Bacterial Community Survey of Wolbachia-Infected Parthenogenetic Parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) Treated with Antibiotics and High Temperature
Abstract
1. Introduction
2. Results
2.1. Reversion to Bisexual Reproduction in T. pretiosum Exposed to Sulfadiazine
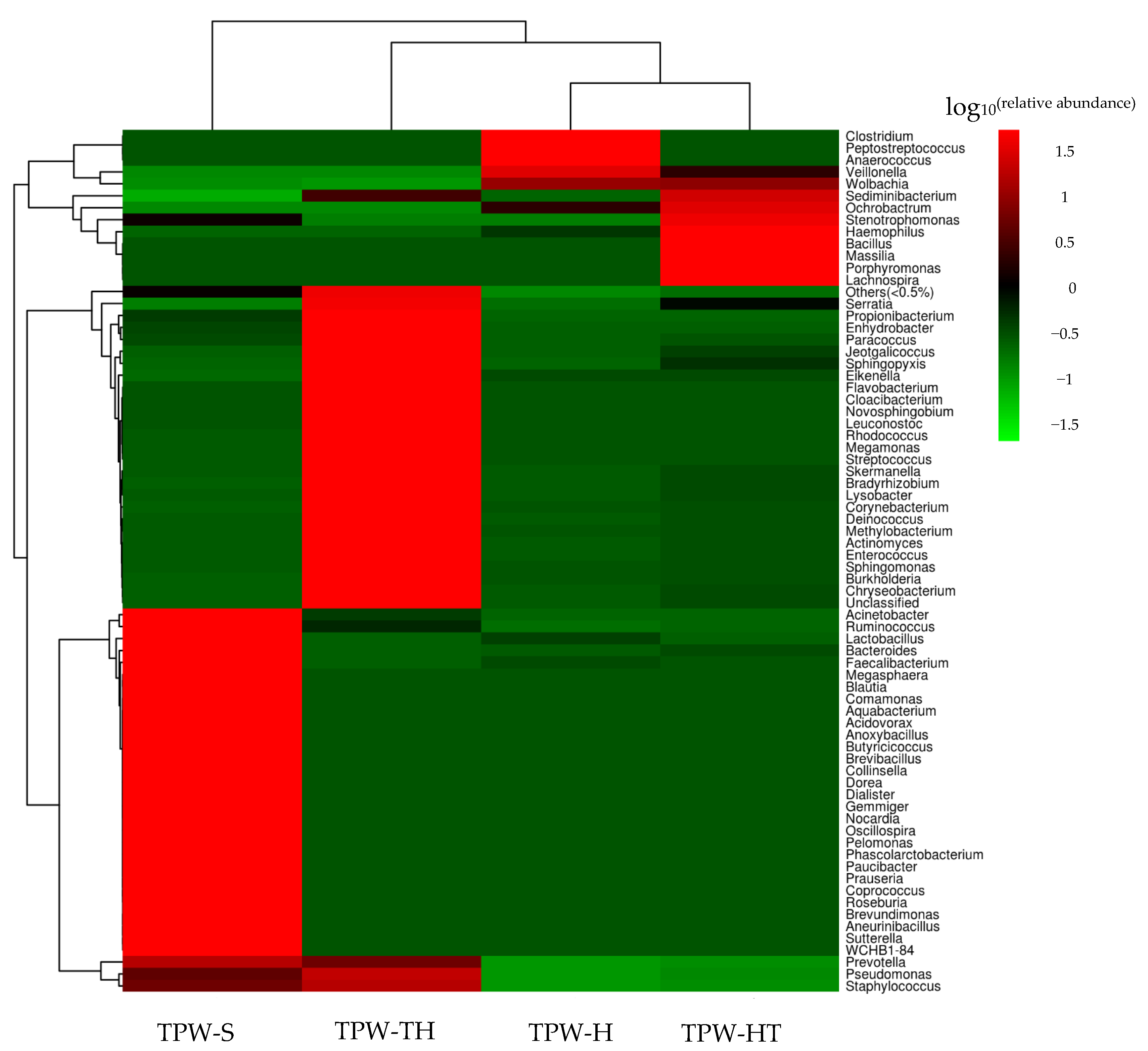
2.2. Bacterial Diversity Analysis in T. pretiosum
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Antibiotics and High-Temperature Treatment
4.3. Crossing Experiments
4.4. Wolbachia Infection Detection
4.5. 16S rRNA-Seq Sample Collection
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stouthamer, R.B.; Breeuwer, J.A.J.; Luck, R.F.; Werren, J.H. Molecular identification of Agrobacterium tumefaciens microorganisms associated with parthenogenesis. Nature 1993, 361, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Biological control with Trichogramma: Advances, successes, and potential of their use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef] [PubMed]
- Gokhman, V.E.; Kuznetsova, V.G. Parthenogenesis in Hexapoda: Holometabolous insects. J. Zool. Syst. Evol. Res. 2018, 56, 23–34. [Google Scholar] [CrossRef]
- Stouthamer, R.; Luck, R.F.; Hamilton, W.D. Antibiotics cause parthenogenetic Trichogramma (Hymenoptera/Trichogrammatidae) to revert to sex. Proc. Natl. Acad. Sci. USA 1990, 87, 2424–2427. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.P.; Stouthamer, R. Phylogeny of the Trichogramma endosymbiont Wolbachia, an alpha-proteobacteria (Rickettsiae). Braz. J. Biol. 2018, 78, 421–428. [Google Scholar] [CrossRef]
- Girin, C.; Boulétreau, M. Microorganism-associated variation in host infestation efficiency in a parasitoid wasp, Trichogramma bourarachae (Hymenoptera: Trichogrammatidae). Experientia 1995, 51, 398–401. [Google Scholar] [CrossRef]
- Pintureau, B.; Chapelle, L.; Delobel, B. Effects of repeated thermic and antibiotic treatments on a Trichogramma (Hym., Trichogrammatidae) symbiont. J. Appl. Entomol. 1999, 123, 473–483. [Google Scholar] [CrossRef]
- Hohmann, C.L.; Luck, R.F. Effect of temperature on the development and thermal requirements of Wolbachia-infected and antibiotically cured Trichogramma kaykai Pinto and Stouthamer (Hymenoptera: Trichogrammatidae). An. Soc. Entomol. Bras. 2000, 29, 497–505. [Google Scholar] [CrossRef]
- Pintureauand, B.; Bolland, P.A. Trichogramma species showing a better adaptation to high temperature than its symbionts. Biocontrol. Sci. Technol. 2001, 11, 13–20. [Google Scholar] [CrossRef]
- Grenier, S.; Gomes, S.M.; Pintureau, B.; Lasslbiere, F.; Bolland, P. Use of tetracycline in larval diet to study the effect of Wolbachia on host fecundity and clarify taxonomic status of Trichogramma species in cured bisexual lines. J. Invertebr. Pathol. 2002, 80, 13–21. [Google Scholar] [CrossRef]
