Polar Metabolites Profiling of Wheat Shoots (Triticum aestivum L.) under Repeated Short-Term Soil Drought and Rewatering
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effect of Repeated Short-Term Soil Drought/Rewatering Cycles on Plant Growth and Development
2.2. Changes in Polar Metabolite Profiles during Control Plant (Well-Watered) Vegetation
2.3. Changes in Polar Metabolite Profiles under Short-Term Soil Drought and Followed by Rewatering
3. Materials and Methods
3.1. Material
3.2. Methods
3.2.1. Analysis of Polar Metabolites
3.2.2. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curtis, B.C. Wheat in the world. In Bread Wheat Improvement and Production; Curtis, B.C., Rajaram, S., Macpherson, H.G., Eds.; FAO Plant Production and Protection Series; FAO: Rome, Italy, 2002; pp. 1–19. [Google Scholar]
- Rosenfelder, P.; Eklund, M.; Mosenthin, R. Nutritive value of wheat and wheat by-products in pig nutrition: A review. Anim. Feed Sci. Technol. 2013, 185, 107–125. [Google Scholar] [CrossRef]
- Dai, A.; Zhao, T.; Chen, J. Climate Change and Drought: A Precipitation and Evaporation Perspective. Curr. Clim. Chang. Rep. 2018, 4, 301–312. [Google Scholar] [CrossRef]
- Helman, D.; Bonfil, D.J. Six decades of warming and drought in the world’s top wheat-producing countries offset the benefits of rising CO2 to yield. Sci. Rep. 2022, 12, 7921. [Google Scholar] [CrossRef] [PubMed]
- Trnka, M.; Feng, S.; Semenov, M.A.; Olesen, J.E.; Kersebaum, K.C.; Rötter, R.P.; Semerádová, D.; Klem, K.; Huang, W.; Ruiz-Ramos, M.; et al. Mitigation efforts will not fully alleviate the increase in water scarcity occurrence probability in wheat-producing areas. Sci. Adv. 2019, 5, eaau2406. [Google Scholar] [CrossRef] [PubMed]
- Elahi, I.; Saeed, U.; Wadood, A.; Abbas, A.; Nawaz, H.; Jabbar, S. Effect of Climate Change on Wheat Productivity. In Wheat; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Varshney, R.K.; Barmukh, R.; Roorkiwal, M.; Qi, Y.; Kholova, Y.; Tuberosa, R.; Reynolds, M.P.; Tardieu, F.; Siddique, K.H.M. Breeding custom-designed crops for improved drought adaptation. Adv. Genet. 2021, 2, e202100017. [Google Scholar] [CrossRef]
- Raza, A.; Mubarik, M.S.; Sharif, R.; Habib, M.; Jabeen, W.; Zhang, C.; Chen, H.; Chen, Z.H.; Siddique, K.H.M.; Zhuang, W.; et al. Developing drought-smart, ready-to-grow future crops. Plant Genome 2022, 16, e20279. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Bowne, J.B.; Erwina, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef]
