Phylogenetical Position versus Pollination Syndromes: Floral Trichomes of Central American and Mexican Pinguicula
Abstract
1. Introduction
1.1. Plant Trichomes and Their Function in General
1.2. Plant Trichomes and Glands in Carnivorous Plants
1.3. State of the Art of Trichomes in Lentibulariaceae, Especially in the Genus Pinguicula
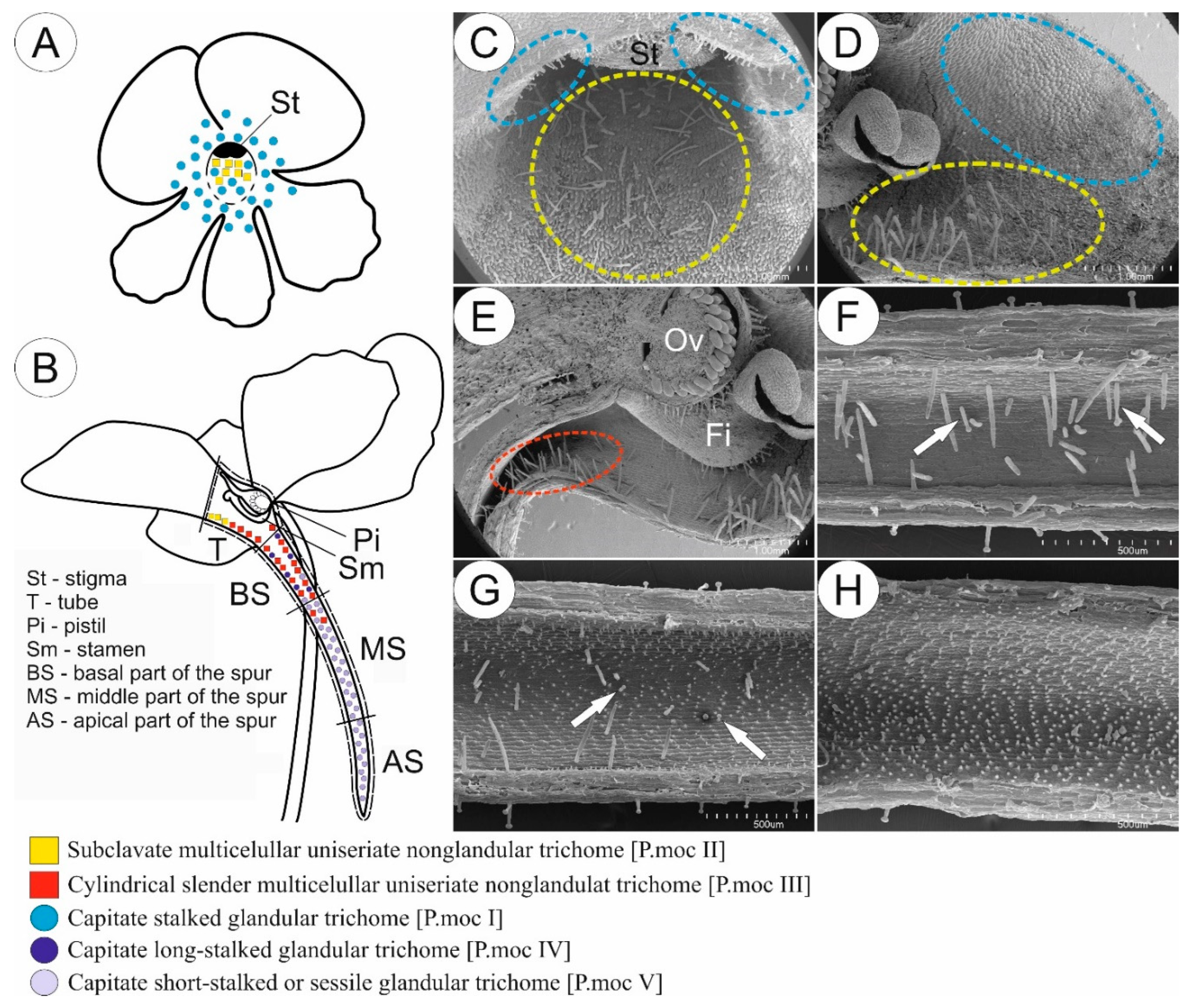
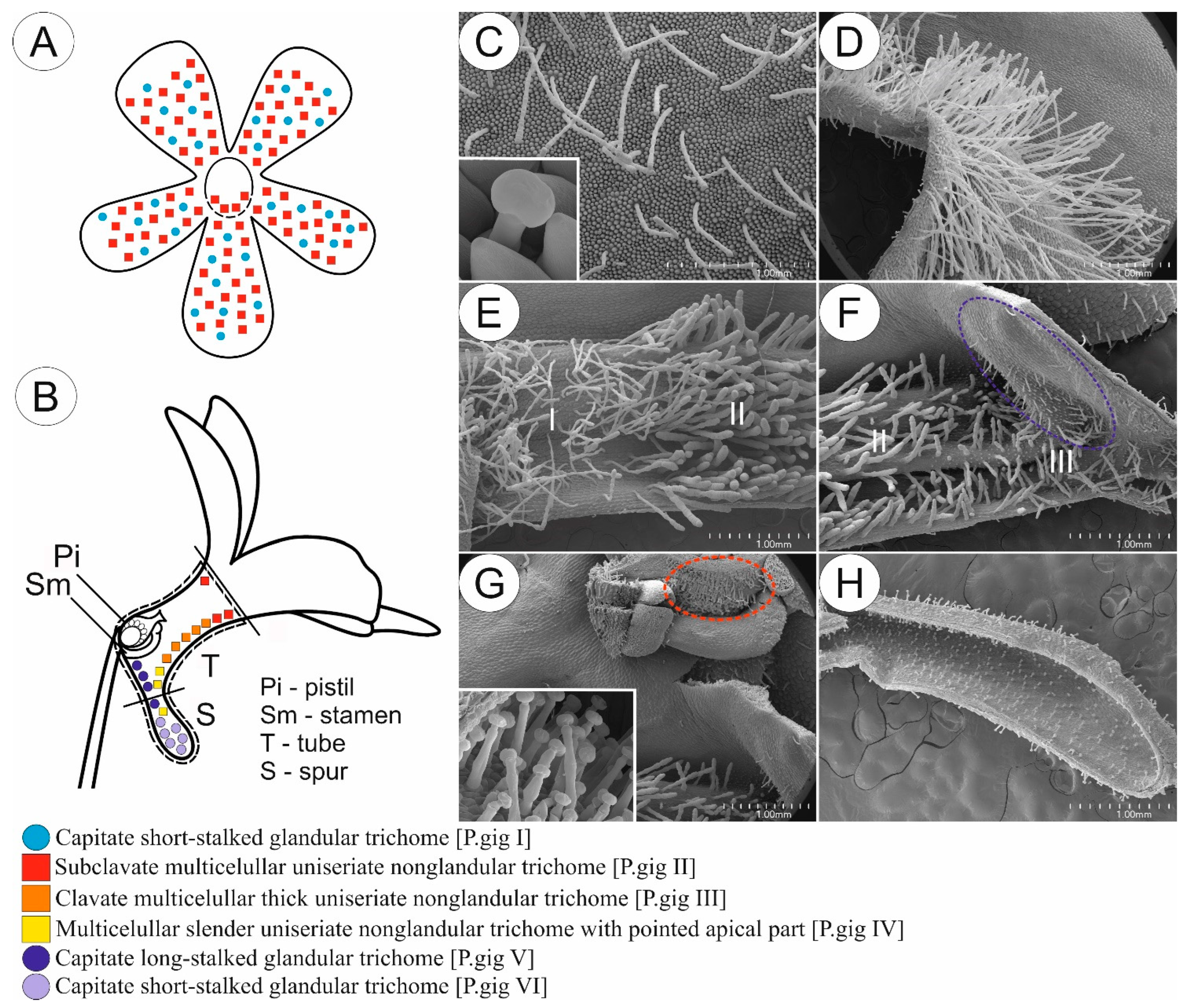
2. Results
2.1. Flowers Micromorphology Analysis
2.1.1. Species with Psychophily Syndrome (a Set of Features Found in Butterfly-Pollinated Flowers)
2.1.2. Species with Myophily/Mellitophily Syndrome (a Set of Features Found in Fly/Bee-Pollinated Flowers)
2.1.3. Species with Ornithophily Syndrome (a Set of Features Found in Bird-Pollinated Flowers)
2.2. Phylogenetic Analysis
2.3. Tracing Analysis of Flower Morphological Characters
3. Discussion
3.1. Diversification and Possible Function of Glandular and Non-Glandular Trichomes of Central American Pinguicula and Mexican Species
3.2. Floral Adaptions to Particular Groups of Pollinators: Pollination Syndrome Concept
3.3. Evolution of Floral Characters: Synapomorphies vs. Homoplasies
4. Materials and Methods
4.1. Plant Material
4.2. Scanning Electron Microscopy
4.3. Trichome Density Assessment
4.4. Phylogenetic Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Werker, E. Trichome diversity and development. In Plant Trichomes; Hallahan, D.L., Gray, J.C., Callow, J.A., Eds.; Academic Press: London, UK, 2000. [Google Scholar]
- Benzing, D.; Henderson, K.; Kessel, B.; Sulak, J. The absortive capacities of bromeliads trichomes. Am. J. Bot. 1976, 63, 1009–1014. [Google Scholar] [CrossRef]
- Fahn, A. Structure and function of secretory cells. In Plant Trichome; Hallahan, D.L., Gray, J.C., Callow, J.A., Eds.; Academic Press: London, UK, 2000. [Google Scholar]
- Dassanayake, M.; Larkin, J.C. Making Plants Break a Sweat: The Structure, Function, and Evolution of Plant Salt Glands. Front. Plant Sci. 2017, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Muravnik, L.E. The structural Peculiarities of the Leaf Glandular Trichomes: A Review. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K., Ekiert, H., Goyal, S., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Zajączkowska, U.; Kucharski, S.; Guzek, D. Are trichomes involved in the biochemical systems of Cucurbita leaf petioles? Planta 2015, 242, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, R. Evolution of unusual morphologies in Lentibulariaceae (bladderworts and allies) and Podostemaceae (river-weeds): A pictorial report at the interface of developmental biology and morphological diversification. Ann. Bot. 2016, 117, 811–832. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Adamec, L.; Świątek, P.; Kapusta, M.; Miranda, V.F.O. Life in the Current: Anatomy and Morphology of Utricularia neottioides. Int. J. Mol. Sci. 2020, 21, 4474. [Google Scholar] [CrossRef] [PubMed]
