Abstract
In the central nervous system (CNS) there are a greater number of glial cells than neurons (between five and ten times more). Furthermore, they have a greater number of functions (more than eight functions). Glia comprises different types of cells, those of neural origin (astrocytes, radial glia, and oligodendroglia) and differentiated blood monocytes (microglia). During ontogeny, neurons develop earlier (at fetal day 15 in the rat) and astrocytes develop later (at fetal day 21 in the rat), which could indicate their important and crucial role in the CNS. Analysis of the phylogeny reveals that reptiles have a lower number of astrocytes compared to neurons and in humans this is reversed, as there have a greater number of astrocytes compared to neurons. These data perhaps imply that astrocytes are important and special cells, involved in many vital functions, including memory, and learning processes. In addition, astrocytes are involved in different mechanisms that protect the CNS through the production of antioxidant and anti-inflammatory proteins and they clean the extracellular environment and help neurons to communicate correctly with each other. The production of inflammatory mediators is important to prevent changes in brain homeostasis. On the contrary, excessive, or continued production appears as a characteristic element in many diseases, such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), and in neurodevelopmental diseases, such as bipolar disorder, schizophrenia, and autism. Furthermore, different drugs and techniques have been developed to reverse oxidative stress and/or excess of inflammation that occurs in many CNS diseases, but much remains to be investigated. This review attempts to highlight the functional relevance of astrocytes in normal and neuropathological conditions by showing the molecular and cellular mechanisms of their role in the CNS.
1. Introduction
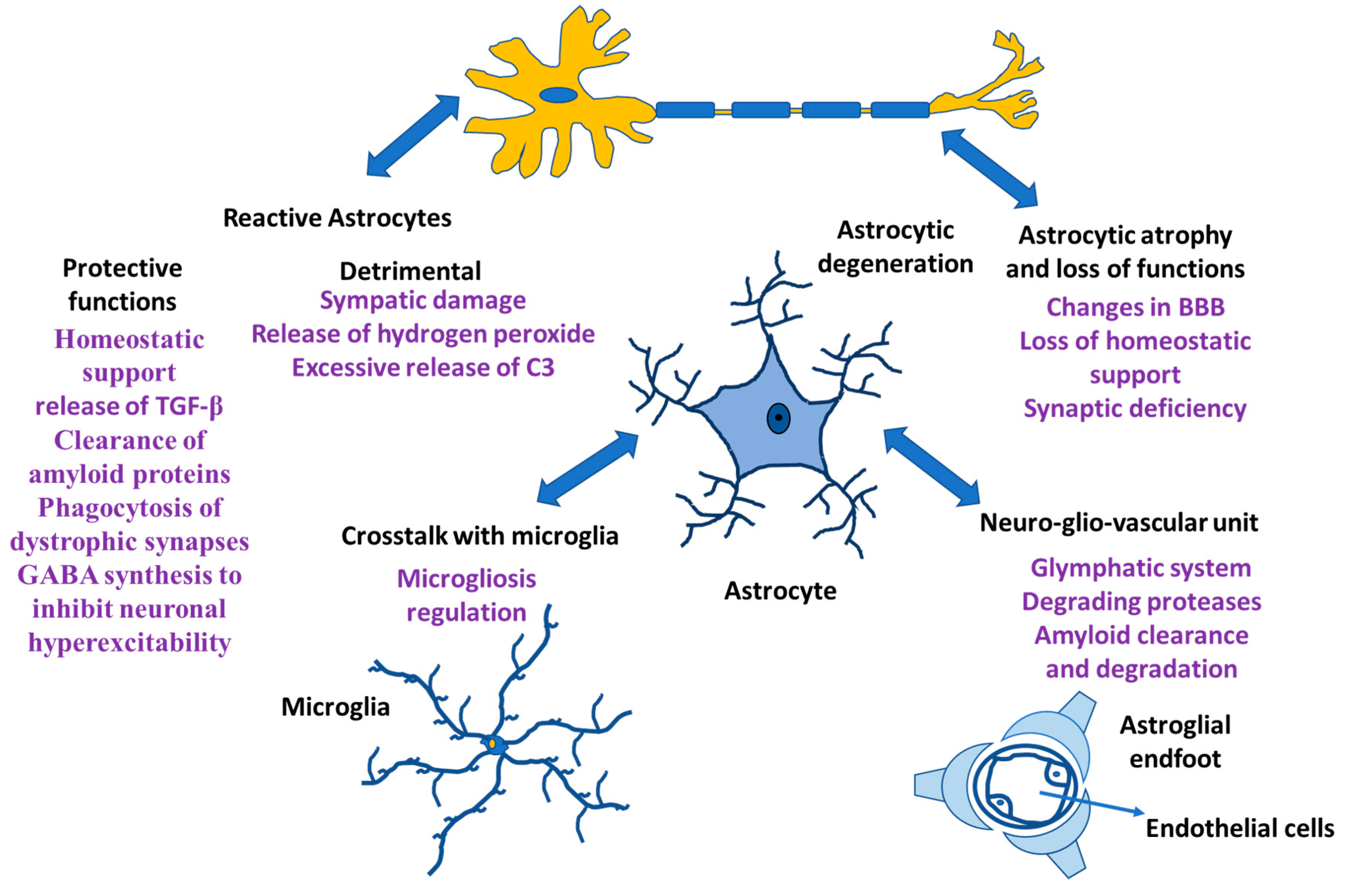
Neurons and glia (astrocytes, radial glia, oligodendroglia, and microglia) are the neural cells of the CNS. Glial cells have different functions; microglia are the resident macrophages in the CNS [1,2,3], oligodendrocytes are responsible for myelin production [4], NG2-positive glia is consistent with an oligodendrocyte progenitor function [5,6], and astrocytes play an important role both in homeostasis and in diseases [7,8,9]. Rudolf Virchow indicated that neuroglia was a connective tissue that embedded all the components of the central nervous system (CNS) [10,11,12]. The morphology and functionality of neuroglial cells were established in the 19th and early 20th century [13,14,15]. In addition, analysis of human evolution during the early 21st century revealed the role of neuroglia in the formation of the human intellect [16,17]. Likewise, the involvement of neuroglia in synaptic regulation and plasticity has been demonstrated, generating transient and even lasting changes in the force that can occur in a synapse [18,19]. At the synapse, astrocytes are involved in the regulation of ionic balance, neurotransmitter clearance and gliotransmitter release and they are involved in the maintenance of the blood–brain barrier (BBB), ion and water homeostasis and immune functions, allowing constant regulation of synapses [8,20,21,22]. Additionally, when functional connections fail, microglia and astrocytes remove dendritic processes and spines. Other glial cells, such as oligodendrocytes, form myelin sheaths in the CNS and regulate the speed of information propagation in the CNS, providing conduction plasticity, speed and efficiency in the exchange of information between neurons in a circuit [23]. In the CNS of Homo sapiens, neuroglia cells are predominant and within them, astrocytes constitute the main cells [24], which maintains the homeostasis of the CNS and failure in their functions causes different pathologies in the CNS (Figure 1).
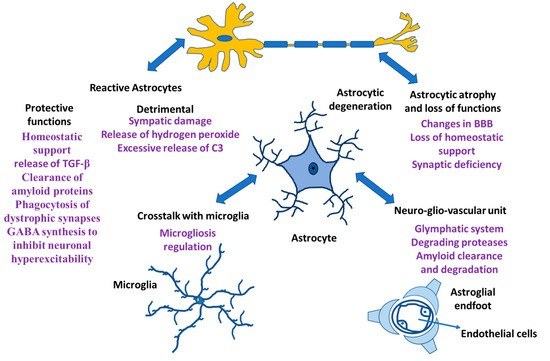
Figure 1.
Astrocyte functions. Astrocytes and their relationship with other cells both in physiological situation and in neurological damage.
In our manuscript, we show that inflammation is an important mechanism in Alzheimer’s disease. Though previous authors indicated that inflammation is characteristic of this disease, the number of proteins involved and the strong relationship with neurons have been poorly studied. Many chemokines and cytokines are involved, and the relationship between these and the different cells inside the CNS could explain the development of many neurodegenerative diseases. Furthermore, the different roles that astrocytes play in disease remain a priority for many researchers.
The importance of this work lies in showing the functions that astrocytes fulfill in the nervous system. This manuscript introduces the idea that astrocytes participate in memory in a notable way, even in neurodegenerative diseases, causing an affectation in the amnesic cognitive deterioration of patients. In addition, it is indicated that the mechanisms and understanding of the relationship between all cells of the CNS could help to achieve a therapeutic pathway in neurodegenerative and developmental disorders. Moreover, in this review we will discuss how astrocytes contribute to CNS functions owing to their structural properties and their specific functions. This review is not exhaustive and tries to highlight the current state of the field.
2. Astrocyte Properties and Functions
Astrocytes contact different populations around neurons, and this is true for protoplasmic astrocytes, which normally contact neurons in the gray matter, and for fibrous astrocytes that contact neuronal extensions [25]. The functions performed by astrocytes are, among others, to control the sleep process, form the extracellular matrix, serve as a support, build and regulate the blood–brain barrier, maintain the balance of extracellular ions, control the production of neurotransmitters, and modulate the synapse (tripartite synapse hypothesis) [26,27]. Memory consolidation occurs during the sleep period and astrocytes can control this process by communicating with other brain cells. During the sleep period they clean toxins, neurotransmitters and release water, ions, and molecules [28].
We believe that neurons and astrocytes are the essential elements in all regulating the processes related to memory of lived situations or dreams. In the CNS, only neurons can produce electrical impulses and therefore help in memory formation and storage. Just like when we turn off a computer and turn it on again after two years, the information stored in it remains, memory created via the communications between neurons remains. We do not know how long-term memory is produced and how memories are maintained but it is established that neurons need to be active all the time to carry out electrical impulses for that memory to be created and stored. Does this imply that we need to have neurons firing constantly if we want to remember something? Astrocytes modulate Ca2+ variations and it is possible that using other pathways and signals they are able to communicate with each other and with neurons over time [29]. In fact, in neurodevelopmental disorders, such as autism [30], different forms of schizophrenia [31], and early onset bipolar disorder [32], the number and morphology of astrocytes changes from one situation to another. In bipolar disorder, patients have an increase in brain functions when they are in the optimistic phase and, when they are in the depressive phase they almost find it impossible to register and develop any work in the brain. The change from an optimistic situation to that of depression is not prompt; it takes days, weeks and/or even months, but it is constant. The functions of the neurons cannot explain the above phenomenon since they are cells that at times are activated or at times not (the cell fires or not), and the modulation of the neuronal action potential is impossible. On the contrary, there is a network between the astrocytes and when necessary, a great communication between them and with the neurons, oligodendroglia and microglia is initiated, which is maintained over time [29]. In the evolution of Homo sapiens, the increase in size and complexity of astrocytes coincided with an increase in intelligence [16]. Furthermore, the difference between humans and rats found is the diameter of the average domain of a protoplasmic astrocyte (2.5 times larger), the volume (bigger in humans) and the arborization. Moreover, fibrous astrocytes are 2.3 times larger in humans than in rats [16,33,34].