- Suprasanna, P.; Nikalje, G.C.; Rai, A.N. Osmolyte accumulation and implications in plant abiotic stress tolerance. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016; pp. 1–12. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Dellero, Y.; Jossier, M.; Schmitz, J.; Maurino, V.G.; Hodges, M. Photorespiratory glycolate-glyoxylate metabolism. J. Exp. Bot. 2016, 67, 3041–3052. [Google Scholar] [CrossRef] [PubMed]
- Hodges, M.; Dellero, Y.; Keech, O.; Betti, M.; Raghavendra, A.S.; Sage, R.; Zhu, X.G.; Allen, D.K.; Weber, A.P.M. Perspectives for a better understanding of the metabolic integration of photorespiration within a complex plant primary metabolism network. J. Exp. Bot. 2016, 67, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef]
- Eisenhut, M.; Brautigam, A.; Timm, S.; Florian, A.; Tohge, T.; Fernie, A.R.; Bauwe, H.; Weber, A.P.M. Photorespiration is crucial for dynamic response of photosynthetic metabolism and stomatal movement to altered CO2 availability. Mol. Plant 2017, 10, 47–61. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef]
- Nikinmaa, E.; Hölttä, T.; Hari, P.; Kolari, P.; Mäkelä, A.; Sevanto, S.; Vesala, T. Assimilate transport in phloem sets conditions for leaf gas exchange. Plant Cell Environ. 2013, 36, 655–669. [Google Scholar] [CrossRef]
- Durand, M.; Porcheron, B.; Hennion, N.; Maurousset, L.; Lemoine, R.; Pourtau, N. Water deficit enhances C export to the roots in Arabidopsis thaliana plants with contribution of sucrose transporters in both shoot and roots. Plant Physiol. 2016, 170, 1460–1479. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Sanders, G.J.; Arndt, S.K. Osmotic adjustment under drought conditions. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–229. [Google Scholar] [CrossRef]
- Khan, M.S.; Ahmad, D.; Khan, M.A. Utilization of genes encoding osmoprotectants in transgenic plants for enhanced abiotic stress tolerance. Electron J. Biotechnol. 2015, 18, 257–266. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Rusilowicz, M.J.; Dickinson, M.; Charlton, A.J.; Bechtold, U.; Mullineaux, P.M.; Wilson, J. Integrating transcriptomic techniques and k-means clustering in metabolomics to identify markers of abiotic and biotic stress in Medicago truncatula. Metabolomics 2018, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef] [PubMed]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef]
- Ma, X.; Xia, H.; Liu, Y.; Wei, H.; Zheng, X.; Song, C.; Chen, L.; Liu, H.; Luo, L. Transcriptomic and metabolomic studies disclose key metabolism pathways contributing to well-maintained photosynthesis under the drought and the consequent drought-tolerance in rice. Front. Plant Sci. 2016, 7, 1886. [Google Scholar] [CrossRef] [PubMed]