- Pintureau, B.; Lassabliere, F.; Daumal, J.; Grenier, S. Does a cyclic natural thermal cure occur in Wolbachia-infected Trichogramma species? Ecol. Entomol. 2002, 27, 366–372. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Heimpel, G.E. Quality assessment of three species of commercially produced Trichogramma and the first report of thelytokous in commercially produced Trichogramma. Biol. Control 2003, 26, 68–73. [Google Scholar] [CrossRef]
- Kazuki, M.; Yohsuke, T. Comparison of life history characters of arrhenotokous and Wolbachia-associated thelytokous Trichogramma kaykai Pinto and Stouthamer (Hymenoptera: Trichogrammatidae). Ann. Entomol. Soc. Am. 2004, 97, 765–769. [Google Scholar]
- Russell, J.E.; Stouthamer, R. The genetics and evolution of obligate reproductive parasitism in Trichogramma pretiosum infected with parthenogenesis-inducing Wolbachia. Heredity 2011, 106, 58–67. [Google Scholar] [CrossRef]
- Tulgetske, G.M.; Stouthamer, R. Characterization of intersex production in Trichogramma kaykai infected with parthenogenesis-inducing Wolbachia. Naturwissenschaften 2012, 99, 143–152. [Google Scholar] [CrossRef]
- Russell, J.E.; Saum, M.; Burgess, V.; Bollavaram, K.; Donnell, T. Influence of parthenogenesis-inducing Wolbachia infection and sexual mode on Trichogramma kaykai (Hymenoptera: Trichogrammatidae) fitness. Ann. Entomol. Soc. Am. 2017, 110, 263–268. [Google Scholar]
- Wang, X.X.; Qi, L.D.; Jiang, R.; Du, Y.Z.; Li, Y.X. Incomplete removal of Wolbachia with tetracycline has two-edged reproductive effects in the thelytokous wasp Encarsia formosa (Hymenoptera: Aphelinidae). Sci. Rep. 2017, 7, 44014. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Lan, R.M.; Ji, D.Z.; Tan, Y.N.; Zhou, X.; Tan, X.F.; Wu, Q.; Jin, L.H. The detection of Wolbachia in tea green leafhopper (Empoasca onukii Matsuda) and its influence on the host. Agriculture 2021, 12, 36. [Google Scholar] [CrossRef]
- Rodriguero, M.S.; Scannapieco, A.S.; Monti, D.S.; Chifflet, L.; Elias-Costa, A.J.; Lanteri, A.A.; Confalonier, V.A. Dependence of egg hatching on Wolbachia density in a parthenogenetic weevil revealed by antibiotic treatment. Entomol. Exp. Appl. 2021, 169, 384–392. [Google Scholar] [CrossRef]
- Taylor, M.J.; Bandi, C.; Hoerauf, A. Wolbachia bacterial endosymbionts of filarial nematodes. Adv. Parasit. 2005, 60, 245–284. [Google Scholar]
- Debrah, A.Y.; Specht, S.; Klarmann-Schulz, U.; Batsa, L.; Mand, S.; Marfo-Debrekyei, Y.; Fimmers, R.; Dubben, B.; Kwarteng, A.; Osei-Atweneboana, M.; et al. Doxycycline leads to sterility and enhanced killing of female Onchocerca volvulus worms in an area with persistent microfilaridermia after repeated ivermectin treatment: A randomized, placebo-controlled, double-blind trial. Clin. Infect. Dis. 2015, 61, 517–526. [Google Scholar] [CrossRef] [PubMed]