Currently, a new field has been opened for the study of the role of astrocytes in the nervous system and their possible role in memory. This could provide a new direction for future interventions in CNS diseases.
3. Astrocytes Functions and Type of Processes
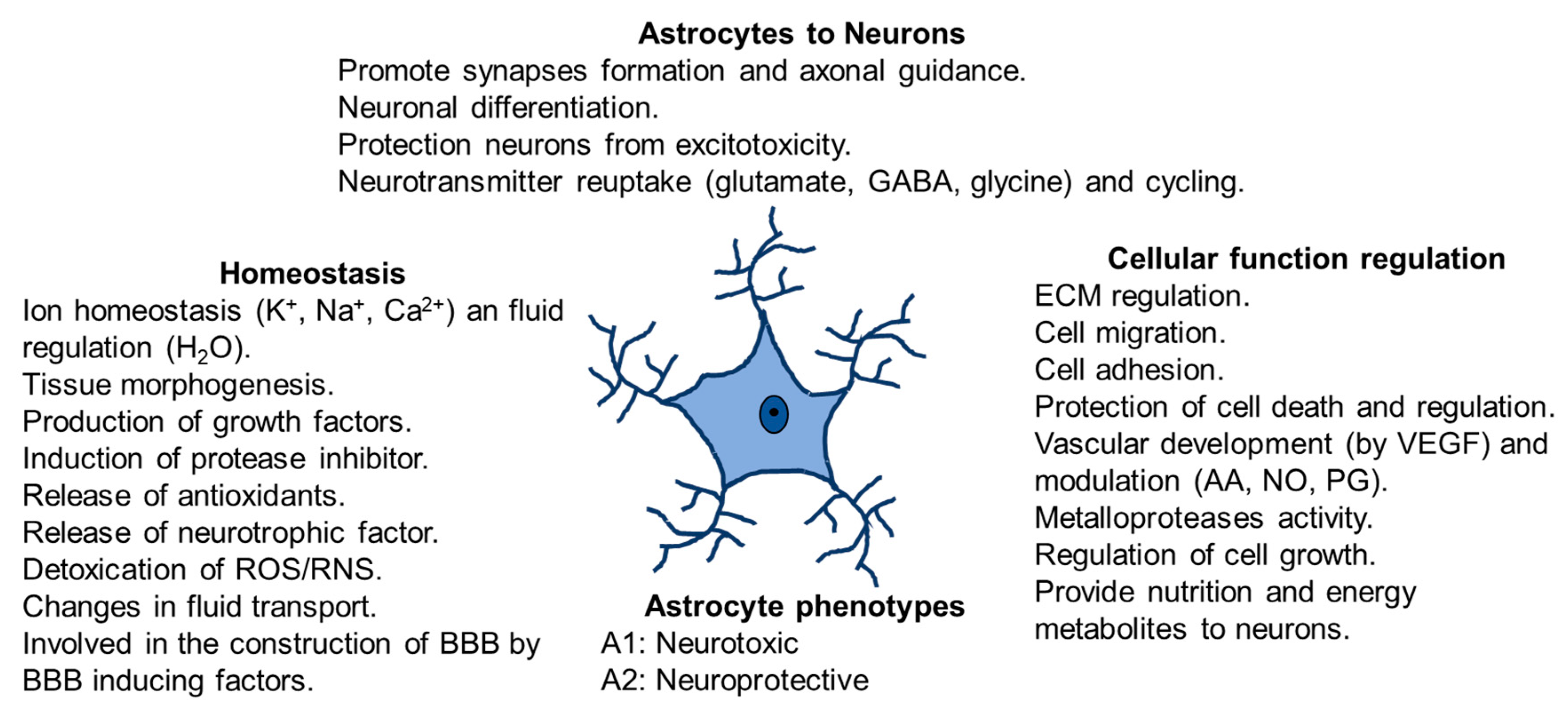
Astrocytes respond to changes in their microenvironment because they have two types of processes: on one hand, perisynaptic processes, with fine extensions to the interneuronal synapse and, on the other hand, vascular processes with final lengthenings (endfeet) communicating with blood vessels. Perisynaptic processes express receptors for neurotransmitters (reuptake glutamate, GABA and glycine), cytokines, chemokines, growth factors, and ion channels. In addition, they possess the glutamate transporters and receptors that control neuronal glutamatergic neurotransmission [35,36]. The endfeet in astrocytes expresses glucose transporters and aquaporins 4 and covers most of the blood vessels [36]. Moreover, astrocytes are territorial cells with processes controlling only a few overlaps between neighboring astrocytes [37], which are interconnected into functional networks [16,35]. It has been demonstrated that astrocyte cells maintain contact with up to 2,000,000 synapses [17,29] and this interaction depends on changes in neuronal activity. Furthermore, these astrocytes offer energy to neurons via lactate shuttle [38,39] and modulate Ca2+ variations [40] (Figure 2). Moreover, they protect neurons from excitotoxicity and help the neuronal differentiation. Astrocytes regulate cellular function controlling extracellular matrix (ECM), cell migration, cell adhesion and cell growth. Furthermore, they control vascular development by producing vascular endothelial growth factor (VEGF) and modulating production of arachidonic acid (AA), nitric oxide (NO) and prostaglandin (PG).
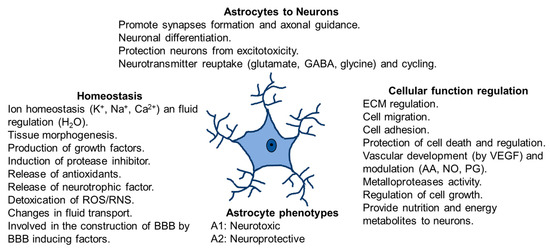
Figure 2.
Astrocytes functions in physiological conditions. Homeostasis, communication astrocytes-neurons, cellular function regulation and astrocytes phenotypes.
4. Toxic Clean Process by Astrocytes
Astrocytes change after inflammation or injury, thus becoming reactive. In this case, there are two types of astrocytes, hypertrophic astrocytes and those that form scars [35,41]. The changes affect different pathologies, such as Alzheimer’s disease (AD), Huntington’s disease, ischemic stroke, and epilepsy. In AD and multiple sclerosis (MS), a reduction in the brain’s weight is detected, with an increase in the grooves and loss of water in general. Astrocytic cells are known to swell with fluid during the day, which it sheds at night, cleaning the brain of toxins, proteins, and unwanted molecules. When the astrocytes are damaged, it is impossible to return water into the astrocytes, thus losing the ability to clean toxins during the wake period and increasing the presence of toxins in the following night [28].
5. Glutamine, Glutathione and Astrocytes
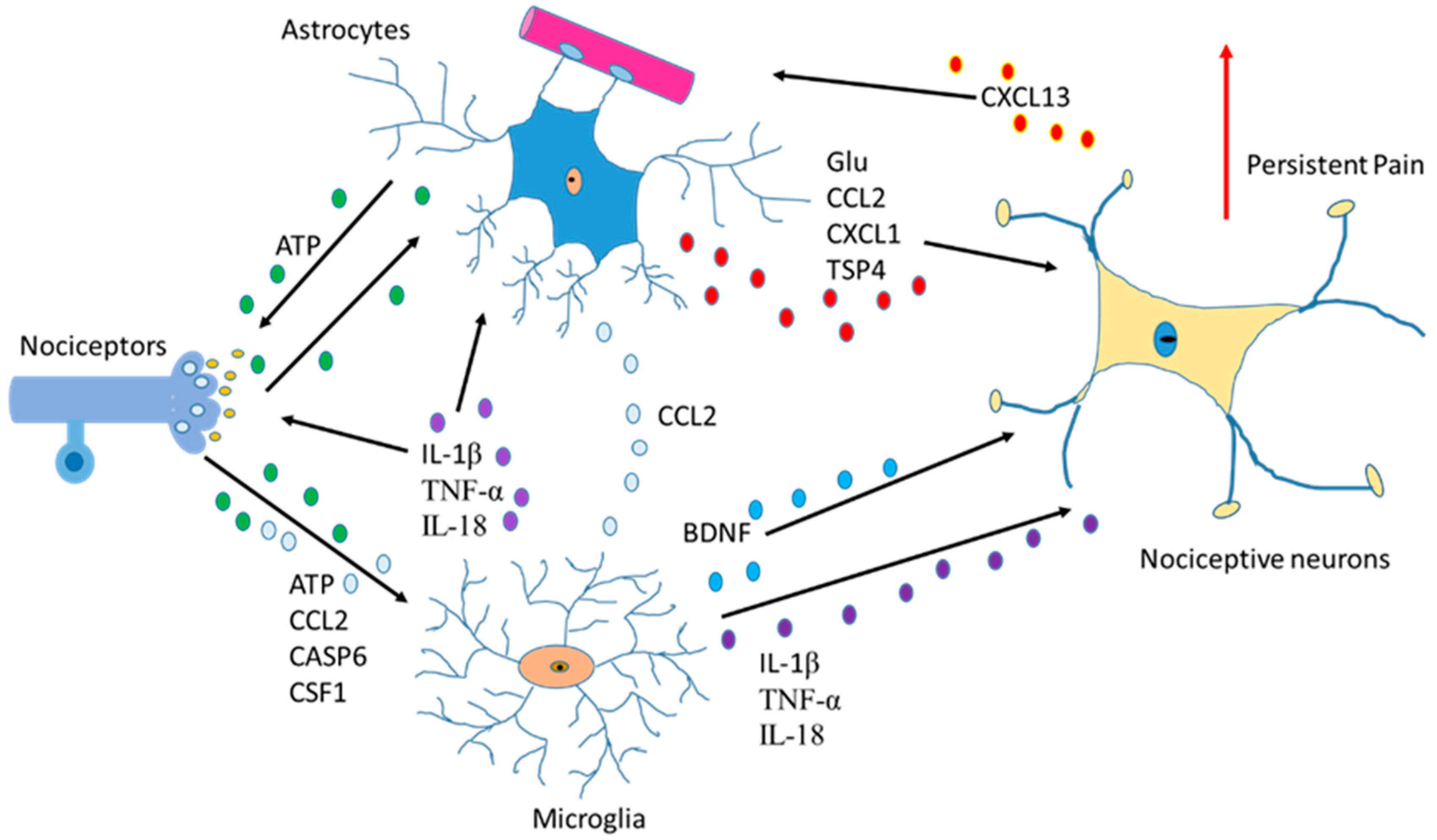
Another function of astrocytes is to supply glutamine to neurons and remove glutathione from the synaptic bouton. In AD, neurons die because of the hyperphosphorylation of TAU (protein π) (pTAU) protein. Astrocytes are probably involved in this process because glutamine reduction and/or glutathione removal may be affected in these patients. In addition, a reduction in ATP produced by damaged mitochondria is detected [28].To function adequately, our brain requires oxygen, which flows through the circulatory system to the extracellular space between brain cells. If the heart function is correct, enough oxygen goes into the brain crossing the blood–brain barrier. On the contrary, if enough oxygen does not go into the brain, problems in brain cell functionality will occur with decrement in the production of adenosine triphosphate (ATP). So, cardiology specialists, neurologists, psychologists, and medical specialists must remain vigilant from now on and in the future while diagnosing AD [42]. Moreover, since astrocytes are involved in non-physiological pain [43], discerning the communication between the cells of the sensory ganglia could be important in the treatment of chronic pain [44,45] (Figure 3).