- Templer, S.E.; Ammon, A.; Pscheidt, D.; Ciobotea, O.; Schuy, C.; McCollum, C.; Sonnewald, U.; Hanemann, A.; Förster, J.; Ordon, F.; et al. Metabolite profiling of barley flag leaves under drought and combined heat and drought stress reveals metabolic QTLs for metabolites associated with antioxidant defense. J. Exp. Bot. 2017, 68, 1697–1713. [Google Scholar] [CrossRef]
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic profiling of soybeans (Glycine max L.) reveals the importance of sugar and nitrogen metabolism under drought and heat stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef]
- Charlton, A.J.; Donarski, J.A.; Harrison, M.; Jones, S.A.; Godward, J.; Oehlschlager, S.; Arques, J.L.; Ambrose, M.; Chinoy, C.; Mullineaux, P.M.; et al. Responses of the pea (Pisum sativum L.) leaf metabolome to drought stress assessed by nuclear magnetic resonance spectroscopy. Metabolomics 2008, 4, 312. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B. Metabolite profiling of semi-leafless pea (Pisum sativum L.) under progressive soil drought and subsequent re-watering. J. Plant Physiol. 2021, 256, 153314. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Horbowicz, M. Changes in Metabolic Profiles of Pea (Pisum sativum L.) as a Result of Repeated Short-Term Soil Drought and Subsequent Re-Watering. Int. J. Mol. Sci. 2022, 23, 1704. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hoque, M.A.; Burritt, D.J.; Fujita, M. Proline protects plants against abiotic oxidative stress: Biochemical and molecular mechanisms. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 477–522. [Google Scholar] [CrossRef]
- Degenkolbe, T.; Do, P.T.; Kopka, J.; Zuther, E.; Hincha, D.K.; Köhl, K.I. Identification of drought tolerance markers in a diverse population of rice cultivars by expression and metabolite profiling. PLoS ONE 2013, 8, e63637. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Chen, X.; Li, H.; Huang, J.; Liu, Y.; Zhao, A. Different adaptive patterns of wheat with different drought tolerance under drought stresses and rehydration revealed by integrated metabolomic and transcriptomic analysis. Front. Plant Sci. 2022, 13, 1008624. [Google Scholar] [CrossRef] [PubMed]
- Večeřová, K.; Oravec, M.; Puranik, S.; Findurová, H.; Veselá, B.; Opoku, E.; Ofori-Amanfo, K.K.; Klem, K.; Urban, O.; Sahu, P.P. Single and interactive effects of variables associated with climate change on wheat metabolome. Front. Plant Sci. 2022, 13, 1002561. [Google Scholar] [CrossRef]
- Marček, T.; Hamow, K.A.; Végh, B.; Janda, T.; Darko, E. Metabolic response to drought in six winter wheat genotypes. PLoS ONE 2019, 14, e0212411. [Google Scholar] [CrossRef]
