int. J. Cancer 2004, 109, 507–515. [Google Scholar] [CrossRef]
- Freund, A.; Chauveau, C.; Brouillet, J.-P.; Lucas, A.; Lacroix, M.; Licznar, A.; Vignon, F.; Lazennec, G. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene 2003, 22, 256–265. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Belluscio, L.; Squinto, S.; Ip, N.Y.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. Neurotrophin-3: A neurotrophic factor related to NGF and BDNF. Science 1990, 247, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, E.; Vanhecke, E.; Saule, P.; Mougel, A.; Page, A.; Romon, R.; Nurcombe, V.; Le Bourhis, X.; Hondermarck, H. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008, 68, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Louie, E.; Chen, X.F.; Coomes, A.; Ji, K.; Tsirka, S.; Chen, E.I. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene 2013, 32, 4064–4077. [Google Scholar] [CrossRef]
- Jin, J.; Gao, Y.; Zhang, J.; Wang, L.; Wang, B.; Cao, J.; Shao, Z.; Wang, Z. Incidence, pattern and prognosis of brain metastases in patients with metastatic triple negative breast cancer. BMC Cancer 2018, 18, 446. [Google Scholar] [CrossRef] [PubMed]
- Tauro, M.; Lynch, C. Cutting to the chase: How matrix metalloproteinase-2 activity controls breast-cancer-to-bone metastasis. Cancers 2018, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Stetler-Stevenson, W.G.; Gavil, N.V. Normalization of the tumor microenvironment: Evidence for tissue inhibitor of metalloproteinase-2 as a cancer therapeutic. Connect Tissue Res. 2014, 55, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.W.; Defamie, V.; Waterhouse, P.; Khokha, R. TIMPs: Versatile extracellular regulators in cancer. Nat. Rev. Cancer 2017, 17, 38–53. [Google Scholar] [CrossRef]
- Peeney, D.; Jensen, S.M.; Castro, N.P.; Kumar, S.; Noonan, S.; Handler, C.; Kuznetsov, A.; Shih, J.; Tran, A.D.; Salomon, D.S.; et al. TIMP-2 suppresses tumor growth and metastasis in murine model of triple-negative breast cancer. Carcinogenesis 2020, 41, 313–325. [Google Scholar] [CrossRef]
- Chen, W.-Q.; Yang, S.-J.; Xu, W.-X.; Deng, F.; Wang, D.-D.; Tang, J.-H. Bioinformatics analysis revealing prognostic significance of TIMP2 gene in breast cancer. Medicine 2021, 100, e27489. [Google Scholar] [CrossRef]
- Dave, H.; Trivedi, S.; Shah, M.; Shukla, S. Transforming growth factor beta 2: A predictive marker for breast cancer. Indian J. Exp. Biol. 2011, 49, 879–887. [Google Scholar]
- Biere, B.; Moses, H. TGF-β and cancer. Cytokine Growth Factor Rev. 2006, 17, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Shipitsin, M.; Campbell, L.L.; Argani, P.; Weremowicz, S.; Bloushtain-Qimron, N.; Yao, J.; Nikolskaya, T.; Serebryiskaya, T.; Beroukhim, R.; Hu, M.; et al. Molecular definition of breast tumor heterogeneity. Cancer Cell 2007, 11, 259–273. [Google Scholar] [CrossRef]
- Katsuno, Y.; Lamouille, S.; Derynck, R. TGF-β signaling and epithelial–mesenchymal transition in cancer progression. Curr. Opin. Oncol. 2013, 25, 76–84. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between Oxidative Stress and Inflammatory Cytokines in Diabetic Nephropathy. Cardiovasc Ther 2012, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, K.A. Oxidant Stress Regulation of IL-8 and ICAM-1 Gene Expression: Differential Activation and Binding of the Transcription Factors AP-1 and NF-KappaB (Review). Int. J. Mol. Med. 1999, 4, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Birsoy, K.; Kong, H.; Martínez-Reyes, I.; Wang, T.; Gao, P.; Sabatini, D.M.; Chandel, N.S. A CRISPR Screen Identifies a Pathway Required for Paraquat-Induced Cell Death. Nat. Chem. Biol. 2017, 13, 1274–1279. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Mohan G., M.; Shaulender, G.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 117727191875539. [Google Scholar] [CrossRef]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M.K. Mechanisms of Cell Death in Oxidative Stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.; Varol, A.; Thakral, F.; Yerer, M.; Sak, K.; Varol, M.; Jain, A.; Khan, M.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cook, N.R.; Albert, C.; Zaharris, E.; Gaziano, J.M.; Van Denburgh, M.; Buring, J.E.; Manson, J.E. Vitamins C and E and Beta Carotene Supplementation and Cancer Risk: A Randomized Controlled Trial. JNCI J. Natl. Cancer Inst. 2009, 101, 14–23. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for Preventing Cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Benhar, M.; Shytaj, I.L.; Stamler, J.S.; Savarino, A. Dual Targeting of the Thioredoxin and Glutathione Systems in Cancer and HIV. J. Clin. Investig. 2016, 126, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniluk, K.; Lange, A.; Wójcik, B.; Zawadzka, K.; Bałaban, J.; Kutwin, M.; Jaworski, S. Effect of Melittin Complexes with Graphene and Graphene Oxide on Triple-Negative Breast Cancer Tumors Grown on Chicken Embryo Chorioallantoic Membrane. Int. J. Mol. Sci. 2023, 24, 8388. https://doi.org/10.3390/ijms24098388
Daniluk K, Lange A, Wójcik B, Zawadzka K, Bałaban J, Kutwin M, Jaworski S. Effect of Melittin Complexes with Graphene and Graphene Oxide on Triple-Negative Breast Cancer Tumors Grown on Chicken Embryo Chorioallantoic Membrane. International Journal of Molecular Sciences. 2023; 24(9):8388. https://doi.org/10.3390/ijms24098388
Chicago/Turabian StyleDaniluk, Karolina, Agata Lange, Barbara Wójcik, Katarzyna Zawadzka, Jaśmina Bałaban, Marta Kutwin, and Sławomir Jaworski. 2023. "Effect of Melittin Complexes with Graphene and Graphene Oxide on Triple-Negative Breast Cancer Tumors Grown on Chicken Embryo Chorioallantoic Membrane" International Journal of Molecular Sciences 24, no. 9: 8388. https://doi.org/10.3390/ijms24098388