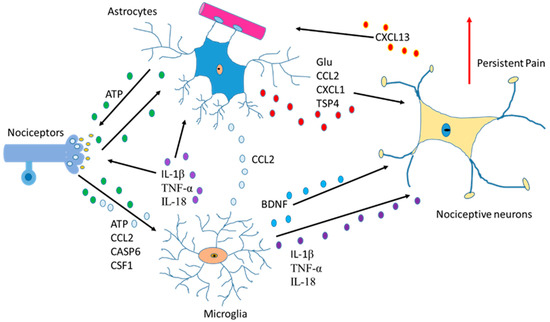
Figure 3.
Communication between astrocytes, neurons, and microglia in pain situations. Activation of nociceptors causes the induction of cytokines, chemokines, BDNF and neurotrophic factors producing changes an increase in persistent pain.
6. Gender Differences and Neuroglia
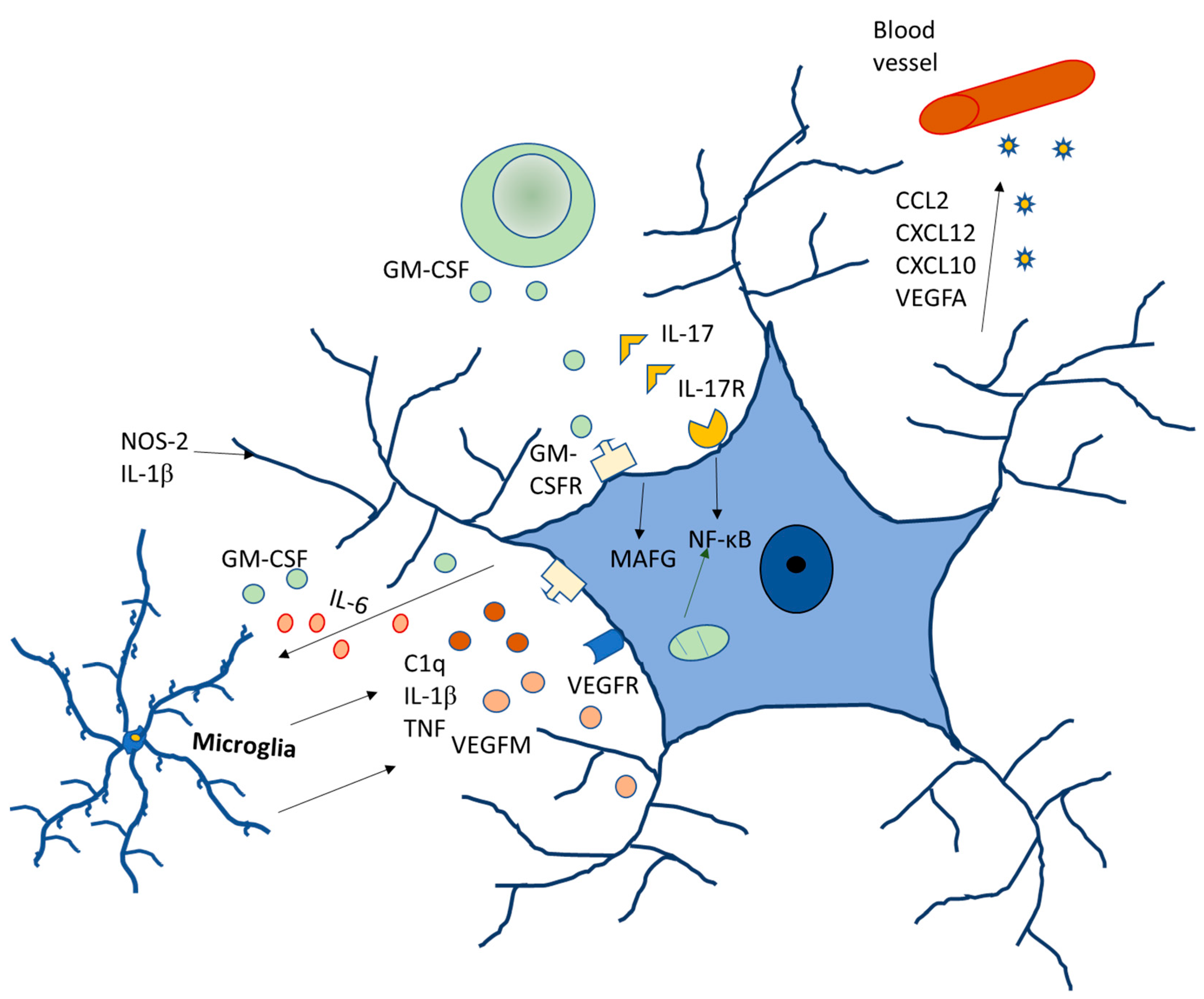
Regarding gender differences, data analyzed in rodents have shown significant differences in the glia between males and females [46,47]. In the male preoptic area, astrocytes are more ramified, with higher dendritic spine density. They also have a higher density of microglia, which has a reduced branching pattern. As compared to females, males are at a higher risk of developing neurodevelopment disorders, such as autism, different forms of schizophrenia and early onset bipolar disorder, and the role of glia in all of these disorders have already been established. Autism is four to eight times more common in males [48,49] with hyper-masculinized phenotypes [50] (Figure 4).
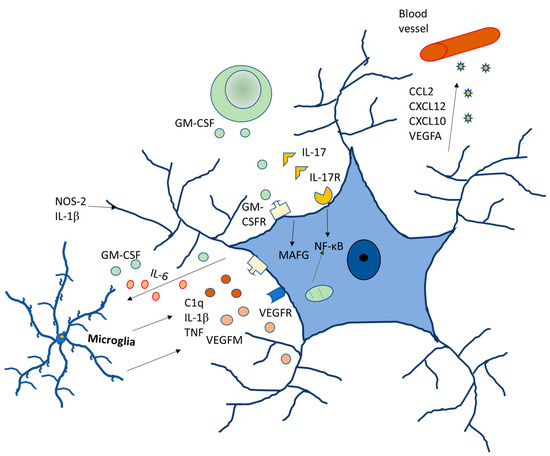
Figure 4.
Molecular signaling of glia modulating neurodegenerative diseases.
7. Astrocytes and Inflammation
Analogous to microglia, the role of astrocytes in inflammation has been studied. While these cells protect against cerebral ischemia, they appear to be ineffective against lipopolysaccharide-mediated inflammation (LPS). However, in retina cells, astrocytes have been demonstrated to induce an anti-inflammatory and neuroprotective effect through the production of lipoxins against acute and chronic lesions [51]. Furthermore, the cytokine IL-33 produced by astrocytes has an essential role in the development of neuronal circuits [52]. Other studies showed that activation of certain transcription factors is involved in producing protective (STAT3) [53] or injurious effects (NF-ᴋB) [54]. Moreover, there is a correlation between IL-1α and the greater number of GFAP-immunoreactive astrocytes [55]. On the other hand, in multiple sclerosis, TNF-α alters synaptic transmission, affecting the cognitive level [56].
Inflammatory signals are present in patients with mild cognition impairment (MCI) before they develop AD [57]. Inflammation is a crucial factor in AD progression as it is seen in activating microglia and increasing reactive astrocytes in these patients. Astrocytes can change their shape during hypertrophy and increase their ramifications, moving to the injury site [58]. Patients with AD present reactive astrocytes, as detected with PET (Positron Emission Tomography) imaging [59,60] and before the formation of plaques in APP transgenic mice [61].
In reactive astrocytes, the level of gliotransmitters (including glutamate, ATP, d-serine, and GABA) can inhibit neuronal activity [62]. In amyloid plaques, an increase in GABA protein has been detected in reactive astrocytes which surround the plaques and that triggers more release into the extracellular space [62,63]. There is a consensus that the role of GABA is protecting neural cells in the brain [63]. Furthermore, Delekate’s group showed that astrocytes in APP/PS1 mice (Mice carrying the human Swedish amyloid precursor protein and the Δe9 presenilin 1 mutation) increase the release of ATP surrounding the plaques. This happens because the Ca2+ concentration rises inside the cell [64]. The latter gives us the idea that an increase in ATP in astrocytes and neurons could help to reduce neuronal death that occurs in AD. The increase in the production of ATP by the mitochondria could help to recover from AD and decrease the development of the illness. The use of a cold laser could elevate the ATP produced by mitochondria as it can act on cytochrome c oxidase, increasing energy in the cells. This could help patients with mild cognitive impairment before they progress into AD [42]. However, in AD patients, the use of the laser could be unproductive in many cases because the cells are already dead. MS is a CNS (Central Nervous System) disorder characterized to be a chronic inflammatory autoimmune disease. Both environmental and genetic factors [65] are involved, and patients develop three states:
- (1)
- Presenting a relapsing–remitting clinical course, characterized by episodes of acute neurological dysfunctions followed by periods of recovery [66] (20–30% of patients).
- (2)
- Progression to a chronic secondary clinical stage, that is characterized by worsening and increased disability (50% of patients) [67].
- (3)
- In 15% of the patients, MS progresses to the clinical gradual reduction in neurological functions [68].
Animal models of MS disease show that it is an autoimmune inflammatory disorder occurring due to the recruitment of reactive lymphocytes, CD4+ T cells (Cluster of Quadruple Differentiation in T cells and the dendritic cells). Focusing on the three states, it seems that in the first state the bipolar relapsing and remitting states look similar to the illness produced by the virus chikungunya (CHIK) [69], or the plasmodium (as malaria) or a bacterial infection, with higher or lower episodes of affectation. The bipolar action will be related to the immune cells and their role in controlling the damage in different diseases.