- Guo, X.; Xin, Z.; Yang, T.; Ma, X.; Zhang, Y.; Wang, Z.; Ren, Y.; Lin, T. Metabolomics response for drought stress tolerance in chinese wheat genotypes (Triticum aestivum). Plants 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Jin, Y.; Li, H.; Amombo, E.; Fu, J. Stress memory induced transcriptional and metabolic changes of perennial ryegrass (Lolium perenne) in response to salt stress. Physiol. Plant 2016, 156, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Silva, P.E.; Sanglard, L.M.V.P.; Ávila, R.T.; Morais, L.E.; Martins, S.C.V.; Nobres, P.; Patreze, C.M.; Ferreira, M.A.; Araújo, W.L.; Fernie, A.R.; et al. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017, 68, 4309–4322. [Google Scholar] [CrossRef]
- Stallmann, J.; Schweiger, R.; Pons, C.A.A.; Müller, C. Wheat growth, applied water use efficiency and flag leaf metabolome under continuous and pulsed deficit irrigation. Sci. Rep. 2020, 10, 10112. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Nemati, F.; Ghanati, F.; Ahmadi Gavlighi, H.; Sharifi, M. Comparison of sucrose metabolism in wheat seedlings during drought stress and subsequent recovery. Biol. Plant 2018, 62, 595–599. [Google Scholar] [CrossRef]
- Kutlu, İ.; Turhan, E.; Yorgancilar, Ö.; Yorgancilar, A. Differences of physiological parameters and sucrose metabolism in bread wheat genotypes exposed to drought stress. J. Anim. Plant Sci. 2021, 31, 998–1006. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Poething, R.S. Vegetative phase change and shoot maturation in plants. Curr. Top. Dev. Biol. 2013, 105, 125–152. [Google Scholar] [CrossRef]
- Wingler, A.; Quick, W.P.; Bungard, R.A.; Bailey, K.J.; Lea, P.J.; Leegood, R.C. The role of photorespiration during drought stress: An analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant Cell Environ. 1999, 22, 361–373. [Google Scholar] [CrossRef]
- Embiale, A.; Hussein, M.; Husen, A.; Sahile, S.; Mohammed, K. Differential sensitivity of Pisum sativum L. cultivars to water-deficit stress: Changes in growth, water status, chlorophyll fluorescence and gas exchange attributes. J. Agron. 2016, 15, 45–57. [Google Scholar] [CrossRef]
- Palmer, L.J.; Dias, D.A.; Boughton, B.; Roessner, U.; Graham, R.D.; Stangoulis, J.C.R. Metabolite profiling of wheat (Triticum aestivum L.) phloem exudate. Plant Methods 2014, 10, 27. [Google Scholar] [CrossRef]
- Heyneke, E.; Watanabe, M.; Erban, A.; Duan, G.; Buchner, P.; Walther, D.; Kopka, J.; Hawkesford, M.J.; Hoefgen, R. Characterization of the Wheat Leaf Metabolome during Grain Filling and under Varied N-Supply. Front. Plant Sci. 2017, 8, 2048. [Google Scholar] [CrossRef]