- GundersonI, E.L.; Vogel, L.; Chappell, L.; Bulman, C.A.; Lim, K.C.; Luo, M.; Whitman, J.D.; Franklin, C.; Choi, Y.J.; Lefoulon, E.; et al. The endosymbiont Wolbachia rebounds following antibiotic treatment. PLoS Pathog. 2020, 16, e1008623. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.O.; Moreau, C.S. Untangling the complex interactions between turtle ants and their microbial partners. Anim. Microbiome 2023, 5, 1. [Google Scholar] [CrossRef]
- Picciotti, U.; Araujo Dalbon, V.; Ciancio, A.; Colagiero, M.; Cozzi, G.; De Bellis, L.; Finetti-Sialer, M.M.; Greco, D.; Ippolito, A.; Lahbib, N.; et al. “Ectomosphere”: Insects and microorganism interactions. Microorganisms 2023, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Gantuya, B.; EI Serag, H.B.; Saruuljavkhlan, B.; Azzaya, D.; Matsumoto, T.; Uchida, T.; Oyuntsetseg, K.; Oyunbileg, N.; Davaadorj, D.; Yamaoka, Y. Advantage of 16S rRNA amplicon sequencing in Helicobacter pylori diagnosis. Helicobacter 2021, 26, e12790. [Google Scholar] [CrossRef]
- Brumfield, K.D.; Raupp, M.J.; Haji, D.; Simon, C.; Graf, J.; Cooley, J.R.; Janton, S.T.; Meister, R.C.; Huq, A.; Colwell, R.R.; et al. Gut microbiome insights from 16S rRNA analysis of 17-year periodical cicadas (Hemiptera: Magicicada spp.) Broods II, VI, and X. Sci. Rep. 2022, 12, 16967. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; An, Y.; Gao, L.; Dong, S.; Zhou, X.; Feng, Y.; Wang, P.; Dimopoulos, G.; Tang, H.; Wang, J. Glucose-mediated proliferation of a gut commensal bacterium promotes Plasmodium infection by increasing mosquito midgut pH. Cell Rep. 2021, 35, 108992. [Google Scholar] [CrossRef]
- Deutscher, A.T.; Burke, C.M.; Darling, A.E.; Riegler, M.; Reynolds, O.L.; Chapman, T.A. Near full-length 16S rRNA gene next-generation sequencing revealed Asaia as a common midgut bacterium of wild and domesticated Queensland fruit fly larvae. Microbiome 2018, 6, 85. [Google Scholar] [CrossRef]
- Pintureau, B.; Louis, C.; Chapelle, L. Symbiose entre microorganismes et Trichogrammes (Hym. Trichogrammatidae): Intérêt pour la lutte biologique. Bull. Soc. Zool. Fr. 1993, 118, 159–167. [Google Scholar]
- Silva, I.M.M.S.; Van Meer, M.M.M.; Roskam, M.M.; Hoogenboom, A.; Gort, G.; Stouthamer, R. Biological control potential of Wolbachia-infected versus uninfected wasps: Laboratory and greenhouse evaluation of Trichogramma cordubensis and T. deion strains. Biocontrol Sci. Technol. 2010, 10, 223–238. [Google Scholar] [CrossRef]
- Kent, M. Advanced Biology; Oxford University Press: Oxford, UK, 2000; p. 46. [Google Scholar]
- Castelli, L.A.; Nguyen, N.P.; Macreadie, I.G. Sulfa drug screening in yeast: Fifteen sulfa drugs compete with p-aminobenzoate in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2001, 199, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Calvori, C.; Frontali, L.; Leoni, L.; Tecce, G. Effect of rifamycin on protein synthesis. Nature 1965, 207, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.N.; Zanuncio, J.C.; Pratissoli, D.; Picanco, M.C. Biological characteristics of Trichogramma maxacalii (Hymenoptera: Trichogrammatidae) on eggs of Anagasta kuehniella (Lepidoptera: Pyralidae). Braz. J. Biol. 2003, 63, 647–653. [Google Scholar] [CrossRef]