- Juniper, B.E.; Robins, R.J.; Joel, D.M. The Carnivorous Plants; Academic Press: London, UK, 1989. [Google Scholar]
- Lloyd, F.D. The Carnivorous Plants; Chronica Botanica Company: Waltham, MA, USA, 1942. [Google Scholar]
- Fineran, B.A.; Lee, M.S.L. Organization of quadrifid and bifid hairs in the trap of Utricularia monanthos. Protoplasma 1975, 84, 43–70. [Google Scholar] [CrossRef]
- Heslop-Harrison, Y.; Heslop-Harrison, J. The Digestive Glands of Pinguicula: Structure and Cytochemistry. Ann. Bot. 1981, 47, 293–319. [Google Scholar] [CrossRef]
- Fineran, B.A. Glandular trichomes in Utricularia: A review of their structure and function. Isr. J. Bot. 1985, 34, 295–333. [Google Scholar]
- Płachno, B.J.; Kozieradzka-Kiszkurno, M.; Świątek, P. Functional ultrastructure of Genlisea (Lentibulariaceae) digestive hairs. Ann. Bot. 2007, 100, 195–203. [Google Scholar] [CrossRef]
- Scatigna, A.V.; Fritsch, P.W.; Souza, V.C.; Simões, A.O. Phylogenetic Relationships and Morphological Evolution in the Carnivorous Genus Philcoxia (Plantaginaceae, Gratioleae). Syst. Bot. 2018, 43, 910–919. [Google Scholar] [CrossRef]
- Płachno, B.J.; Świątek, P.; Adamec, L.; Carvalho, S.; Miranda, V.F.O. The trap architecture of Utricularia multifida and Utricularia westonii (subg. Polypompholyx). Front. Plant Sci. 2019, 10, 336. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M.; Pellmyr, O. Plant Animal Interactions: An Evolutionary Approach; Wiley-Blackwell: Oxford, UK, 2002. [Google Scholar]
- Giuliani, C.; Ascrizzi, R.; Lupi, D.; Tassera, G.; Santagostini, L.; Giovanetti, M.; Flamini, G.; Fico, G. Salvia verticillata: Linking glandular trichomes, volatiles and pollinators. Phytochemistry 2018, 155, 53–60. [Google Scholar] [CrossRef] [PubMed]
- González-Teuber, M.; Heil, M. Nectar chemistry is tailored for both attraction of mutualists and protection from exploiters. Plant Signal Behav. 2009, 4, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Nepi, M.; Grasso, D.A.; Mancuso, S. Nectar in Plant–Insect Mutualistic Relationships: From Food Reward to Partner Manipulation. Front. Plant Sci. 2018, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Bogo, G.; Fisogni, A.; Rabassa-Juvanteny, J.; Bortolotti, L.; Nepi, M.; Guarnieri, M.; Conte, L.; Galloni, M. Nectar chemistry is not only a plant’s affair: Floral visitors affect nectar sugar and amino acid composition. OIKOS 2021, 130, 1180–1192. [Google Scholar] [CrossRef]
- Bernardello, G. A systematic survey of floral nectaries. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Nepi, M. Nectary structure and ultrastructure. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Albert, V.A.; Williams, S.E.; Chase, M.W. Carnivorous Plants: Phylogeny and Structural Evolution. Science 1992, 257, 1491–1495. [Google Scholar] [CrossRef]