The gliotransmitter glutamate is released by astrocytes when beta-amyloid is detected in the medium and causes loss of the neural spine and synaptic damage due to activation of NMDA (N-Methyl-D-Aspartate) receptors [70]. In addition, astrocytes release purines that can influence the development of AD or other diseases and activate the production of inflammatory proteins, decreasing anti-inflammatory proteins [71]. In a 7-month-old APP/PS1 mice, an increase in chemokines and their receptors has been detected compared to wild-type mice, demonstrating the role of cytokines and chemokines produced by microglia and astrocytes [71,72]. In addition, Valles’ group detected, for the first time, changes in the expression of CCR5 (Chemokine (C-C motif) Receptor 5) and CCR8 (Chemokine (C-C motif) Receptor 8) in these mice with a high production of β-amyloid [72]. These results demonstrate that changes in inflammatory protein expressions could affect neurodegeneration, brain development, cognition and memory [71,73]. Furthermore, using lipopolysaccharide (LPS) as an inducer, astrocytes increase the expression of many genes in the complement cascade [74]. On the other hand, the upregulation of trophic factors after ischemic damage has been shown to be a protective mechanism. However, the role of astrocytes could be different depending on the age and situation of these cells in our body and there are probably not only two states of astrocytes. Neurotrophic factors (NTF), and their action on neuronal survival have led to the search for new treatments for neurodegenerative diseases. One of them is the mesencephalic astrocyte-derived neurotrophic factor (MANF). This treatment is unique because its amino acid sequence and three-dimensional structure is different from other NTFs. MANF is secreted after stress or cell injury to promote neuronal survival. Its mechanism of action is unknown as well as its receptors. MANF is a neuroprotective protein with great therapeutic potential for PD [75] and AD [76]. PD is characterized by tremors, rigidity and bradykinesia, caused by the death of dopaminergic neurons in the substantia nigra of the brain. MANF protects against cell death through the PI3K/Akt/GSK3β pathway and by upregulating stress genes, such as HSP70 [77].
8. Astrocytes and Oxidative Stress
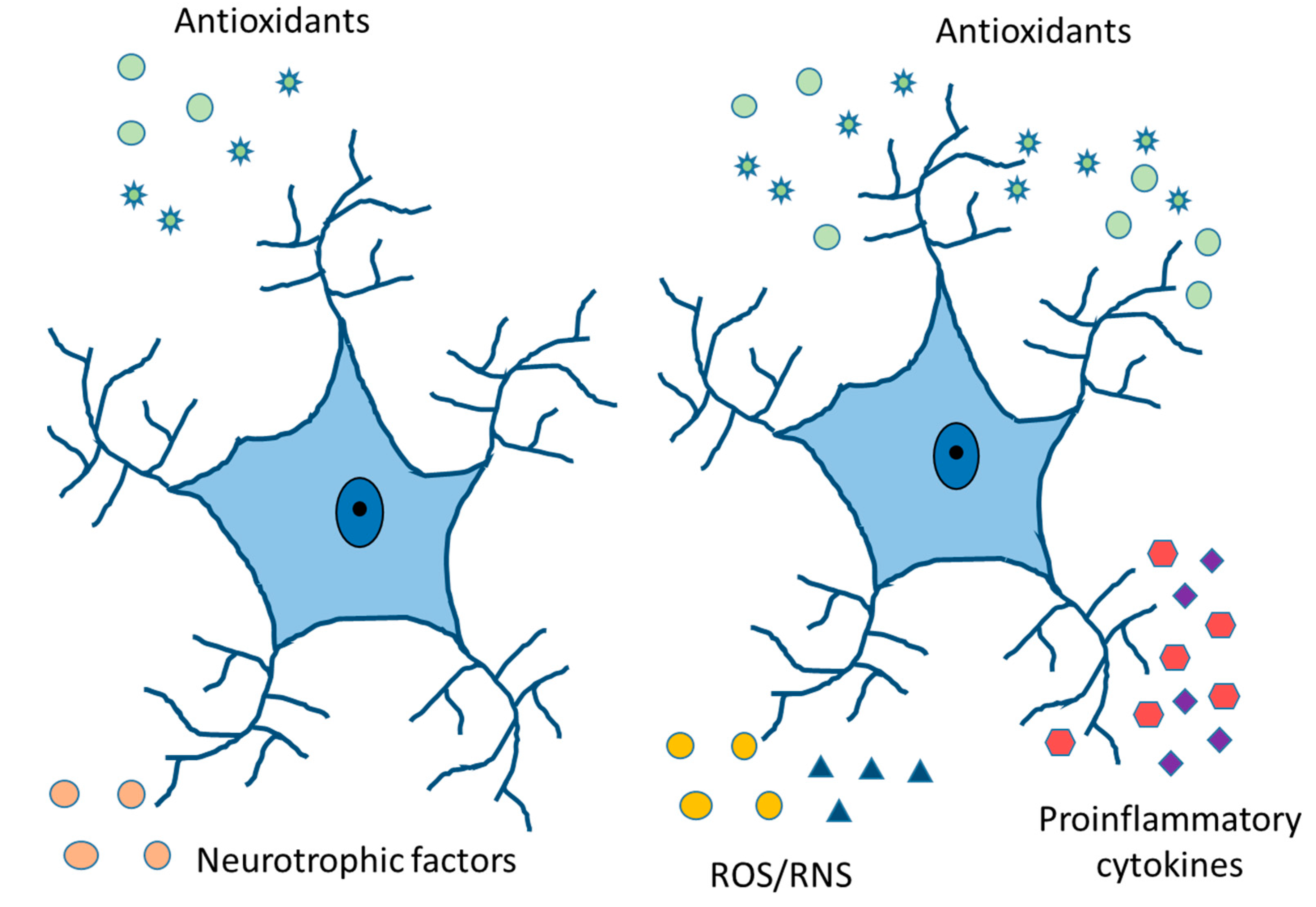
Free radicals are pro-oxidant molecules that contain one or more unpaired electrons. Depending on the molecules, free radicals can come from oxygen, nitrogen, lipids, etc. Furthermore, they can take electrons from other molecules, making them highly reactive [78]. The increase in ROS levels (Reactive Oxygen Species) is related to many neurodegenerative diseases [79,80]. The effects of antioxidants in clinical studies have been very disappointing because the high concentration of antioxidants acts in many cases as a pro-oxidant [81], because oxidative stress occurs relatively early in the course of diseases, and/or the combination of antioxidants falls into a clinical situation [82]. Activated immune cells can produce ROS that contributes to mitochondrial dysfunction and neural cell death by apoptosis (Figure 5). Superoxide dismutase (SOD) converts superoxide free radicals into molecular oxygen and hydrogen peroxide, which are broken down by the enzyme catalase [83,84]. Microglial cells are the primary immune cells in the CNS and the role of oxidative stress has been well studied in this cell type. In contrast, studies on the role of astrocytes in this process is scarce. Astrocytes protect neurons from oxidative stress by producing antioxidant proteins [41].
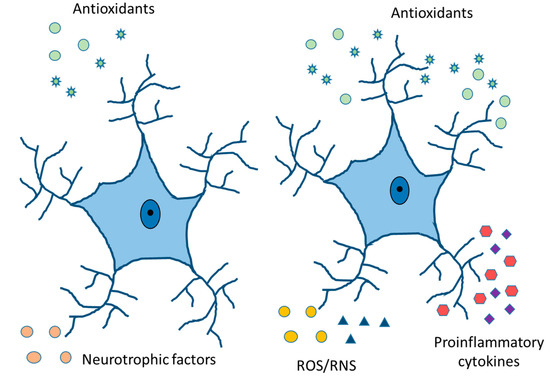
Figure 5.
Physiologically healthy and reactive astrocytes. Release of neurotrophic and antioxidant factors, ion homeostasis, ROS/RNS detoxification, fluid transport, vasodilation, and neurotransmitter reuptake in healthy astrocytes. Cytokine and chemokine release, ROS/RNS production, increased expression of GFAP, vimentin, and Glu, and compensatory release of antioxidants in reactive astrocytes.
The toxic peptide beta-amyloid causes the production of hydrogen peroxide by astrocytes [85], as it has been indicated before [80], and these ROS are released in response to beta-amyloid through the pentose phosphate pathway. Drugs that can protect astrocytes and neurons from inflammation and/or oxidative stress damage have been used in AD cells in culture, in animal models and in humans [73,86,87], but none of them have obtained a recovery and/or less development of AD. The results are the same in studies conducted for other diseases.
Due to the high metabolic rate of neurons, these cells produce more free radicals than other cells in the nervous system. Neurons also have a reduced ability to eliminate reactive oxygen species, which makes them highly vulnerable to oxidative stress [88]. In many neurodegenerative disorders, such as PD, AD, ALS, HD, etc., an increase of free radicals that can induce aggregations of proteins has been detected. [89,90,91,92]. In sporadic cases of Alzheimer’s disease, age factors are important and can influence the Aβ-amyloid processing by weakening mitochondrial function and producing excessive ROS [93]. Alternatively, α-synuclein aggregates caused by dopaminergic neurons in Parkinson’s disease disrupt mitochondrial function and then produce oxidative stress [94,95]. Moreover, α-synuclein aggregation can be produced by an increase in oxidative stress [90]. One form of amyotrophic lateral sclerosis (ALS) caused by superoxide dismutase 1 (SOD1) mutation [96], presents the affectation of the antioxidant molecules balance with the increase in oxidative stress [97] due to aggregation of SOD 1 [98]. The diseases indicated above, along with HD, share aggregation processes and oxidative stress that together engender a vicious cycle [99].
9. Neurodegeneration Mechanisms
In the neurodegenerative cascade, several basic mechanisms can join in, such as apoptosis, necrosis, autophagy, retrograde neurodegeneration, Wallerian degeneration, demyelination and astrogliopathy [100]. There is evidence of apoptotic mechanisms in animal models of various neurodegenerative diseases, but the evidence in human tissues is limited. The activation of caspase 1, 3, 8 and 9 and the release of cytochrome c observed in models of Huntington’s disease (HD) is demonstrated in human striated brain tissue [101,102]. Similarly, caspases activation and neuronal apoptosis have been detected in ALS [103] and HIV [104]. In necrosis, with non-caspases-dependent death, two major effector proteins act, serine/threonine-protein kinase 1 (RIPK1) and the mixed lineage kinase domain (MLKL). In murine ALS models, release of TNF-α, FasL and TRAIL by astrocytes has been detected that can trigger necrosis through the activation of RIPK1 and MLKL [104]. Furthermore, in humans with ALS, a normal pathology mediated by RIPK1 has been detected [105]. On the other hand, in MS, necrotic mechanisms are also observed in pathological samples [106].
Apathy has multifactorial symptoms, such as behavioral, cognitive, and emotional facets including impaired motivation and reduced goal-directed behavior. Apathy belongs to schizophrenia, bipolar disorders, and autism’s negative symptomatology, although the molecular mechanisms are still poorly studied [107,108]. Correlations between apathy with specific brain regions and executive functions have been shown (the anterior cingulate cortex, orbitofrontal cortex, and the ventral and dorsal striatum). It is considered the major neuropsychiatric symptom in both acquired and neurodegenerative disorders, such as strokes [109], AD [110], ELA [111] or Parkinson’s disease [112]. All these disorders have a disturbance in the normal balance of neurotransmitters and are associated with anomalies in specific brain regions and inflammatory pathways leading to glia activation and finally neuronal and neural loss [113]. In MS there is a decomposition of the blood–brain barrier (BBB), death without regeneration of oligodendrocytes, loss of myelin, axonal degeneration and reactive gliosis of astrocytes and activation of microglia [114,115]. In this disease, inflammation plays an important role by increasing in cytokines and chemokines. In the pathophysiology of MS, the BBB is compromised, causing the activation of the microglia and the immune cells of the periphery. The microglia not only produces pro-inflammatory cytokines and chemokines secretion with decreased anti-inflammatory agents but also releases reactive oxygen and glutamate species [116]. Each type of cell of the innate and adaptive immune system can organize the inflammatory response within the CNS and the autoreactive CD4 + T cells make an important contribution in the MS.