- Lü, X.; Han, S.; Li, Z.; Li, L. Biological characters of Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae) reared in vitro versus in vivo for thirty generations. Sci. Rep. 2017, 7, 17928. [Google Scholar] [CrossRef]
- King, B.H. Offspring sex ratios in parasitoid wasps. Q. Rev. Biol. 1987, 62, 367–396. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hiehata, K. Mating systems and sex ratios in the egg parasitoids, Trichogramma dendrolimi and T. papilionis (Hymenoptera: Trichogrammatidae). Anim. Behav. 1985, 33, 1223–1227. [Google Scholar] [CrossRef]
- Arakaki, N.; Miyoshi, T.; Noda, H. Wolbachia-mediated parthenogenesis in the predatory thrips Franklinothrips vespiformis (Thysanoptera: Insecta). Proc. Biol. Sci. 2001, 268, 1011–1016. [Google Scholar] [CrossRef]
- Jeong, G.; Stouthamer, R. Genetics of female functional virginity in the parthenogenesis-Wolbachia infected parasitoid wasp Telenomus nawai (Hymenoptera: Scelionidae). Heredity 2005, 94, 402–407. [Google Scholar] [CrossRef]
- Kremer, N.; Charif, D.; Henri, H.; Bataille, M.; Prevost, G.; Kraaijeveld, K.; Vavre, F. A new case of Wolbachia dependence in the genus Asobara: Evidence for parthenogenesis induction in Asobara japonica. Heredity 2009, 103, 248–256. [Google Scholar] [CrossRef]
- Pijls, J.W.A.N.; van Steenbergen, H.J.; van Alphen, J.M. Asexuality cured: The relations and differences between sexual and asexual Apoanagyrus diversicornis. Heredity 1996, 76, 506–513. [Google Scholar] [CrossRef]
- Pannebakker, B.A.; Schidlo, N.S.; Boskamp, G.J.F.; Dekker, L.; Van Dooren, T.J.M.; Beukeboom, L.W.; Zwaan, B.J.; Brakefield, P.M.; Van Alphen, J.J.M. Sexual functionality of Leptopilina clavipes (Hymenoptera: Figitidae) after reversing Wolbachia-induced parthenogenesis. J. Evolution. Biol. 2005, 18, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Balvín, O.; Roth, S.; Talbot, B.; Reinhardt, K. Co-speciation in bedbug Wolbachia parallel the pattern in nematode hosts. Sci. Rep. 2018, 8, 8797. [Google Scholar] [CrossRef]
- Firake, D.M.; Khan, M.A. Alternating temperatures affect the performance of Trichogramma species. J. Insect. Sci. 2014, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Foerster, M.R.; Marchioro, C.A.; Foerster, L.A. Temperature-dependent parasitism, survival, and longevity of five species of Trichogramma Westwood (Hymenoptera: Trichogrammatidae) associated with anticarsia gemmatalis hiibner (Lepidoptera: Noctuidae). Neotrop. Entomol. 2014, 43, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, C.L.; Luck, R.F.; Stouthamer, R. Effect of Wolbachia on the survival and reproduction of Trichogramma kaykai Pinto and Stouthamer (Hymenoptera: Trichogrammatidae). Neotrop. Entomol. 2001, 30, 607–612. [Google Scholar] [CrossRef]