- Sedlacko, E.M.; Heuberger, A.L.; Chaparro, J.M.; Cath, T.Y.; Higgins, C.P. Metabolomics reveals primary response of wheat (Triticum aestivum) to irrigation with oilfield produced water. Environ. Res. 2022, 212, 113547. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Głowacka, K.; Stałanowska, K.; Railean-Plugaru, V.; Horbowicz, M.; Pomastowski, P.; Buszewski, B. The Effect of Bio-Synthesized Silver Nanoparticles on Germination, Early Seedling Development, and Metabolome of Wheat (Triticum aestivum L.). Molecules 2022, 27, 2303. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Stałanowska, K.; Głowacka, K.; Horbowicz, M. The Size-Dependent Effects of Silver Nanoparticles on Germination, Early Seedling Development and Polar Metabolite Profile of Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 13255. [Google Scholar] [CrossRef] [PubMed]
- Kameli, A.; Lösel, D.M. Growth and sugar accumulation in durum wheat plants under water stress. New Phytol. 1996, 132, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Valluru, R.; Van den Ende, W. Plant fructans in stress environments: Emerging concepts and future prospects. J. Exp. Bot. 2008, 59, 2905–2916. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W.; Michiels, A.; De Roover, J.; Van Laere, A. Fructan biosynthetic and breakdown enzymes in dicots evolved from different invertases. Expression of fructan genes throughout chicory development. Sci. World J. 2002, 11, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, P.S.A.; Wasonga, D.O.; Solano Hernandez, A.; Santanen, A. Seedling growth and phosphorus uptake in response to different phosphorus sources. Agronomy 2020, 10, 1089. [Google Scholar] [CrossRef]
- Römer, W.; Schilling, G. Phosphorus requirements of the wheat plant in various stages of its life cycle. Plant Soil. 1986, 91, 221–229. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Influence of drought and heat stress, applied independently or in combination during seed development, on qualitative and quantitative aspects of seeds of lentil (Lens culinaris Medikus) genotypes, differing in drought sensitivity. Plant Cell Environ. 2019, 42, 198–211. [Google Scholar] [CrossRef]
- Pelleschi, S.; Rocher, J.P.; Prioul, K.L. Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environ. 1997, 20, 493–503. [Google Scholar] [CrossRef]
- Silvente, S.; Sobolev, A.P.; Lara, M. Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. PLoS ONE 2012, 7, e38554. [Google Scholar] [CrossRef]
- Matos, M.C.; Campos, P.S.; Passarinho, J.A.; Semedo, J.N.; Marques, N.M.; Ramalho, J.C.; Ricardo, C.P. Drought effect on photosynthetic activity, osmolyte accumulation and membrane integrity of two Cicer arietinum genotypes. Photosynthetica 2010, 48, 303–312. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Schwabe, A.F.; Erban, B.A.; Udvardi, M.K.; Kopka, J. Comparative metabolomics of drought acclimation in model and forage legumes. Plant Cell Environ. 2012, 35, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Yüce, M.; Gökçe, Z.N.Ö.; Budak, H. Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genom. 2017, 18, 969. [Google Scholar] [CrossRef] [PubMed]