- Cameron, K.M.; Wurdack, K.J.; Jobson, R.W. Molecular Evidence for the Common Origin of Snap-Traps among Carnivorous Plants. Am. J. Bot. 2002, 89, 1503–1509. [Google Scholar] [CrossRef]
- Fleischmann, A.; Schlauer, J.; Smith, S.A.; Givnish, T.J. Evolution of Carnivory in Angiosperms. In Carnivorous Plants: Physiology, Ecology, and Evolution; Ellison, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Lin, Q.; Ané, C.; Givnish, T.J.; Graham, S.W. A New Carnivorous Plant Lineage (Triantha) with a Unique Sticky-Inflorescence Trap. Proc. Natl. Acad. Sci. USA 2021, 118, e2022724118. [Google Scholar] [CrossRef]
- Renner, T.; Specht, C.D. A sticky situation: Assessing adaptations for plant carnivory in the Caryophyllales by means of stochastic character mapping. Int. J. Plant Sci. 2011, 172, 889–901. [Google Scholar] [CrossRef]
- Renner, T.; Specht, C.D. Inside the trap: Gland morphologies, digestive enzymes, and the evolution of plant carnivory in the Caryophyllales. Curr. Opin. Plant Biol. 2013, 16, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Muravnik, L.E. Functional Anatomy of Carnivorous Traps. In Carnivorous Plants: Physiology, Ecology, and Evolution; Ellison, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Freund, M.; Graus, D.; Fleischmann, A.; Gilbert, K.J.; Lin, Q.; Renner, T.; Stigloher, C.; Albert, V.A.; Hedrich, R.; Fukushima, K. The digestive systems of carnivorous plants. Plant Physiol. 2022, 190, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Fenner, C.A. Beiträge zur Kenntnis der Anatomie, Entwicklungsgeschichte und Biologie der Laubblätter und Drüsen einiger Insektivoren. Flora 1904, 93, 335–434. [Google Scholar]
- Green, S.; Green, T.L.; Heslop-Harrison, Y. Seasonal heterophylly and leaf gland features in Triphyophyllum (Dioncophyllaceae), a new carnivorous plant genus. Bot. J. Linn. Soc. 1979, 78, 99–116. [Google Scholar] [CrossRef]
- Heslop-Harrison, Y. Enzyme release in carnivorous plants. In Lysozymes in Biology and Pathology; Dingle, J.T., Dean, R.T., Eds.; North Holland Publishing Company: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Pereira, C.G.; Almenara, D.P.; Winter, C.E.; Fritsch, P.W.; Lambers, H.; Oliveira, R.S. Underground leaves of Philcoxia trap and digest nematodes. Proc. Natl. Acad. Sci. USA 2012, 109, 1154–1158. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P.; Strzemski, M.; Miranda, V.F.O. Immunocytochemical Analysis of the Wall Ingrowths in the Digestive Gland Transfer Cells in Aldrovanda vesiculosa L. (Droseraceae). Cells 2022, 11, 2218. [Google Scholar] [CrossRef]