- Vereijssen, J.; Silva, I.; Honda, J.; Stouthamer, R. Development of a method to predict the biological control quality of Trichogramma strains. Proc. Sect. Exp. Appl. Entomol. Neth. Entomol. Soc. 1997, 8, 145–149. [Google Scholar]
- Wang, Z.; Smith, S.M. Phenotypic differences between thelytokous and arrhenotokous Trichogramma minutum from Zeiraphera canadensis. Entomol. Exp. Appl. 1996, 78, 315–323. [Google Scholar] [CrossRef]
- Smith, T.E.; Moran, N.A. Coordination of host and symbiont gene expression reveals a metabolic tug-of-war between aphids and Buchnera. Proc. Natl. Acad Sci. USA 2020, 117, 2113–2121. [Google Scholar] [CrossRef]
- Taylor, M.J.; Bordenstein, S.R.; Slatko, B. Microbe Profile: Wolbachia: A sex selector, a viral protector and a target to treat filarial nematodes. Microbiology 2018, 164, 1345–1347. [Google Scholar] [CrossRef]
- Itoh, H.; Jang, S.; Takeshita, K.; Ohbayashi, T.; Ohnishi, N.; Meng, X.Y.; Mitani, Y.; Kikuchi, Y. Host-symbiont specificity determined by microbe-microbe competition in an insect gut. Proc. Natl. Acad. Sci. USA 2019, 116, 22673–22682. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, S.; Foley, E. Host-microbe-pathogen interactions: A review of vibrio cholerae pathogenesis in Drosophila. Front. Immunol. 2019, 10, 3128. [Google Scholar] [CrossRef] [PubMed]
- Zug, R.; Hammerstein, P. Wolbachia and the insect immune system: What reactive oxygen species can tell us about the mechanisms of Wolbachia-host interactions. Front. Microbiol. 2015, 6, 1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; He, K.; Yang, Q.; Gong, M.; Ji, M.; Chen, L. Wolbachia limits pathogen infections through induction of host innate immune responses. PLoS ONE 2020, 15, e0226736. [Google Scholar] [CrossRef] [PubMed]
- Ourry, M.; Crosland, A.; Lopez, V.; Derocles, S.A.P.; Mougel, C.; Cortesero, A.M.; Poinsot, D. Influential insider: Wolbachia, an intracellular symbiont, manipulates bacterial diversity in its insect host. Microorganisms 2021, 9, 1313. [Google Scholar] [CrossRef]
- Russell, J.E.; Nunney, L.; Saum, M.; Stouthamer, R. Host and symbiont genetic contributions to fitness in a Trichogramma-Wolbachia symbiosis. PeerJ 2018, 6, e4655. [Google Scholar] [CrossRef]
- Duan, X.Z.; Sun, J.T.; Wang, L.T.; Shu, X.H.; Guo, Y.; Keiichiro, M.; Zhu, Y.X.; Bing, X.L.; Hoffmann, A.A.; Hong, X.Y. Recent infection by Wolbachia alters microbial communities in wild Laodelphax striatellus populations. Microbiome 2020, 8, 104. [Google Scholar] [CrossRef]
- Ourry, M.; Lopez, V.; Herve, M.; Lebreton, L.; Mougel, C.; Outreman, Y.; Poinsot, D.; Cortesero, A.M. Long-lasting effects of antibiotics on bacterial communities of adult flies. FEMS Microbiol. Ecol. 2020, 96, fiaa028. [Google Scholar] [CrossRef]
- Srinatha, H.S.; Jalali, S.K.; Sriram, S.; Chakravarthy, A.K. Isolation of microbes associated with field-collected populations of the egg parasitoid, Trichogramma chilonis capable of enhancing biotic fitness. Biocontrol Sci. Technol. 2015, 25, 789–802. [Google Scholar] [CrossRef]