10. Astrocytes, Sleep Process and Diseases
A century ago, Santiago Ramón y Cajal proposed astrocytes as cells that regulate the process of sleep [28]. He detected several processes in the synapses during sleep and observed retraction of those processes during wakefulness. In 2009, scientists began to take an interest in sleep and the role of astrocytes in it and discovered that the influence of astrocyte on the sleep/wake cycle [117] and then, astrocytes were postulated as modulator members of the homeostasis of the sleep cycle [118]. Astrocytes release adenosine units to its receptor, adenosine A1, occasioning sleep and driving to total sleep [119]. Furthermore, it is known that astrocytes clean the brain during sleep, releasing solutes and water inside the brain, cleaning it through astrocytic aquaporins 4. In addition, in vivo microdialysis studies have shown that β-amyloid (the toxic peptide in Alzheimer’s brain) increases within the interstitial fluid during wakefulness and decreases during the sleep process [119]. Thus, the brain cleaning that occurs during the sleep period is decreased in Alzheimer’s patients compared to control individuals [120,121]. In addition, the changes observed in the amount of toxic peptide decrease during the development of AD [122], so detection in the glymphatic pathway could be affected in neurodegenerative patients and in other diseases, such as bipolar disorders, chronic fatigue syndrome, MS and schizophrenia [123]. Furthermore, the sleep/wake cycle, modulated by astrocytes, is also altered in those diseases [65,124,125]. Many people suffering from these diseases present a REM (Rapid Eye Movement) alteration process during the sleep period. This data could indicate alterations in the sleep period of the active zone of the brain that initiates the off-switch in the brain. In fact, the patients cannot sleep well, and they feel tired during the awake period, with problems in attention, memory, spatial recognition, and so on.
11. Therapeutic Effects to Combat Diseases
At present, there are many therapeutic drugs used against diseases of the nervous system, however, it is also important to test the use of new drugs and approaches against those diseases to treat them more effectively (Table 1). Future therapeutics against brain diseases must develop specific drugs against reactive astrocytes and microglia activation. Studies on the mechanisms that eliminate amyloid beta toxic peptide, the decrement of the pTAU inside the neurons, the ATP changes in the brain controlled by astrocytes and the production of metabolites, will be necessary for finding therapeutic targets in AD and other diseases [126].
Crises due to sensory overstimulation in people with autism are involuntary. The body of a person with autism react to sensory stimuli and the person being unable to bear the overstimulation suffers from a nervous breakdown. Some approaches could produce beneficial physiological effects, such as the reduction in deep pressure to achieve a decrement in anxiety in children with autism spectrum disorders (ASD) [127,128]. Furthermore, the use of new drugs, such as monoclonal antibodies designed to elicit an immune response to eliminate senile plaques, which damage communication between brain cells and end up killing the neurons, will be necessary. In chronic pain, drugs controlling the mechanisms of SG cells and the interaction of these cells with the neuronal body will be important to assume the relationship between astrocytes and the other cells in the sensorial ganglia and chronic pain treatment. In the future, for better health, brain changes in sleep-inducing proteins and the sleep/wake cycle could help fighting sleeping disorders and neurodegenerative diseases as well. In addition, the influence of astrocytes in the brain cleaning process will be a therapeutic approach to eliminate the toxic elements detected in many diseases, such as in AD, Parkinson’s, or ALS (Amyotrophic Lateral Sclerosis) and many other neurodegenerative diseases in which toxic proteins are present. Moreover, altered mRNA expression profile in ALS and/or other neurodegenerative diseases has been detected with an increase in inflammation produced by microglia and astrocyte reactivities, which are potential mediators of neurodegenerative processes [97,129].

Table 1.
Drugs and their effects on glia.
Table 1.
Drugs and their effects on glia.
| DRUGS | EFFECT ON ASTROCYTES | REFERENCES |
|---|---|---|
| IFN-β | Downregulation of cytokine, NO production, and MMP generation | Bhat et al., 2019 [130] |
| Pioglitazones | Inhibit inflammation reducing glial activation | Dhapola et al., 2021 [131] |
| Fingolimod | Immune-modulator inhibitor actions on activated microglia | Pitteri et al., 2018 [132] |
| Minocycline | Immune-modulator inhibitor actions on activated microglia | Wang et al., 2020 [133] |
| Xaliproden | Production of increased amount of neurotrophic factors | Lacomblez et al., 2004 [134] |
| Cyclooxygenase (COX) inhibitors | Improved memory and decrease amyloid deposition | Lopez-Ramirez et al., 2021 [135] |
| Propentofylline | Decrement of neuroinflammation relative to glial cell activation | Sweitzer and De Leo, 2011 [136] |
| Glatiramer acetate | Anti-inflammatory cytokine and neurotrophic factors increase | Kasindi et al., 2022 [137] |
| Verapamil + magnesium sulphate | Block overactivation of L-Type calcium channels | Zhang et al., 2019 [138] |
| Rapamycin | Astrogliosis inhibition | Selvarani et al., 2021 [139] |
| Fuoxetine | Decrement of antigen-presenting | Barakat et al., 2018 [140] |
| Teriflunomide | NO synthesis downregulation | Hauser et al., 2020 [141] |
| Methotrexate | Induction of astrogliosis, injury | Shao et al., 2019 [142] |
| Tacrolimus | Inhibition of pro-inflammatory cytokines | La Maestra et al., 2018 [143] |
| Mycophenolate mofetil | Downregulation of NO synthesis | Ebrahimi et al., 2012 [144] |
| Glutocorticoids | Dwonregulation of pro-inflammatory cytokines, and astrogliosis | Nichols et al., 2001 [145] |
It is necessary to promote the clearance of toxic proteins by astrocytes (such as amyloid beta) via different mechanisms, such as autophagy or ubiquitin systems. In addition, in reactive astrocytes, an increase in antioxidant proteins, such as Nrf2 (Nuclear Factor Erythroid 2-related factor 2), could benefit our brain. On the other hand, astrocytes can clear toxic peptides from the brain leading to reactive astrocytes and increased inflammation, toxic proteins, and oxidative stress molecules. Regulation of the oxidative stress state and inflammation could help neurons located near astrocytes to survive. Additionally, astrocytes can increase GABA levels after damage, so controlling MAO-B (monoamine oxidase-B) activity could help rescue the brain from memory problems, such as those found in the AD. Cerebral blood flow (CBF) decreases with age. Between the ages of 20 and 60, the CBF falls by 16% and continues to fall by 0.4% each year. A reduction in oxygen and glucose supply to the brain occurs, and this drop in CBF reduces ATP energy production. Mitochondrial loss or damage with reduced ATP worsens when vascular risk factors (VRFs) develop during Alzheimer’s disease and may accelerate CBF declination and mitochondrial deficiency where mild cognitive impairment (MCI) develops [42]. One form of photobiomodulation (PBM), transcranial infrared brain stimulation (TIBS), is planned in a randomized, placebo-controlled study of MCI patients which is to be conducted at our university. Photobiomodulation has been used in Parkinson’s disease, depression, traumatic brain injury and stroke with reported benefits. Medical interventions, pharmacological approach, etc. have been used in AD, but TIBS will be a better technique for the future. The study of the effects of photobiomodulation on the brain during aging has been studied and reviewed by many authors [146,147].
The heterogeneity of astrocytes is poorly understood and has not been sufficiently studied. The study of the different forms that astrocytes acquire to activate and their actions during the processes in different neurological diseases must be deepened and has not been sufficiently explained in this manuscript. Nor has it been addressed whether it is possible to act therapeutically on the different types of astrocytes in neurological diseases.
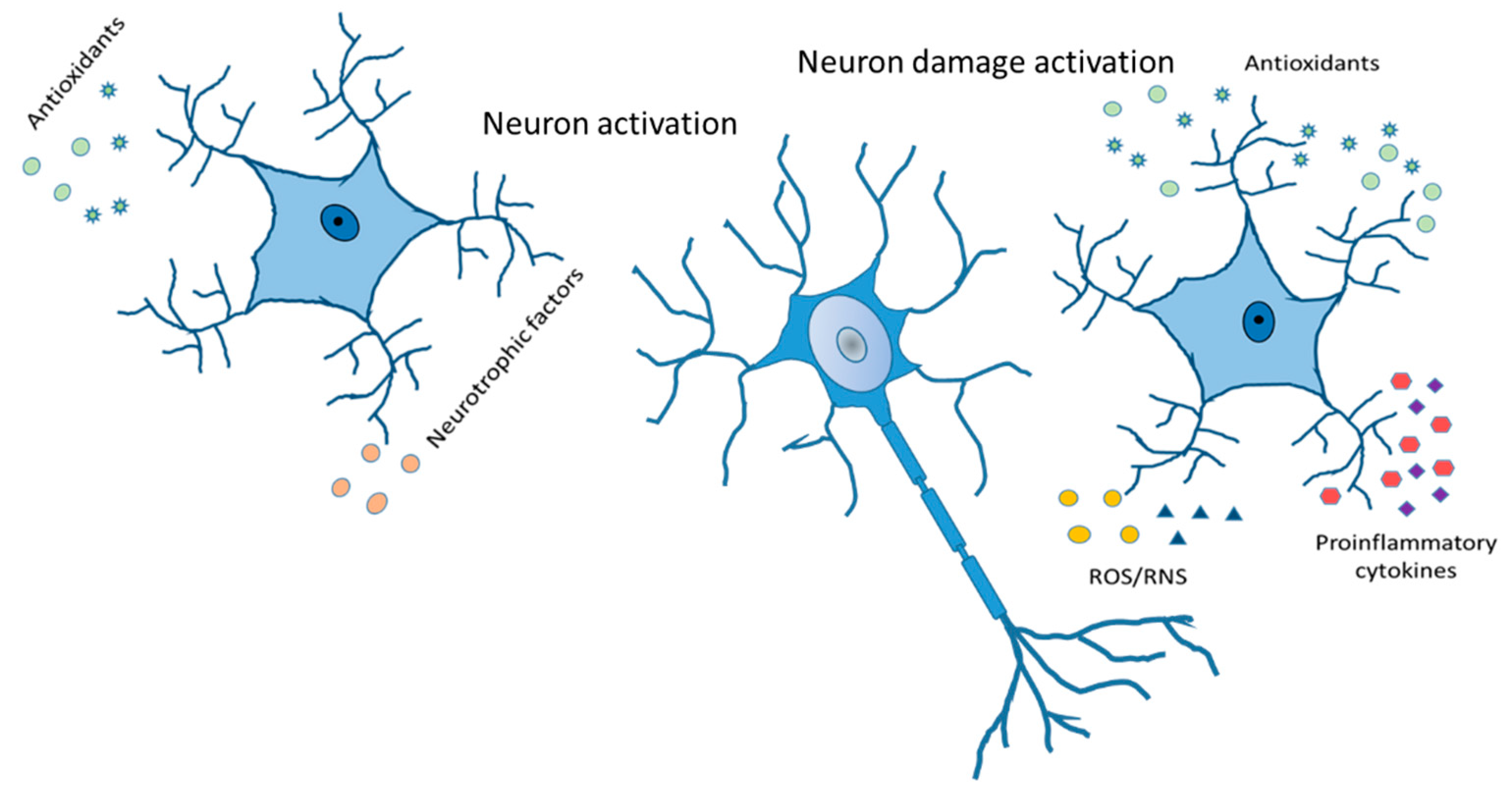
12. Conclusions
Controlling mechanisms and understanding the relationship between astrocytes, neurons, oligodendroglia, and microglia could become the therapeutic track to some neurodegenerative disorders and diseases, such as bipolar disorder, schizophrenia, and autistic spectrum. Thus, different strategies can be considered to bridge the gap between human disorders and astrocytes and glia intervention. Furthermore, the presence of different types of astrocytes and increases or decreases in them depending on the disease and age should be a principal focus of studies in the future (Figure 6). Future investigations must be carried out to understand the general mechanisms that produce morphological deficits of astrocytes in neurodegeneration. It is imperative to know if these deficits produce functional changes in astrocytes and if neurodegeneration can be slowed down. Hypothesis looking for the role of astrocytes in mammalian functions will be necessary as a new field in the astrocytes’ job in the nervous system has been opened now. This will provide a new direction for future interventions in CNS diseases.