- Fineran, B.A.; Lee, M.S.L. Organization of mature external glands on the trap and other organs of the bladderwort Utricularia monanthos. Protoplasma 1980, 103, 17–34. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Wójciak, M.; Świątek, P. Immunocytochemical Analysis of Bifid Trichomes in Aldrovanda vesiculosa L. Traps. Int. J. Mol. Sci. 2023, 24, 3358. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P. Stellate Trichomes in Dionaea muscipula Ellis (Venus Flytrap) Traps, Structure and Functions. Int. J. Mol. Sci. 2023, 24, 553. [Google Scholar] [CrossRef]
- Adlassnig, W.; Lendl, T.; Peroutka, M.; Lang, I. Deadly glue—Adhesive traps of carnivorous plants. In Biological Adhesive Systems; Von Byern, J., Grunwald, I., Eds.; Springer: Vienna, NY, USA, 2010. [Google Scholar]
- Lichtscheidl, I.; Lancelle, S.; Weidinger, M.; Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Krammer, S.; Hepler, P.K. Gland cell responses to feeding in Drosera capensis, a carnivorous plant. Protoplasma 2021, 258, 1291–1306. [Google Scholar] [CrossRef]
- Płachno, B.J.; Stpiczyńska, M.; Adamec, L.; Miranda, V.F.O.; Świątek, P. Nectar trichome structure of aquatic bladderworts from the section Utricularia (Lentibulariaceae) with observation of flower visitors and pollinators. Protoplasma 2018, 255, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Stpiczyńska, M.; Świątek, P.; Lambers, H.; Cawthray, G.R.; Nge, F.J.; Silva, S.R.; Miranda, V.F.O. Floral micromorphology and nectar composition of the early evolutionary lineage Utricularia (subgenus Polypompholyx, Lentibulariaceae). Protoplasma 2019, 256, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Aranguren, Y.; Płachno, B.J.; Stpiczyńska, M.; Miranda, V.F.O. Reproductive biology and pollination of the carnivorous Genlisea violaceae (Lentibulariaceae). Plant Biol. 2018, 20, 591–601. [Google Scholar] [CrossRef]
- Lustofin, K.; Świątek, P.; Miranda, V.; Płachno, B.J. Flower nectar trichome structure of carnivorous plants from the genus butterworts Pinguicula L. (Lentibulariaceae). Protoplasma 2020, 257, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Stpiczyńska, M.; Krajewski, Ł.; Świątek, P.; Adamec, L.; Miranda, V.F.O. Flower palate structure of the aquatic bladderworts Utricularia bremii Heer and U. minor L. from section Utricularia (Lentibulariaceae). Protoplasma 2017, 254, 2007–2015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Płachno, B.J.; Świątek, P.; Stpiczyńska, M.; Miranda, V.F.O. Flower palate ultrastructure of the carnivorous plant Genlisea hispidula Stapf with remarks on the structure and function of the palate in the subgenus Genlisea (Lentibulariaceae). Protoplasma 2018, 255, 1139–1146. [Google Scholar] [CrossRef]
- Casper, S.J. Monographie der Gattung Pinguicula L. In Bibliotheca Botanica; Schweizerbart: Stuttgart, Germany, 1966. [Google Scholar]