- da Silva, H.; Oliveira, T.M.P.; Sallum, M.A.M. Bacterial community diversity and bacterial interaction network in eight mosquito species. Genes 2022, 13, 2052. [Google Scholar] [CrossRef]
- Du, S.J.; Ye, F.Y.; Wang, Q.J.; Liang, Y.X.; Wan, W.J.; Guo, J.Y.; Liu, W.X. Multiple data demonstrate that bacteria regulating reproduction could be not the cause for the thelytoky of Diglyphus wani (Hymenoptera: Eulophidae). Insects 2022, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Noel, H.R.; Petrey, J.R.; Palmer, L.D. Mobile genetic elements in Acinetobacter antibiotic-resistance acquisition and dissemination. Ann. N. Y. Acad. Sci. 2022, 1518, 166–182. [Google Scholar] [CrossRef]
- Nian, X.G.; Tao, X.B.; Xiao, Z.T.; Wang, D.S.; He, Y.R. Effects of sublethal concentrations of tetracycline hydrochloride on the biological characteristics and Wolbachia titer in parthenogenesis Trichogramma pretiosum. Insects 2022, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Hu, L. Advances in the research of tetracyclines antibiotics. J. Pharm. Res. 2017, 36, 1–5. [Google Scholar]
- Cubas, A.; Dutra, A.; Alves, T.; Bianchet, R.; Rambo, A.; Debacher, N. Evaluation of antimicrobial sensitivity to tetracycline exposed to non-thermal plasma. Química Nova 2023, XY, 1–5. [Google Scholar] [CrossRef]
- Brust, R.A. Gynandromorphs and intersexes in mosquitoes (Diptera: Culicidae). Can. J. Zool. 1966, 44, 911–921. [Google Scholar] [CrossRef]
- Stouthamer, R.; Hu, J.G.; van Kan, F.J.P.M.; Platner, G.R.; Pinto, J.D. The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma. BioControl 1999, 43, 421–440. [Google Scholar] [CrossRef]
- Wen, S.Y.; He, X.F. A method of rapid preparation of trace-DNA templates of insects for PCR. Entomol. Knowl. 2003, 40, 276–279. [Google Scholar]
- Zhou, W.; Rousset, F.; O’Neill, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microb. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Huws, S.A.; Edwards, J.E.; Kingston-Smith, A.H.; Siu-Ting, K.; Hughes, M.; Rubino, F.; Friedersdorff, M.; Creevey, C.J. CowPI: A rumen microbiome focussed version of the PICRUSt functional inference software. Front. Microbiol. 2018, 9, 1095. [Google Scholar] [CrossRef]

 The red symbol with a circle indicates male-female hybridization. F2S was generated after F1 was fed with sulfadiazine. F2H was generated by F1 fed with 30% honey water. F3S: the F2 male hybridized with the F1 female and fed 5.0 mg/mL sulfadiazine to produce F3S. F3H: the F2 male hybridized with the F1 female and fed 30% honey water to produce F3H.
The red symbol with a circle indicates male-female hybridization. F2S was generated after F1 was fed with sulfadiazine. F2H was generated by F1 fed with 30% honey water. F3S: the F2 male hybridized with the F1 female and fed 5.0 mg/mL sulfadiazine to produce F3S. F3H: the F2 male hybridized with the F1 female and fed 30% honey water to produce F3H.