- Winter, H.; Huber, S.C. Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 253–289. [Google Scholar] [CrossRef] [PubMed]
- De Roover, J.; Vandenbranden, K.; Van Laere, A.; Van den Ende, W. Drought induces fructan synthesis and 1-SSS (sucrose:sucrose fructosyltransferase) in roots and leaves of chicory seedlings (Cichorium intybus L.). Planta 2000, 210, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; James, A.R. Partitioning of sugars in Lolium perenne (perennial ryegrass) during drought and on rewatering. New Phytol. 1999, 142, 295–305. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Zhang, J.Y.; de Carvalho, M.H.C.; Torres-Jerez, I.; Kang, Y.; Allen, S.N.; Huhman, D.V.; Tang, Y.; Murray, J.; Sumner, L.W.; Udvardi, M.K. Global reprogramming of transcription and metabolism in Medicago truncatula during progressive drought and after rewatering. Plant Cell Environ. 2014, 37, 2553–2576. [Google Scholar] [CrossRef]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef]
- Simon-Sarkadia, L.; Kocsy, G.; Várhegyi, A.; Galiba, G.; de Ronde, J.A. Effect of drought stress at supraoptimal temperature on polyamine concentrations in transgenic soybean with increased proline levels. Z. Nat. C 2006, 61, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Jacques, C.; Salon, C.; Barnard, R.L.; Vernoud, V.; Prudent, M. Drought Stress Memory at the Plant Cycle Level: A Review. Plants 2021, 10, 1873. [Google Scholar] [CrossRef]
- Fernie, A.R.; Martinoia, E. Malate: Jack of all trades or master of a few? Phytochemistry 2009, 70, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Q. Methods for the study of water relations under desiccation stress. In Desiccation and Survival in Plants; Black, M., Pritchard, H.W., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 47–91. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B.; Stałanowska, K.; Horbowicz, M. The Imbibition of Pea (Pisum sativum L.) Seeds in Silver Nitrate Reduces Seed Germination, Seedlings Development and Their Metabolic Profile. Plants 2022, 11, 1877. [Google Scholar] [CrossRef]
- Sun, X.; Weckwerth, W. COVAIN: A toolbox for uni- and multivariate statistics, time-series and correlation network analysis and inverse estimation of the differential Jacobian from metabolomics covariance data. Metabolomics 2012, 8, 81–93. [Google Scholar] [CrossRef]

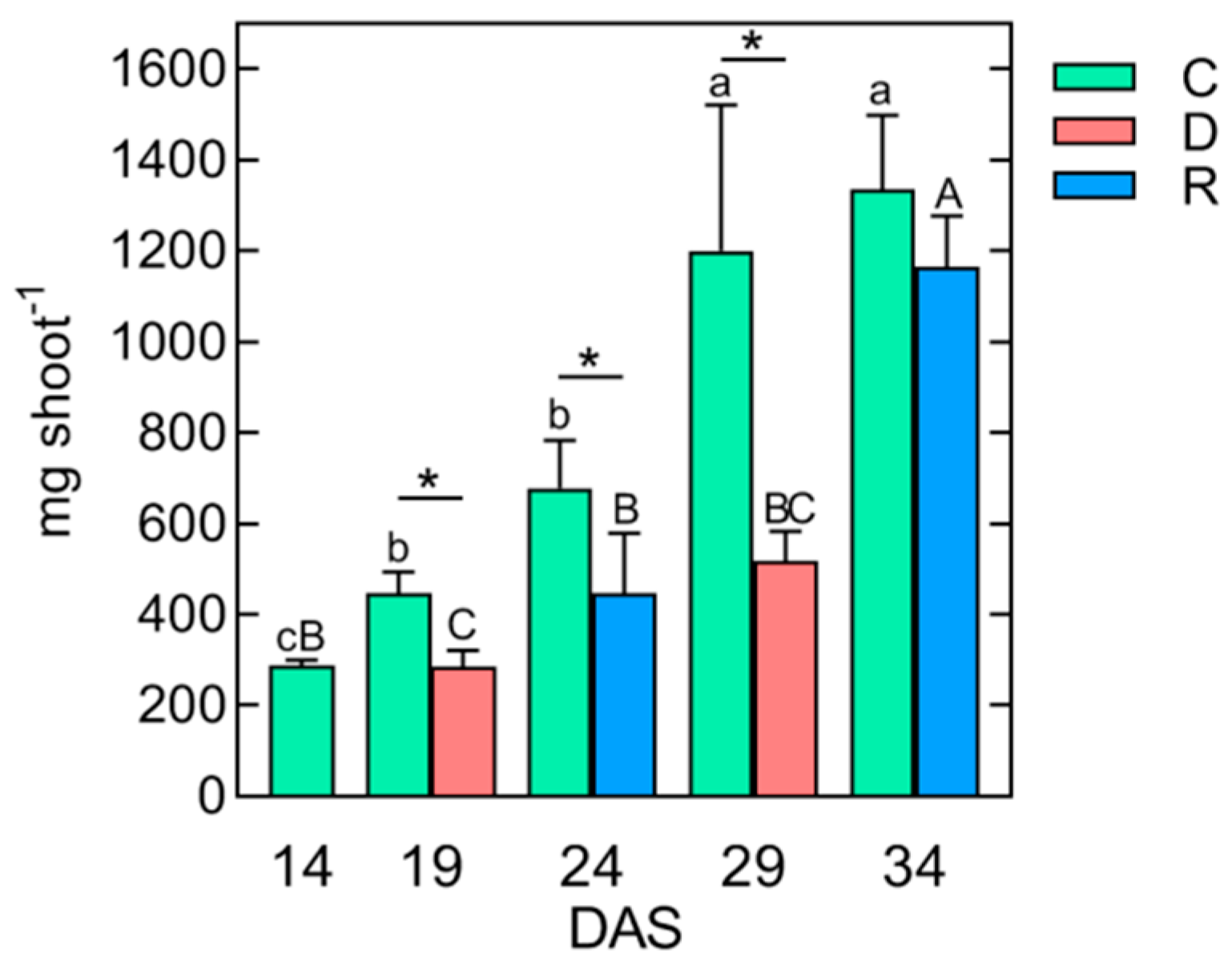