- Lustofin, K.; Świątek, P.; Stolarczyk, P.; Miranda, V.; Płachno, B.J. Do food trichomes occur in Pinguicula (Lentibulariaceae) flowers? Ann. Bot. 2020, 126, 1039–1048. [Google Scholar] [CrossRef]
- Shimai, H.; Setoguchi, H.; Roberts, D.L.; Sun, M. Biogeographical patterns and speciation of the genus Pinguicula (Lentibulariaceae) inferred by phylogenetic analyses. PLoS ONE 2021, 19, e0252581. [Google Scholar]
- Dominguez, Y.; Silva, S.R.; Valdés, C.M.P.; Miranda, V.F.O. Inter- and intraspecific diversity of Cuban Pinguicula (Lentibulariaceae) based on morphometric analyses and its relation with geographical distribution. Plant Ecol. Divers. 2014, 7, 519–531. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Kessler, M.; Hanley, D.; Karger, D.N.; Muller, M.P.J.; Knauer, A.C.; Keller, F.; Schwerdtfeger, M.; Humphreys, A.M. Pollinator adaptation and the evolution of floral nectar sugar composition. J. Evol. Biol. 2017, 30, 112–127. [Google Scholar] [CrossRef]
- Villegas, S.G.; Alcalá, R.E. Reproductive ecology of the carnivorous plant Pinguicula moranensis (Lentibulariaceae). Plant Biol. 2018, 20, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Lampard, S.; Gluch, O.; Robinson, A. Pinguicula of Latin America; Redfern Natural History: Dorset, UK, 2016. [Google Scholar]
- Roccia, A.; Gluch, O.; Lampard, S. Pinguicula of the Temperate North; Redfern Natural History: Dorset, UK, 2016. [Google Scholar]
- Fleischmann, A.; Roccia, A. Systematics and evolution of Lentibulariaceae: I. Pinguicula. In Carnivorous Plants, Physiology, Ecology and Evolution; Elison, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Fleischmann, A. On the infrageneric classification of Pinguicula. CPN 2021, 50, 174–188. [Google Scholar] [CrossRef]
- Zamudio, S.; Rosas, M.M.; Salinas-Rodríguez, M.M.; Rendón, J.H. Pinguicula warijia sp. nov. (Lentibulariaceae), a newly rediscovered species from the Sierra Obscura, northern Mexico. Phytotaxa 2023, 578, 219–227. [Google Scholar] [CrossRef]
- Davies, K.L.; Roberts, D.L.; Turner, M.P. Pseudopollen and Food-hair Diversity in Polystachya Hook. (Orchidaceae). Ann. Bot. 2002, 90, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.L.; Stpiczyńska, M.; Kamińska, M. Dual deceit in pseudopollen-producing Maxillaria s.s. (Orchidaceae: Maxillariinae). Bot. J. Linn. Soc. 2013, 173, 744–763. [Google Scholar] [CrossRef]
- Davies, K.L.; Turner, M.P. Pseudopollen in Dendrobium unicum Seidenf. (Orchidaceae): Reward or Deception? Ann. Bot. 2004, 94, 129–132. [Google Scholar] [CrossRef]
- Pansarin, E.R.; Maciel, A.A. Evolution of pollination systems involving edible trichomes in orchids. AoB Plants 2017, 9, plx033. [Google Scholar] [CrossRef]
- Pansarin, E.R. Vanilla flowers: Much more than food-deception. Bot. J. Linn. Soc. 2021, 198, 57–73. [Google Scholar] [CrossRef]