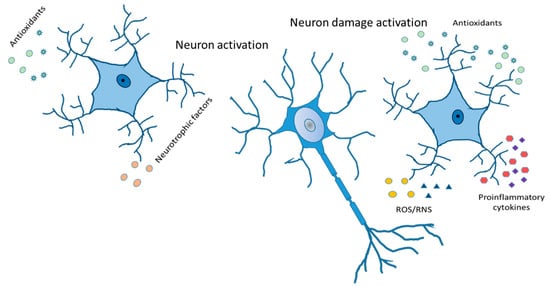
Figure 6.
Reactive astrocytes. Release of antioxidants and neurotrophic factor activate neurons in a good way. Cytokines and chemokines, ROS/RNS production, increased expression of GFAP, vimentin and Glu, and compensatory release of antioxidants leading to neuron damage activation.
Author Contributions
A.J. designed the review, critically revised the manuscript, and corrected English in the manuscript. J.C.-C. critically revised the manuscript and improved it. S.K.S. interpreted the results, critically revised manuscript, and corrected English in the manuscript. A.J., K.A.-G., C.C. and I.C.-P. conducted experiments and interpreted results, O.C. critically anayzed the information and worked on the bibliography, S.L.V. conceived and designed the review, drafted the manuscript, and proofread it. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All animal procedures were carried out in accordance with the European legislation on the use and care of laboratory animals (CEE 86/609). Experimental research on mice was performed with the approval of the ethics committee on animal research of the University of Valencia (Spain). All procedures were performed in accordance with the 1964 Helsinki declaration and its later amendments.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created.
Conflicts of Interest
All participants provided written informed consent. Participants have declared that no competing interest exists.
References
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Hubbard, P.; Butt, A.M. Cytology and lineage of NG2-positive glia. J. Neurocytol. 2002, 31, 457–467. [Google Scholar] [CrossRef]
- Seeker, L.A.; Williams, A. Oligodendroglia heterogeneity in the human central nervous system. Acta Neuropathol. 2022, 143, 143–157. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef]
- Khakh, B.S.; Deneen, B. The Emerging Nature of Astrocyte Diversity. Annu. Rev. Neurosci. 2019, 8, 187–207. [Google Scholar] [CrossRef]
- Sims, S.G.; Cisney, R.N.; Lipscomb, M.M.; Meares, G.P. The role of endoplasmic reticulum stress in astrocytes. Glia 2022, 70, 5–19. [Google Scholar] [CrossRef]
- Kettenmann, H.; Verkhratsky, A. Neuroglia: The 150 years after. Trends Neurosci. 2008, 31, 653–659. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. Die Cellularpathologie in ihrer Begründung aufphysiologische and pathologische Gewebelehre. In Zwanzig Vorlesungen Gehalten Während der Monate Februar, März und April 1858 im Pathologischen Institut zu Berlin; August Hirschwald: Berlin, Germany, 1858; p. 440. [Google Scholar]
- Gong, Y.H.; Parsadanian, A.S.; Andreeva, A.; Snider, W.D.; Elliott, J.L. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 660–665. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Ho, M.S.; Parpura, V. Evolution of Neuroglia. Adv. Exp. Med. Biol. 2019, 1175, 15–44. [Google Scholar] [PubMed]
- Verkhratsky, A.; Ho, M.S.; Zorec, R.; Parpura, V. The Concept of Neuroglia. Adv. Exp. Med. Biol. 2019, 1175, 1–13. [Google Scholar]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Wang, X.; Goldman, S.; Nedergaard, M. Astrocytic complexity distinguishes the human brain. Trends Neurosci 2006, 29, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Herculano-Houzel, S. The glia/neuron ratio: How it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia 2014, 62, 1377–1391. [Google Scholar] [CrossRef]
- Reemst, K.; Noctor, S.C.; Lucassen, P.J.; Hol, E.M. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front. Hum. Neurosci. 2016, 10, 566. [Google Scholar] [CrossRef]
- Sibille, J.; Pannasch, U.; Rouach, N. Astroglial potassium clearance contributes to short-term plasticity of synaptically evoked currents at the tripartite synapse. J. Physiol. 2014, 592, 87–102. [Google Scholar] [CrossRef]
- Ziff, O.J.; Clarke, B.E.; Taha, D.M.; Crerar, H.; Luscombe, N.M.; Patani, R. Meta-analysis of human and mouse ALS astrocytes reveals multi-omic signatures of inflammatory reactive states. Genome Res. 2022, 32, 71–84. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Ronzano, R.; Thetiot, M.; Lubetzki, C.; Desmazieres, A. Myelin Plasticity and Repair: Neuro-Glial Choir Sets the Tuning. Front. Cell. Neurosci. 2020, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Nieh, E.H.; Kim, S.Y.; Namburi, P.; Tye, K.M. Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res. 2013, 1511, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.; Dossi, E.; Rouach, N. Human astrocytes: Structure and functions in the healthy brain. Brain Struct. Funct. 2017, 2017222, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Khurana, S.; Vats, A.; Sahu, B.; Ganguly, N.K.; Chakraborti, P.; Gourie-Devi, M.; Taneja, V. Neuromuscular Junction Dysfunction in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2022, 59, 1502–1527. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G. Dynamic signaling between astrocytes and neurons. Annu. Rev. Physiol. 2001, 63, 795–813. [Google Scholar] [CrossRef]
- Corkrum, M.; Covelo, A.; Lines, J.; Bellocchio, L.; Pisansky, M.; Loke, K.; Quintana, R.; Rothwell, P.E.; Lujan, R.; Marsicano, G.; et al. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron 2020, 105, 1036–1047.e5. [Google Scholar] [CrossRef]
- Wang, Q.; Kong, Y.; Wu, D.Y.; Liu, J.H.; Jie, W.; You, Q.L.; Huang, L.; Hu, J.; Chu, H.D.; Gao, F.; et al. Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice. Nat. Commun. 2021, 12, 3321. [Google Scholar] [CrossRef]
- Notter, T. Astrocytes in schizophrenia. Brain Neurosci. Adv. 2021, 27, 23982128211009148. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Rivera, A.D. Astrocytes in Bipolar Disorder. Adv. Neurobiol. 2021, 26, 95–113. [Google Scholar] [PubMed]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. The tripartite synapse: Roles for gliotransmission in health and disease. Trends Mol. Med. 2007, 13, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Fellin, T.; Takano, H.; Dong, J.H.; Haydon, P.G. Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 2007b, 27, 6473–6477. [Google Scholar] [CrossRef]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Iadecola, C.; Nedergaard, M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007, 10, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Kacem, K.; Lacombe, P.; Seylaz, J.; Bonvento, G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: A confocal microscopy study. Glia 1998, 23, 1–10. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Hurley, J.B.; Lindsay, K.J.; Du, J. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. J. Neurosci. Res. 2015, 93, 1079–1092. [Google Scholar] [CrossRef]
- Peteri, U.K.; Niukkanen, M. Castrén ML. Astrocytes in Neuropathologies Affecting the Frontal Cortex. Front Cell Neurosci. 2019, 13, 44. [Google Scholar] [CrossRef]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Astrocyte-neuron metabolic relationships: For better and for worse. Trends Neurosci. 2011, 34, 76–87. [Google Scholar] [CrossRef]
- de la Torre, J.C.; Olmo, A.D.; Valles, S. Can mild cognitive impairment be stabilized by showering brain mitochondria with laser photons? Neuropharmacology 2019, 171, 107841. [Google Scholar] [CrossRef] [PubMed]
- Mapps, A.A.; Thomsen, M.B.; Boehm, E.; Zhao, H.; Hattar, S.; Kuruvilla, R. Diversity of satellite glia in sympathetic and sensory ganglia. Cell Rep. 2022, 38, 110328. [Google Scholar] [CrossRef] [PubMed]
- Ledda, M.; Blum, E.; De Palo, S.; Hanani, M. Augmentation in gap junction-mediated cell coupling in dorsal root ganglia following sciatic nerve neuritis in the mouse. Neuroscience 2009, 164, 1538–1545. [Google Scholar] [CrossRef]
- Uta, D.; Kato, G.; Doi, A.; Andoh, T.; Kume, T.; Yoshimura, M.; Koga, K. Animal models of chronic pain increase spontaneous glutamatergic transmission in adult rat spinal dorsal horn in vitro and in vivo. Biochem. Biophys. Res. Commun. 2019, 512, 352–359. [Google Scholar] [CrossRef]
- Vegeto, E.; Villa, A.; Della Torre, S.; Crippa, V.; Rusmini, P.; Cristofani, R.; Galbiati, M.; Maggi, A.; Poletti, A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr. Rev. 2020, 41, 273–319. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; McCarthy, M.M. A starring role for microglia in brain sex differences. Neuroscientist 2015, 21, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S. Autism: The empathizing-systemizing (E-S) theory. Ann. N. Y. Acad. Sci. 2009, 1156, 68–80. [Google Scholar] [CrossRef]
- Fombonne, E. Epidemiological trends in rates of autism. Mol. Psychiatry 2002, 7 (Suppl. S2), S4–S6. [Google Scholar] [CrossRef]
- Khakh, B.S.; Beaumont, V.; Cachope, R.; Munoz-Sanjuan, I.; Goldman, S.A.; Grantyn, R. Unravelling and Exploiting Astrocyte Dysfunction in Huntington’s Disease. Trends Neurosci. 2017, 40, 422–437. [Google Scholar] [CrossRef]
- Livne-Bar, I.; Wei, J.; Liu, H.-H.; Alqawlaq, S.; Won, G.-J.; Tuccitto, A.; Gronert, K.; Flanagan, J.G.; Sivak, J.M. Astrocytederived lipoxins A4 and B4 promote neuroprotection from acute and chronic injury. J. Clin. Investig. 2017, 127, 4403–4414. [Google Scholar] [CrossRef]