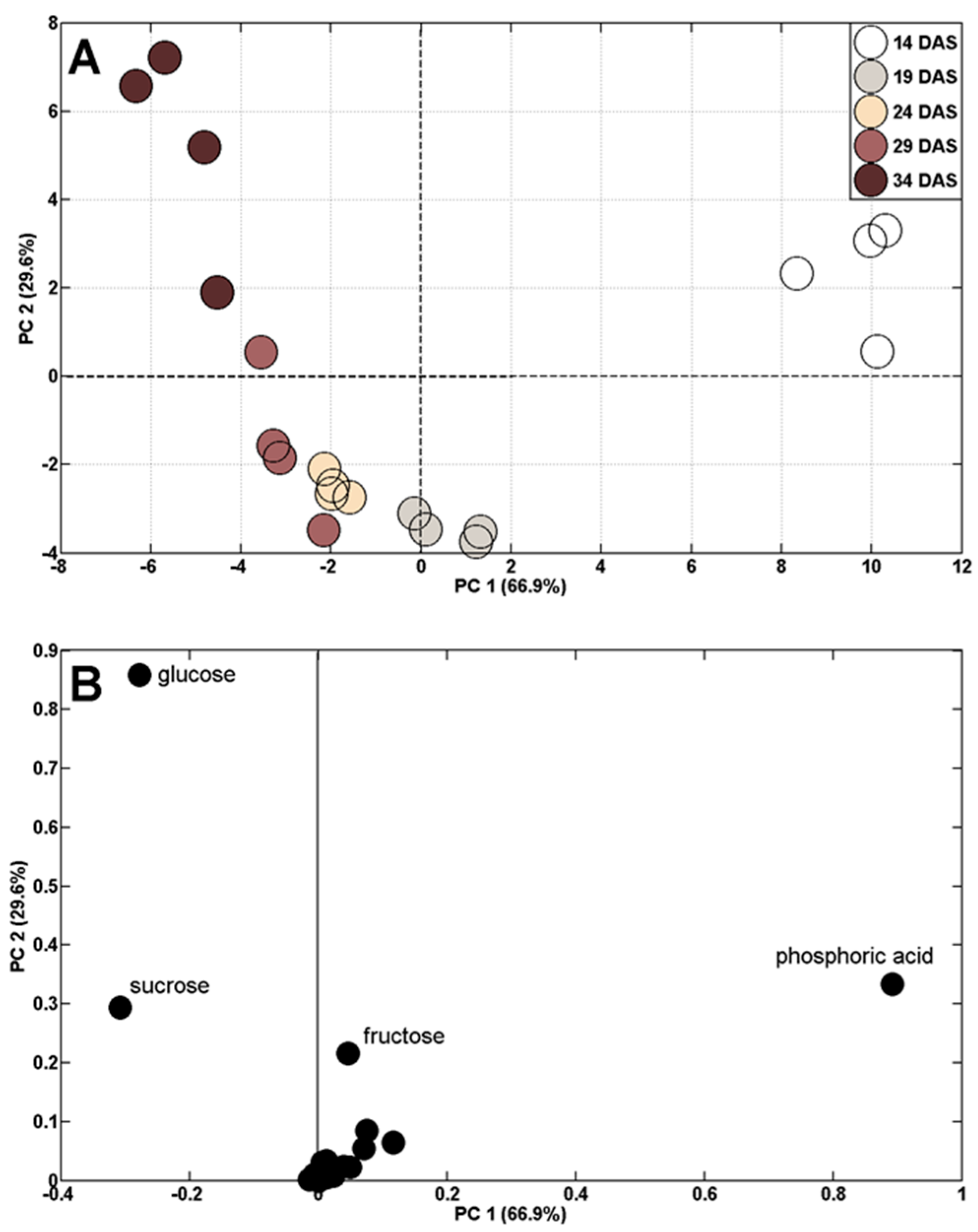
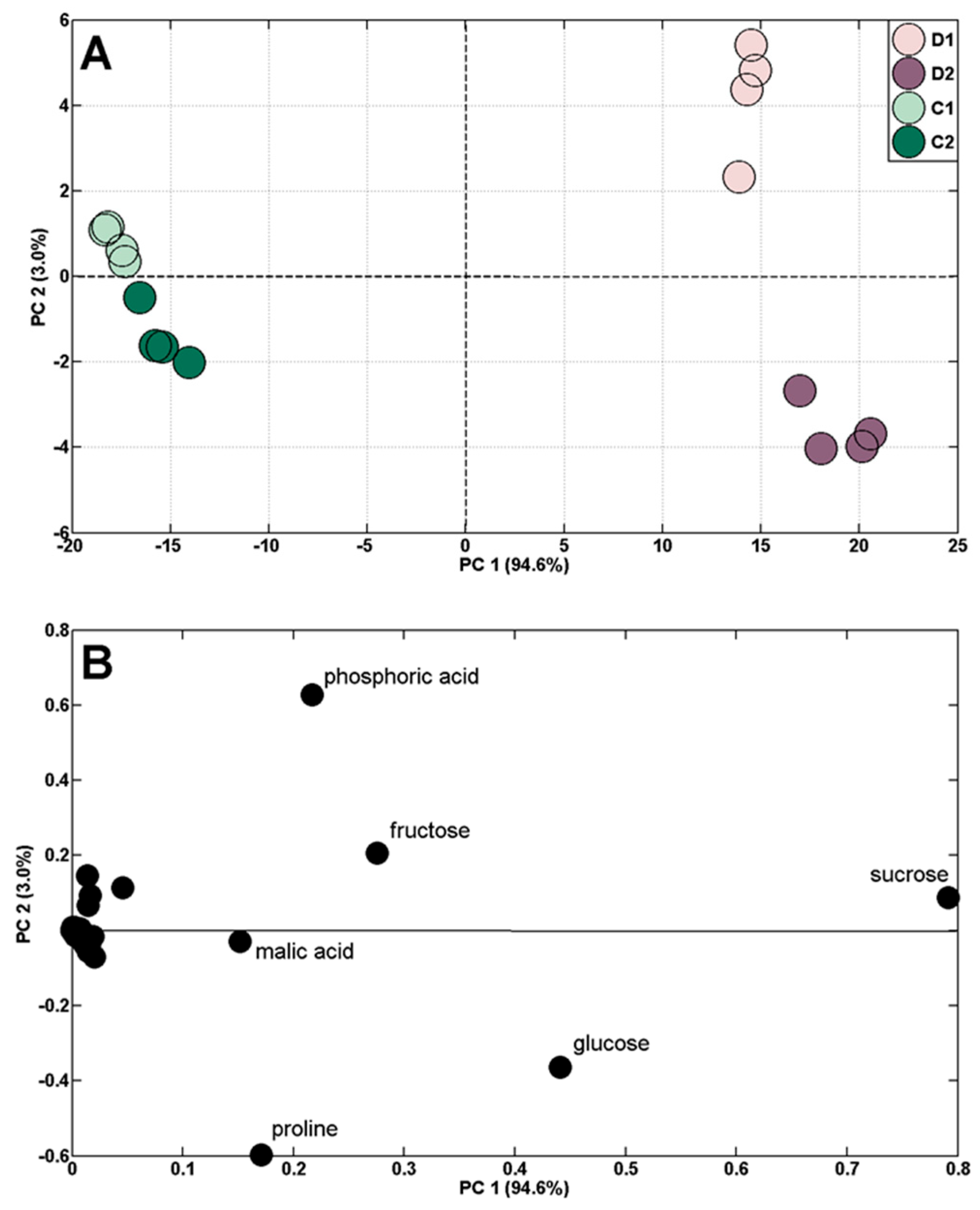

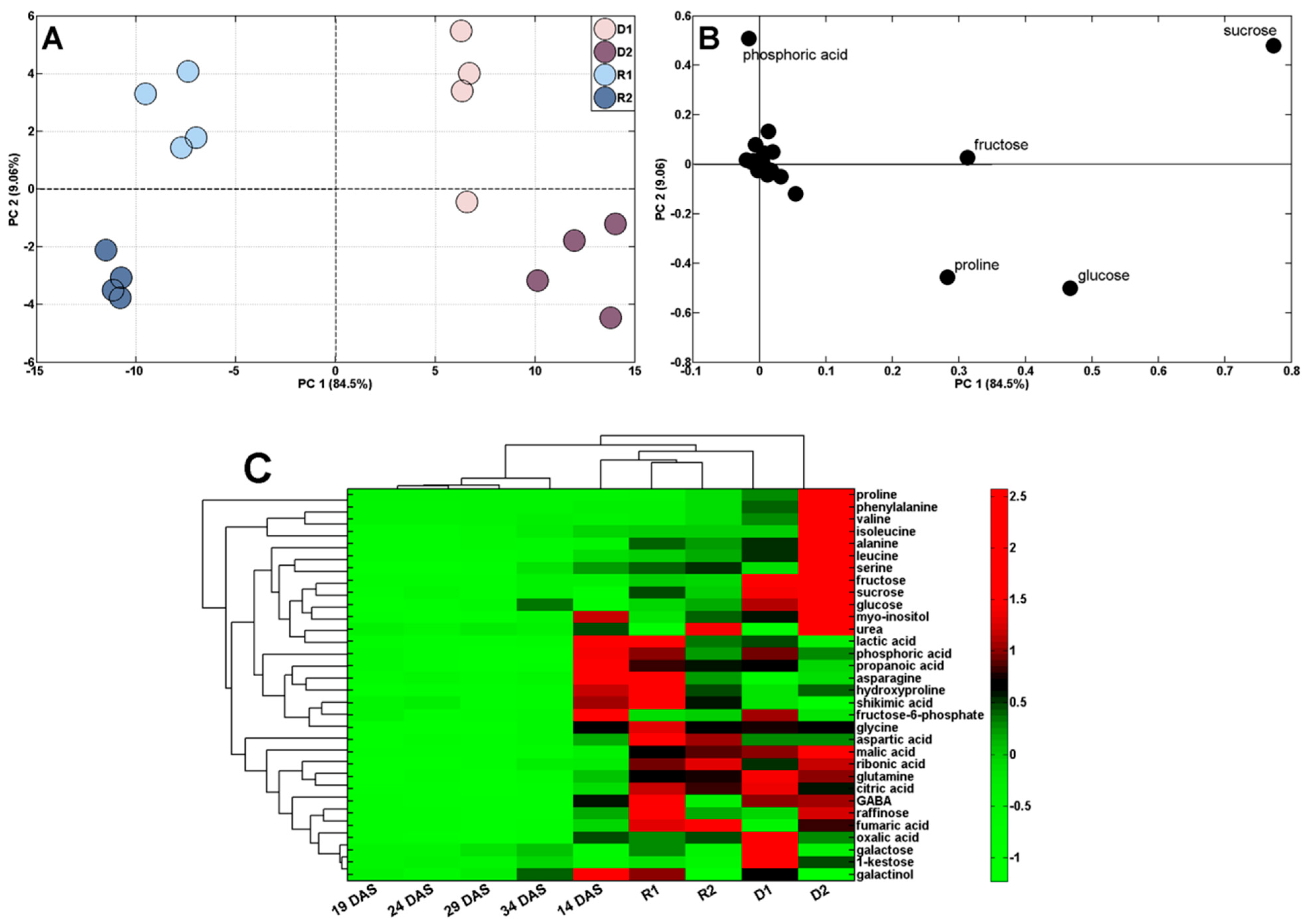
| Control | Drought (D)/Rewatering (R) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DAS | 14 | 19 | 24 | 29 | 34 | D1 14–19 | R1 19–24 | D2 24–29 | R2 29–34 |
| Metabolites | mg g−1 DW | ||||||||
| TIPMs, including: | 33.74 aE | 11.99 c | 14.47 c | 14.19 c | 26.96 b | 84.18 B* | 57.56 C* | 95.65 A* | 48.52 D* |
| TSCs, including: | 12.05 bE | 6.12 c | 9.75 bc | 10.84 b | 22.17 a | 59.18 B* | 33.09 C* | 64.12 A* | 28.20 D* |
| fructose | 2.50 aB | 1.16 b | 0.85 b | 1.14 b | 2.45 a | 10.85 A* | 4.18 B* | 10.09 A* | 3.97 B* |
| galactose | 0.00 aB | 0.04 a | 0.05 a | 0.18 a | 0.27 a | 1.21 A* | 0.37 B | 0.10 B | 0.07 B |
| glucose | 3.92 bD | 2.00 b | 2.53 b | 4.35 b | 10.70 a | 15.45 B* | 8.06 C* | 19.76 A* | 9.45 C |
| sucrose | 2.43 cD | 2.17 c | 5.73 b | 4.56 b | 7.63 a | 28.24 A* | 17.70 B* | 31.22 A* | 12.40 C* |
| galactinol | 0.10 aA | 0.03 c | 0.02 c | 0.03 c | 0.06 b | 0.07 B* | 0.08 AB* | 0.00 C* | 0.00 C* |
| raffinose | 0.53 aBC | 0.19 b | 0.23 b | 0.17 b | 0.17 b | 0.45 C* | 1.11 A* | 0.89 AB* | 0.52 BC* |
| 1-kestose | 0.00 bC | 0.02 b | 0.04 b | 0.02 b | 0.14 a | 0.78 A* | 0.11 BC* | 0.27 B* | 0.00 C* |
| myo-inositol | 0.40 aA | 0.09 bc | 0.06 c | 0.09 bc | 0.15 b | 0.32 B* | 0.20 C* | 0.46 A* | 0.29 B* |
| ribonic acid | 0.40 aC | 0.11 b | 0.17 b | 0.20 b | 0.39 a | 0.66 B* | 0.80 A* | 0.87 A* | 0.91 A* |
| fructose-6-phosphate | 1.77 aA | 0.31 b | 0.08 b | 0.09 b | 0.21 b | 1.14 B* | 0.49 C* | 0.46 C* | 0.58 C* |
| TPAAs, including: | 2.18 aC | 0.42 c | 0.56 c | 0.60 c | 1.05 b | 4.27 B* | 2.78 C* | 13.40 A* | 3.33 BC* |
| alanine | 0.05 aB | 0.02 a | 0.02 a | 0.04 a | 0.03 a | 0.13 B* | 0.12 B* | 0.26 A* | 0.11 B* |
| asparagine | 0.68 aA | 0.10 b | 0.13 b | 0.10 b | 0.14 b | 0.11 C | 0.67 A* | 0.25 BC* | 0.33 B* |
| aspartic acid | 0.29 aC | 0.05 c | 0.10 bc | 0.09 bc | 0.13 b | 0.31 C* | 0.58 A* | 0.30 C* | 0.44 B* |
| glutamine | 0.05 aA | 0.01 c | 0.02 c | 0.01 c | 0.03 b | 0.10 A* | 0.08 A* | 0.09 A* | 0.08 A* |
| glycine | 0.24 aB | 0.06 c | 0.07 c | 0.06 c | 0.11 b | 0.24 B* | 0.30 A* | 0.24 B* | 0.24 B* |
| isoleucine | 0.20 aB | 0.04 c | 0.05 c | 0.08 c | 0.15 b | 0.21 B* | 0.21 B* | 0.78 A* | 0.22 B* |
| leucine | 0.12 aB | 0.02 b | 0.03 b | 0.03 b | 0.04 b | 0.22 B* | 0.14 B* | 0.50 A* | 0.17 B* |
| phenylalanine | 0.03 aC | 0.01 b | 0.01 b | 0.01 b | 0.01 b | 0.16 B* | 0.03 C* | 0.53 A* | 0.07 C* |
| proline | 0.04 aD | 0.02 b | 0.01 b | 0.01 b | 0.01 b | 2.17 B* | 0.09 D* | 8.50 A* | 1.02 C* |
| serine | 0.43 aB | 0.09 b | 0.10 b | 0.14 b | 0.33 a | 0.34 B* | 0.48 B* | 0.93 A* | 0.52 B* |