- Kinoshita, M.; Arikawa, K. Color and polarization vision in foraging Papilio. J. Comp. Physiol. A 2014, 200, 513–526. [Google Scholar] [CrossRef]
- Arikawa, K. The eyes and vision of butterflies. J. Physiol. 2017, 595, 5457–5464. [Google Scholar] [CrossRef]
- Rajchard, J. Ultraviolet (UV) light perception by birds: Review. Vet. Med. 2009, 54, 351–359. [Google Scholar] [CrossRef]
- Stoddard, M.C.; Eyster, H.N.; Hogan, B.G.; Morris, D.H.; Soucy, E.R.; Inouye, D.W. Wild hummingbirds discriminate nonspectral colors. Proc. Natl. Acad. Sci. USA 2020, 117, 15112–15122. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, K. Repellence of nectar-thieving ants by a physical barrier: Adaptive role of petal hairs on Menyanthes trifoliata (Menyanthaceae). J. Asia Pac. Entomol. 2018, 21, 1211–1214. [Google Scholar] [CrossRef]
- Fleischmann, A. Pinguicula flowers with pollen imitations close at night—Some observations on butterwort flower biology. CPN 2016, 45, 84–92. [Google Scholar] [CrossRef]
- Płachno, B.J.; Stpiczyńska, M.; Davies, K.L.; Świątek, P.; Miranda, V.F.O. Floral ultrastructure of two Brazilian aquatic-epiphytic bladderworts: Utricularia cornigera Studnička and U. nelumbifolia Gardner (Lentibulariaceae). Protoplasma 2017, 254, 353–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Płachno, B.J.; Stpiczyńska, M.; Świątek, P.; Davies, K.L. Floral micromorphology of the Australian carnivorous bladderwort Utricularia dunlopii, a putative pseudocopulatory species. Protoplasma 2016, 253, 1463–1473. [Google Scholar] [CrossRef][Green Version]
- Johnson, S.D.; Linder, H.P.; Steiner, K.E. Phylogeny and radiation of pollination systems in Disa (Orchidaceae). Am. J. Bot. 1998, 85, 402–411. [Google Scholar] [CrossRef]
- Roguz, K.; Bajguz, A.; Chmur, M.; Gołębiewska, A.; Roguz, A.; Zych, M. Diversity of nectar amino acids in the Fritillaria (Liliaceae) genus: Ecological and evolutionary implications. Sci. Rep. 2019, 9, 15209. [Google Scholar] [CrossRef]
- Roguz, K.; Hill, L.; Roguz, A.; Zych, M. Evolution of Bird and Insect Flower traits in Fritillaria L. (Liliaceae). Front. Plant Sci. 2021, 12, 656783. [Google Scholar] [CrossRef]
- Johnson, S.D.; Steiner, K.E.; Kaiser, R. Deceptive pollination in two subspecies of Disa spathulata (Orchidaceae) differing in morphology and floral fragrance. Plant Syst. Evol. 2005, 255, 87–98. [Google Scholar] [CrossRef]
- Perez-Alva, B.R.; Jesus, S.G.; Galindo-Flores, G.L.; Valencia-Quintana, R.; Perez-Flores, G.A. Effect of the environment and morphological characteristics on biotic and reproductive variables of Pinguicula moranensis var. Neovolcanica Zamudio (Lentibulariaceae) in Tlaxcala, Mexico. Bot. Sci. 2022, 100, 550–562. [Google Scholar] [CrossRef]