- Vainchtein, I.D.; Chin, G.; Cho, F.S.; Kelley, K.W.; Miller, J.G.; Chien, E.C.; Liddelow, S.A.; Nguyen, P.T.; Nakao-Inoue, H.; Dorman, L.C.; et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 2018, 359, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.J.; Avramopoulos, D.; Jantzie, L.L.; McCallion, A.S. Neuroinflammation represents a common theme amongst genetic and environmental risk factors for Alzheimer and Parkinson diseases. J. Neuroinflamm. 2022, 19, 223. [Google Scholar] [CrossRef] [PubMed]
- Habbas, S.; Santello, M.; Becker, D.; Stubbe, H.; Zappia, G.; Liaudet, N.; Klaus, F.R.; Kollias, G.; Fontana, A.; Pryce, C.R.; et al. Neuroinflammatory TNFalpha impairs memory via astrocyte signaling. Cell 2015, 163, 1730–1741. [Google Scholar] [CrossRef]
- Obrenovich, M.E.; Smith, M.A.; Siedlak, S.L.; Chen, S.G.; de la Torre, J.C.; Perry, G.; Aliev, G. Overexpression of GRK2 in Alzheimer disease and in a chronic hypoperfusion rat model is an early marker of brain mitochondrial lesions. Neurotox. Res. 2006, 10, 43–56. [Google Scholar] [CrossRef]
- Bardehle, S.; Krüger, M.; Buggenthin, F.; Schwausch, J.; Ninkovic, J.; Clevers, H.; Snippert, H.J.; Theis, F.J.; Meyer-Luehmann, M.; Bechmann, I.; et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat. Neurosci. 2013, 16, 580–586. [Google Scholar] [CrossRef]
- Carter, S.F.; Herholz, K.; Rosa-Neto, P.; Pellerin, L.; Nordberg, A.; Zimmer, E.R. Astrocyte Biomarkers in Alzheimer’s Disease. Trends Mol. Med. 2019, 25, 77–95. [Google Scholar] [CrossRef]
- Edison, P.; Brooks, D.J. Role of Neuroinflammation in the Trajectory of Alzheimer’s Disease and in vivo Quantification Using PET. J. Alzheimer’s Dis. 2018, 64, S339–S351. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; An, H.; Lim, J.; Woo, J.; Lee, J.; Ryu, H.; Lee, C.J. Astrocytic proBDNF and Tonic GABA Distinguish Active versus Reactive Astrocytes in Hippocampus. Exp. Neurobiol. 2018, 27, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Delekate, A.; Füchtemeier, M.; Schumacher, T.; Ulbrich, C.; Foddis, M.; Petzold, G.C. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Commun. 2014, 5, 5422. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of multiple sclerosis: Progress and challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef]
- Lorscheider, J.; Buzzard, K.; Jokubaitis, V.; Spelman, T.; Havrdova, E.; Horakova, D.; Trojano, M.; Izquierdo, G.; Girard, M.; Duquette, P.; et al. MSBase Study Group Defining secondary progressive multiple sclerosis. Brain A J. Neurol. 2016, 139, 2395–2405. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef]
- Vu, D.M.; Jungkind, D. Angelle Desiree LaBeaud. Chikungunya Virus. Clin. Lab. Med. 2017, 37, 371–382. [Google Scholar] [CrossRef]
- Talantova, M.; Sanz-Blasco, S.; Zhang, X.; Xia, P.; Akhtar, M.W.; Okamoto, S.; Dziewczapolski, G.; Nakamura, T.; Cao, G.; Pratt, A.E.; et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. USA 2013, 110, E2518–E2527. [Google Scholar] [CrossRef]
- Sung, P.S.; Lin, P.Y.; Liu, C.H.; Su, H.C.; Tsai, K.J. Neuroinflammation and Neurogenesis in Alzheimer’s Disease and Potential Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 701. [Google Scholar] [CrossRef]
- Jorda, A.; Aldasoro, M.; Aldasoro., C.; Valles, S.L. Inflammatory Chemokines Expression Variations and Their Receptors in APP/PS1 Mice. J Alzheimer’s Dis. 2021, 83, 1051–1060. [Google Scholar] [CrossRef]
- Silvin, A.; Uderhardt, S.; Piot, C.; Da Mesquita, S.; Yang, K.; Geirsdottir, L.; Mulder, K.; Eyal, D.; Liu, Z.; Bridlance, C.; et al. Dual ontogeny of disease-associated microglia and disease inflammatory macrophages in aging and neurodegeneration. Immunity 2022, 55, 1448–1465.e6. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Vallés, S.L.; Pascual, M.; Guerri, C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J. Immunol. 2005, 175, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, M.H.; Arumäe, U.; Airavaara, M.; Saarma, M. Therapeutic potential of the endoplasmic reticulum located and secreted CDNF/MANF family of neurotrophic factors in Parkinson’s disease. FEBS Lett. 2015, 589, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Di, Z.; He, Y.; Wang, R.; Ma, Y.; Sun, R.; Li, J.; Wang, T.; Shen, Y.; Fang, S.; et al. Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects against Aβ toxicity via attenuating Aβ-induced endoplasmic reticulum stress. J. Neuroinflamm. 2019, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, D.Y.; Chen, X.S.; Zhu, L.; Wan, L.H. MANF: A Novel Endoplasmic Reticulum Stress Response Protein-The Role in Neurological and Metabolic Disorders. Oxidative Med. Cell. Longev. 2021, 2021, 6467679. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. -Cell Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress, and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Miller, M.B.; Huang, A.Y.; Kim, J.; Zhou, Z.; Kirkham, S.L.; Maury, E.A.; Ziegenfuss, J.S.; Reed, H.C.; Neil, J.E.; Rento, L.; et al. Somatic genomic changes in single Alzheimer’s disease neurons. Nature 2022, 604, 714–722. [Google Scholar] [CrossRef]
- Herbert, V. Prooxidant effects of antioxidant vitamins. Introduction. J. Nutr. 1996, 126, 1197S-200S. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Zarkovic, N.; Zarkovic, K. Oxidative stress and regeneration. Free Radic. Biol. Med. 2022, 181, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Rueda, D.; Guerra-Ojeda, S.; Aldasoro, M.; Iradi, A.; Obrador, E.; Ortega, A.; Mauricio, M.D.; Vila, J.M.; Valles, S.L. Astrocytes protect neurons from Aβ1-42 peptide-induced neurotoxicity increasing TFAM and PGC-1 and decreasing PPAR-γ and SIRT-1. Int. J. Med. Sci. 2015, 12, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Desagher, S.; Glowinski, J.; Premont, J. Astrocytes protect neurons from hydrogen peroxide toxicity. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Valles, S.L.; Dolz-Gaiton, P.; Gambini, J.; Borras, C.; Lloret, A.; Pallardo, F.V.; Viña, J. Estradiol or genistein prevent Alzheimer’s disease-associated inflammation correlating with an increase PPAR gamma expression in cultured astrocytes. Brain Res. 2010, 1312, 138–144. [Google Scholar] [CrossRef]
- Aguirre-Rueda, D.; Guerra-Ojeda, S.; Aldasoro, M.; Iradi, A.; Obrador, E.; Mauricio, M.D.; Vila, J.M.; Marchio, P.; Valles, S.L. WIN 55,212-2, agonist of cannabinoid receptors, prevents amyloid β1-42 effects on astrocytes in primary culture. PLoS ONE 2015, 10, e0122843. [Google Scholar] [CrossRef]
- Aldasoro, M.; Guerra-Ojeda, S.; Aguirre-Rueda, D.; Mauricio, M.D.; Vila, J.M.; Marchio, P.; Iradi, A.; Aldasoro, C.; Jorda, A.; Obrador, E.; et al. Effects of Ranolazine on Astrocytes and Neurons in Primary Culture. PLoS ONE 2016, 11, e0150619. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective Neuronal Vulnerability to Oxidative Stress in the Brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Hsu, L.J.; Sagara, Y.; Arroyo, A.; Rockenstein, E.; Sisk, A.; Mallory, M.; Wong, J.; Takenouchi, T.; Hashimoto, M.; Masliah, E. α-Synuclein Promotes Mitochondrial Deficit and Oxidative Stress. Am. J. Pathol. 2000, 157, 401–410. [Google Scholar] [CrossRef]
- Scudamore, O.; Ciossek, T. Increased Oxidative Stress Exacerbates α-Synuclein Aggregation In Vivo. J. Neuropathol. Exp. Neurol. 2018, 77, 443–453. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. The critical role of methionine 35 in Alzheimer’s amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2005, 1703, 149–156. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of Oxidative Stress in Neurodegeneration. Oxidative Med. Cell. Longev. 2012 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Tapias, V.; Hu, X.; Luk, K.C.; Sanders, L.H.; Lee, V.M.; Greenamyre, J.T. Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell. Mol. Life Sci. 2017, 74, 2851–2874. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, L.; Liu, J.; Xie, F.; Su, B.; Wang, X. Abnormalities of Mitochondrial Dynamics in Neurodegenerative Diseases. Antioxidants 2017, 6, 25. [Google Scholar] [CrossRef]
- Cudkowicz, M.E.; McKenna-Yasek, D.; Sapp, P.E.; Chin, W.; Geller, B.; Hayden, D.L.; Schoenfeld, D.A.; Hosler, B.A.; Horvitz, H.R.; Brown, R.H. Epidemiology of mutations in superoxide dismutase in amyotrophic lateal sclerosis. Ann. Neurol. 1997, 41, 210–221. [Google Scholar] [CrossRef]
- Beckman, J.S.; Carson, M.; Smith, C.D.; Koppenol, W.H. ALS, SOD and peroxynitrite. Nature 1993, 364, 584. [Google Scholar] [CrossRef]
- Forsberg, K.; Jonsson, P.A.; Andersen, P.M.; Bergemalm, D.; Graffmo, K.S.; Hultdin, M.; Jacobsson, J.; Rosquist, R.; Marklund, S.L.; Brännström, T. Novel Antibodies Reveal Inclusions Containing Non-Native SOD1 in Sporadic ALS Patients. PLoS ONE 2010, 5, e11552. [Google Scholar] [CrossRef]
- Goswami, A.; Dikshit, P.; Mishra, A.; Mulherkar, S.; Nukina, N.; Jana, N.R. Oxidative stress promotes mutant huntingtin aggregation and mutant huntingtin-dependent cell death by mimicking proteasomal malfunction. Biochem. Biophys. Res. Commun. 2006, 342, 184–190. [Google Scholar] [CrossRef]
- Morris, G.; Stubbs, B.; Köhler, C.A.; Walder, K.; Slyepchenko, A.; Berk, M.; Carvalho, A.F. The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: Focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med. Rev. 2018, 41, 255–265. [Google Scholar] [CrossRef]
- Bortolon, C.; Macgregor, A.; Capdevielle, D.; Raffard, S. Apathy in schizophrenia: A review of neuropsychological and neuroanatomical studies. Neuropsychologia 2018, 118, 22–33. [Google Scholar] [CrossRef]