| valine | 0.05 abC | 0.01 c | 0.01 c | 0.04 bc | 0.07 a | 0.27 B* | 0.08 C* | 1.01 A* | 0.15 C* |
| NTPAAs, including: | 1.61 aAB | 0.32 b | 0.34 b | 0.35 b | 0.47 b | 0.98 C* | 2.02 A* | 1.27 BC* | 1.14 BC* |
| hydroxyproline | 1.39 aAB | 0.27 b | 0.31 b | 0.32 b | 0.43 b | 0.70 C* | 1.69 A* | 0.99 BC* | 1.04 BC* |
| GABA | 0.22 aB | 0.05 b | 0.04 b | 0.03 b | 0.04 b | 0.27 AB* | 0.34 A* | 0.28 AB* | 0.10 C* |
| TOAs, including: | 3.96 aB | 1.47 bc | 1.60 bc | 1.26 c | 1.82 b | 8.10 A* | 7.66 A* | 8.43 A* | 7.62 A* |
| citric acid | 0.96 aB | 0.33 b | 0.28 b | 0.23 b | 0.29 b | 2.18 A* | 2.06 A* | 1.55 AB* | 1.77 AB* |
| fumaric acid | 0.07 aC | 0.04 b | 0.04 b | 0.03 b | 0.04 b | 0.05 C* | 0.14 AB* | 0.11 B* | 0.15 A* |
| malic acid | 2.26 aC | 0.93 c | 1.13 bc | 0.92 c | 1.36 b | 5.39 B* | 4.77 B* | 6.53 A* | 5.22 B* |
| oxalic acid | 0.06 aB | 0.02 b | 0.01 c | 0.01 c | 0.02 b | 0.13 A* | 0.06 B* | 0.06 B* | 0.07 B* |
| lactic acid | 0.13 aAB | 0.03 b | 0.02 b | 0.02 b | 0.03 b | 0.09 ABC* | 0.14 A* | 0.05 C* | 0.08 BC* |
| propanoic acid | 0.23 aA | 0.06 b | 0.04 bc | 0.02 c | 0.04 bc | 0.15 B* | 0.16 B* | 0.10 C* | 0.14 BC* |
| shikimic acid | 0.24 aB | 0.07 b | 0.09 b | 0.03 c | 0.05 c | 0.11 C | 0.34 A* | 0.03 D | 0.19 B* |
| TRCs, including: | 13.94 aA | 3.65 b | 2.21 c | 1.14 d | 1.45 cd | 11.65 A* | 12.00 A* | 8.43 B* | 8.23 B* |
| phosphoric acid | 13.93 aA | 3.65 b | 2.21 c | 1.13 d | 1.44 cd | 11.65 A* | 12.00 A* | 8.40 B* | 8.21 B* |
| urea | 0.01 aB | 0.00 a | 0.00 a | 0.01 a | 0.00 a | 0.00 C | 0.00 C* | 0.02 A | 0.02 A* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szablińska-Piernik, J.; Lahuta, L.B. Polar Metabolites Profiling of Wheat Shoots (Triticum aestivum L.) under Repeated Short-Term Soil Drought and Rewatering. Int. J. Mol. Sci. 2023, 24, 8429. https://doi.org/10.3390/ijms24098429
Szablińska-Piernik J, Lahuta LB. Polar Metabolites Profiling of Wheat Shoots (Triticum aestivum L.) under Repeated Short-Term Soil Drought and Rewatering. International Journal of Molecular Sciences. 2023; 24(9):8429. https://doi.org/10.3390/ijms24098429
Chicago/Turabian StyleSzablińska-Piernik, Joanna, and Lesław Bernard Lahuta. 2023. "Polar Metabolites Profiling of Wheat Shoots (Triticum aestivum L.) under Repeated Short-Term Soil Drought and Rewatering" International Journal of Molecular Sciences 24, no. 9: 8429. https://doi.org/10.3390/ijms24098429
APA StyleSzablińska-Piernik, J., & Lahuta, L. B. (2023). Polar Metabolites Profiling of Wheat Shoots (Triticum aestivum L.) under Repeated Short-Term Soil Drought and Rewatering. International Journal of Molecular Sciences, 24(9), 8429. https://doi.org/10.3390/ijms24098429