- Roguz, K.; Hill, L.; Koethe, S.; Lunau, K. Visibility and attractiveness of Fritillaria (Liliaceae) flowers to potential pollinators. Sci. Rep. 2021, 11, 11006. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Alarcon, R.; Waser, N.M.; Price, M.V.; Watts, S.; Cranmer, L.; Hingston, A.; Peter, C.I.; Rotenberry, J. A global test of the pollination syndrome hypothesis. Ann. Bot. 2009, 103, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Knapp, S. On ‘various contrivances’: Pollination, phylogeny and flower form in the Solanaceae. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Krakos, K.N.; Austin, M.W. Testing pollination syndromes in Oenothera (Onagraceae). J. Pollinat. Ecol. 2020, 26, 52–66. [Google Scholar] [CrossRef]
- Johnson, S.D.; Steiner, K.E. Generalization versus specialization in plant pollination systems. Trends Ecol. Evol. 2000, 15, 140–143. [Google Scholar] [CrossRef]
- Rosas-Guerrero, V.; Aguilar, R.; Martén-Rodríguez, S.; Ashworth, L.; Lopezaraiza-Mikel, M.; Bastida, J.M.; Quesada, M. A quantitative review of pollination syndromes: Do floral traits predict effective pollinators? Ecol. Lett. 2014, 17, 388–400. [Google Scholar] [CrossRef]
- Dellinger, A.S. Pollination syndromes in the 21st century: Where do we stand and where may we go? New Phytol. 2020, 228, 1193–1213. [Google Scholar] [CrossRef]
- Shimai, H.; Kondo, K. Phylogenetic analysis of Mexican and Central American Pinguicula (Lentibulariaceae) based on internal transcribed spacer (ITS) sequence. Chromosome Bot. 2007, 2, 67–77. [Google Scholar] [CrossRef]
- Fleischmann, A. Monograph of the Genus Genlisea; Redfern Natural History: Poole, UK, 2012. [Google Scholar]
- Hobbhahn, N.; Küchmeister, H.; Porembski, S. Pollination biology of mass flowering terrestrial Utricularia species (Lentibulariaceae) in the Indian Western Ghats. Plant. Biol. 2006, 8, 791–804. [Google Scholar] [CrossRef]
- Clivati, D.; Cordeiro, G.D.; Płachno, B.J.; Miranda, V.F.O. Reproductive biology and pollination of Utricularia reniformis A.St.-Hil. (Lentibulariaceae). Plant Biol. 2014, 16, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Stpiczyńska, M.; Świątek, P.; Lambers, H.; Miranda, V.F.O.; Nge, F.J.; Stolarczyk, P.; Cawthray, G.R. Floral micromorphology of the bird-pollinated carnivorous plant species Utricularia menziesii R.Br. (Lentibulariaceae). Ann. Bot. 2019, 123, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest 2.4. Program Distributed by the Author. Evolutionary Biology Centre; Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In International Symposium on Information Theory; Akadémiai Kiadó: Budapest, Hungary, 1973. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van der Mark, P.; Ayers, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Agnarsson, I.; Miller, J.A. Is ACCTRAN better than DELTRAN? Cladistics 2008, 24, 1032–1038. [Google Scholar] [CrossRef] [PubMed]






| General Morphology of the Flower |