- Caeiro, L.; Ferro, J.M.; Costa, J. Apathy secondary to stroke: A systematic review and meta-analysis. Cerebrovasc. Dis. 2013, 35, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, T.; Dedeoglu, A.; Kubilus, J.; Kowall, N.W.; Beal, M.F.; Friedlander, R.M.; Hersch, S.M.; Ferrante, R.J. Cytochrome C and caspase-9 expression in Huntington’s disease. Neuromol. Med. 2002, 1, 183–195. [Google Scholar]
- Li, S.H.; Lam, S.; Cheng, A.L.; Li, X.J. Intranuclear huntingtin increases the expression of caspase-1 and induces apoptosis. Hum. Mol. Genet. 2000, 9, 2859–2867. [Google Scholar] [CrossRef]
- Pasinelli, P.; Houseweart, M.K.; Brown, R.H.; Cleveland, D.W. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2000, 97, 13901–13906. [Google Scholar] [CrossRef]
- Garden, G.A.; Budd, S.L.; Tsai, E.; Hanson, L.; Kaul, M.; D’Emilia, D.M.; Friedlander, R.M.; Yuan, J. Masliah, E.; Lipton, S.A. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 4015–4024. [Google Scholar] [CrossRef]
- Re, D.B.; Le Verche, V.; Yu, C.; Amoroso, M.W.; Politi, K.A.; Phani, S.; Ikiz, B.; Hoffmann, L.; Koolen, M.; Nagata, T.; et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron 2014, 81, 1001–1008. [Google Scholar] [CrossRef]
- Ito, Y.; Ofengeim, D.; Najafov, A.; Das, S.; Saberi, S.; Li, Y.; Hitomi, J.; Zhu, H.; Chen, H.; Mayo, L.; et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 2016, 353, 603–608. [Google Scholar] [CrossRef]
- Picon, C.; Jayaraman, A.; James, R.; Beck, C.; Gallego, P.; Witte, M.E.; van Horssen, J.; Mazarakis, N.D.; Reynolds, R. Neuron-specific activation of necroptosis signaling in multiple sclerosis cortical grey matter. Acta Neuropathol. 2021, 141, 585–604. [Google Scholar] [CrossRef]
- Levenson, R.W.; Sturm, V.E.; Haase, C.M. Emotional and behavioral symptoms in neurodegenerative disease: A model for studying the neural bases of psychopathology. Annu. Rev. Clin. Psychol. 2014, 10, 581–606. [Google Scholar] [CrossRef]
- Pagonabarraga, J.; Kulisevsky, J.; Strafella, A.P.; Krack, P. Apathy in Parkinson’s disease: Clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015, 14, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Carnes-Vendrell, A.; Deus, J.; Molina-Seguin, J.; Pifarré, J.; Purroy, F. Depression and Apathy after Transient Ischemic Attack or Minor Stroke: Prevalence, Evolution and Predictors. Sci. Rep. 2019, 9, 16248. [Google Scholar] [CrossRef]
- Cortes, N.; Andrade, V.; Maccioni, R.B. Behavioral and Neuropsychiatric Disorders in Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 63, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Christoforidou, E.; Joilin, G.; Hafezparast, M. Potential of activated microglia as a source of dysregulated extracellular microRNAs contributing to neurodegeneration in amyotrophic lateral sclerosis. J. Neuroinflamm. 2020, 17, 135. [Google Scholar] [CrossRef]
- Takahashi, M.; Tabu, H.; Ozaki, A.; Hamano, T.; Takeshima, T. Antidepressants for Depression, Apathy, and Gait Instability in Parkinson’s disease: A Multicenter Randomized Study. Intern. Med. 2019, 58, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Florian, C.; Fellin, T.; Munoz, J.R.; Lee, S.Y.; Abel, T.; Haydon, P.G.; Frank, M.G. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 2009, 61, 213–219. [Google Scholar] [CrossRef]
- Brown, R.E.; Basheer, R.; McKenna, J.T.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087–1187. [Google Scholar] [CrossRef]
- Kang, J.E.; Lim, M.M.; Bateman, R.J.; Lee, J.J.; Smyth, L.P.; Cirrito, J.R.; Fujiki, N.; Nishino, S.; Holtzman, D.M. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009, 326, 1005–1007. [Google Scholar] [CrossRef]
- Haydon, P.G. Astrocytes and the modulation of sleep. Curr. Opin. Neurobiol. 2017, 44, 28–33. [Google Scholar] [CrossRef]
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Galecki, P.; Walder, K.; Maes, M. The Neuro-Immune Pathophysiology of Central and Peripheral Fatigue in Systemic Immune-Inflammatory and Neuro-Immune Diseases. Mol. Neurobiol. 2016, 53, 1195–1219. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Nave, K.A. Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple sclerosis: Mechanisms and immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Liu, S.; Park, S.; Allington, G.; Prelli, F.; Sun, Y.; Martá-Ariza, M.; Scholtzova, H.; Biswas, G.; Brown, B.; Verghese, P.B.; et al. Targeting Apolipoprotein E/Amyloid β Binding by Peptoid CPO_Aβ17-21 P Ameliorates Alzheimer’s Disease Related Pathology and Cognitive Decline. Sci. Rep. 2017, 7, 8009. [Google Scholar] [CrossRef]
- Afif, I.Y.; Manik, A.R.; Munthe, K.; Maula, M.I.; Ammarullah, M.I.; Jamari, J.; Winarni, T.I. Physiological Effect of Deep Pressure in Reducing Anxiety of Children with ASD during Traveling: A Public Transportation Setting. Bioengineering 2022, 9, 157. [Google Scholar] [CrossRef]
- Afif, I.Y.; Farkhan, M.; Kurdi, O.; Maula, M.I.; Ammarullah, M.I.; Setiyana, B.; Jamari, J.; Winarni, T.I. Effect of Short-Term Deep-Pressure Portable Seat on Behavioral and Biological Stress in Children with Autism Spectrum Disorders: A Pilot Study. Bioengineering 2022, 9, 48. [Google Scholar] [CrossRef]
- Chang, W.S.; Wang, Y.H.; Zhu, X.T.; Wu, C.J. Genome-Wide Profiling of miRNA and mRNA Expression in Alzheimer’s Disease. Medical science monitor. Int. Med. J. Exp. Clin. Res. 2017, 23, 2721–2731. [Google Scholar] [CrossRef]
- Bhat, H.; Lang, K.S.; Hardt, C.; Lang, J. Interferon in the CNS. Neuro-Signals 2019, 27, 44–53. [Google Scholar]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef]
- Pitteri, M.; Magliozzi, R.; Bajrami, A.; Camera, V.; Calabrese, M. Potential neuroprotective effect of Fingolimod in multiple sclerosis and its association with clinical variables. Expert Opin. Pharmacother. 2018, 19, 387–395. [Google Scholar] [CrossRef]
- Wang, B.; Huang, X.; Pan, X.; Zhang, T.; Hou, C.; Su, W.J.; Liu, L.L.; Li, J.M.; Wang, Y.X. Minocycline prevents the depressive-like behavior through inhibiting the release of HMGB1 from microglia and neurons. Brain Behav. Immun. 2020, 88, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Lacomblez, L.; Bensimon, G.; Douillet, P.; Doppler, V.; Salachas, F.; Meininger, V. Xaliproden in amyotrophic lateral sclerosis: Early clinical trials. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. Off. Publ. World Fed. Neurol. Res. Group Mot. Neuron Dis. 2004, 5, 99–106. [Google Scholar] [CrossRef]
- Lopez-Ramirez, M.A.; Lai, C.C.; Soliman, S.I.; Hale, P.; Pham, A.; Estrada, E.J.; McCurdy, S.; Girard, R.; Verma, R.; Moore, T.; et al. Astrocytes propel neurovascular dysfunction during cerebral cavernous malformation lesion formation. J. Clin. Investig. 2021, 131, e139570. [Google Scholar] [CrossRef] [PubMed]
- Sweitzer, S.; De Leo, J. Propentofylline: Glial modulation, neuroprotection, and alleviation of chronic pain. In Methylxanthines; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 200, pp. 235–250. [Google Scholar]
- Kasindi, A.; Fuchs, D.T.; Koronyo, Y.; Rentsendorj, A.; Black, K.L.; Koronyo-Hamaoui, M. Glatiramer Acetate Immunomodulation: Evidence of Neuroprotection and Cognitive Preservation. Cells 2022, 11, 1578. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Lu, K.; Wang, F.; Deng, J.; Xu, Z.; Wang, X.; Zhou, Q.; Le, W.; Zhao, Y. Verapamil Ameliorates Motor Neuron Degeneration and Improves Lifespan in the SOD1G93A Mouse Model of ALS by Enhancing Autophagic Flux. Aging Dis. 2019, 10, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Selvarani, R.; Mohammed, S.; Richardson, A. Effect of rapamycin on aging and age-related diseases-past and future. GeroScience 2021, 43, 1135–1158. [Google Scholar] [CrossRef]
- Barakat, A.; Hamdy, M.M.; Elbadr, M.M. Uses of fluoxetine in nociceptive pain management: A literature overview. Eur. J. Pharmacol. 2018, 829, 12–25. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Cohen, J.A.; Comi, G.; Correale, J.; Coyle, P.K.; Cross, A.H.; de Seze, J.; Leppert, D.; Montalban, X.; et al. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N. Engl. J. Med. 2020, 383, 546–557. [Google Scholar] [CrossRef]
- Shao, Y.; Tan, B.; Shi, J.; Zhou, Q. Methotrexate induces astrocyte apoptosis by disrupting folate metabolism in the mouse juvenile central nervous system. Toxicol. Lett. 2019, 301, 146–156. [Google Scholar] [CrossRef]
- La Maestra, S.; Frosina, G.; Micale, R.T.; D’Oria, C.; Garibaldi, S.; Daga, A.; Pulliero, A.; Izzotti, A. Brain microglia activation induced by intracranial administration of oligonucleotides and its pharmacological modulation. Drug Deliv. Transl. Res. 2018, 8, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Koch, M.; Pieroh, P.; Ghadban, C.; Hobusch, C.; Bechmann, I.; Dehghani, F. Time dependent neuroprotection of mycophenolate mofetil: Effects on temporal dynamics in glial proliferation, apoptosis, and scar formation. J. Neuroinflamm. 2012, 9, 89. [Google Scholar] [CrossRef]
- Nichols, N.R.; Zieba, M.; Bye, N. Do glucocorticoids contribute to brain aging?. Brain research. Brain Res. Rev. 2001, 37, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.D.S.; Gonzalez-Lima, F.; Gomes da Silva, S. Photobiomodulation for the aging brain. Ageing Res. Rev. 2021, 70, 101415. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).