| 1. Elongated (apical pointed part) spur |
| 2. Shortened (apical rounded part) spur |
| 3. Short tube |
| 4. Elongated tube |
| 5. Occurrence of contrast color signal at the entrance to the tube |
| Distribution of trichomes on corolla and papillae inside of the flower |
| 6. Glandular trichomes on middle part and edges of corolla petals |
| 7. Non-glandular trichomes on middle part and edges of corolla petals |
| 8. Short-stalked capitate glandular trichomes at the base of corolla petals of upper lip (at the entrance to the tube) |
| 9. Short-stalked capitate glandular trichomes at the base of corolla petals of lower lip (at the entrance to the tube) |
| 10. Long-stalked capitate glandular trichomes at the base of corolla petals (around the entrance to the tube) |
| 11. Non-glandular trichomes on the corolla petals of lower lip (before the entrance to the tube) |
| 12. Papillae at the entrance to the spur |
| 13. Papillae in spur |
| Non-glandular trichome morphological types |
| 14. Apical to obtuse pointed slender non-glandular trichomes |
| 15. Subclavate non-glandular trichomes |
| 16. Clavate non-glandular trichomes |
| 17. Non-glandular trichomes, with elongated cylinder concave-shaped cells |
| 18. Non-glandular trichomes with compact rounded cells |
| 19. Non-glandular trichomes, with rounded apical “head” cell |
| 20. Moniliform non-glandular trichomes |
| Non-glandular trichomes features |
| 21. Non-glandular trichomes with cuticle striations |
| 22. Non-glandular trichomes with (almost) smooth cuticle surface |
| 23. Non-glandular trichomes with outgrowths |
| 24. Non-glandular trichomes with few celled branches |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lustofin, K.; Świątek, P.; Miranda, V.F.O.; Płachno, B.J. Phylogenetical Position versus Pollination Syndromes: Floral Trichomes of Central American and Mexican Pinguicula. Int. J. Mol. Sci. 2023, 24, 8423. https://doi.org/10.3390/ijms24098423
Lustofin K, Świątek P, Miranda VFO, Płachno BJ. Phylogenetical Position versus Pollination Syndromes: Floral Trichomes of Central American and Mexican Pinguicula. International Journal of Molecular Sciences. 2023; 24(9):8423. https://doi.org/10.3390/ijms24098423
Chicago/Turabian StyleLustofin, Krzysztof, Piotr Świątek, Vitor F. O. Miranda, and Bartosz J. Płachno. 2023. "Phylogenetical Position versus Pollination Syndromes: Floral Trichomes of Central American and Mexican Pinguicula" International Journal of Molecular Sciences 24, no. 9: 8423. https://doi.org/10.3390/ijms24098423
APA StyleLustofin, K., Świątek, P., Miranda, V. F. O., & Płachno, B. J. (2023). Phylogenetical Position versus Pollination Syndromes: Floral Trichomes of Central American and Mexican Pinguicula. International Journal of Molecular Sciences, 24(9), 8423. https://doi.org/10.3390/ijms24098423