 The red symbol with a circle indicates male-female hybridization. F2S was generated after F1 was fed with sulfadiazine. F2H was generated by F1 fed with 30% honey water. F3S: the F2 male hybridized with the F1 female and fed 5.0 mg/mL sulfadiazine to produce F3S. F3H: the F2 male hybridized with the F1 female and fed 30% honey water to produce F3H.
The red symbol with a circle indicates male-female hybridization. F2S was generated after F1 was fed with sulfadiazine. F2H was generated by F1 fed with 30% honey water. F3S: the F2 male hybridized with the F1 female and fed 5.0 mg/mL sulfadiazine to produce F3S. F3H: the F2 male hybridized with the F1 female and fed 30% honey water to produce F3H.
| Treatments | Generations | The Average Number of Parasitic Eggs | The Average Emergence Rate (%) | The Average Male Percentage (%) | Wolbachia Infection (n = 10) | N |
|---|---|---|---|---|---|---|
| Control | F0 | 29.40 ± 0.83 | 94.37 ± 1.12 | 0.00 ± 0.00 | 100% | 294 |
| Sulfadiazine | F0 | 26.90 ± 1.10 | 105.39 ± 2.31 ** | 0.00 ± 0.00 | 10% | 269 |
| F1(S) | 29.30 ± 1.38 | 101.83 ± 1.78 * | 100.00 ± 0.00 ** | 0% | 293 | |
| F1(H) | 29.60 ± 1.17 | 97.29 ± 1.15 * | 100.00 ± 0.00 ** | 0% | 296 | |
| F2(S) bisexual | 28.60 ± 0.52 | 105.25 ± 3.51 ** | 29.66 ± 7.04 ** | 0% | 286 | |
| F2(H) bisexual | 29.90 ± 0.50 | 104.14 ± 3.85 * | 29.20 ± 7.55 ** | 0% | 299 | |
| Tetracycline Hydrochloride | F0 | 14.40 ± 1.43 ** | 84.08 ± 5.86 * | 6.87 ± 2.76 * | 100% | 144 |
| F1 | 20.30 ± 0.93 ** | 82.29 ± 2.48 ** | 15.24 ± 3.02 ** | 100% | 203 | |
| F2 | 24.30 ± 1.80 * | 80.52 ± 3.28 ** | 17.31 ± 2.23 ** | 100% | 243 | |
| F3 | 21.80 ± 1.67 ** | 85.87 ± 2.53 * | 14.92 ± 3.89 ** | 100% | 218 | |
| F4 | 23.70 ± 2.30 * | 84.05 ± 2.99 * | 28.14 ± 10.53 ** | 100% | 237 | |
| High Temperature | F0 | 13.20 ± 0.93 ** | 97.59 ± 1.24 | 0.71 ± 0.71 | 100% | 132 |
| F1 | 28.40 ± 0.50 | 95.33 ± 1.64 | 2.54 ± 2.16 * | 100% | 284 | |
| F2 | 34.60 ± 0.58 ** | 89.90 ± 1.82 | 2.35 ± 1.24 * | 100% | 346 | |
| F3 | 33.70 ± 0.50 ** | 85.00 ± 2.80 * | 8.20 ± 1.96 ** | 100% | 337 | |
| F4 | 36.70 ± 0.54 ** | 84.83 ± 2.17 * | 9.22 ± 2.20 ** | 100% | 367 |
| Sample | Diversity Index | Coverage Rate (%) | ||||
|---|---|---|---|---|---|---|
| OTUs | Chao | Ace | Shannon | Simpson | ||
| TPW-H-F | 60 | 35.42 ± 2.79 | 49.17 ± 9.50 | 0.27 ± 0.078 | 0.92 ± 0.023 | 100.00 ± 0.00 |
| TPW-S-F | 157 | 77.00 ± 24.48 | 80.05 ± 23.21 | 1.84 ± 0.40 | 0.32 ± 0.060 * | 100.00 ± 0.00 |
| TPW-TH-F | 87 | 87.00 ± 17.59 * | 87.00 ± 10.60 | 3.41 ± 0.54 * | 0.07 ± 0.016 ** | 100.00 ± 0.00 |
| TPW-HT-F | 103 | 104.64 ± 7.80 ** | 106.40 ± 13.25 ** | 0.29 ± 0.11 | 0.93 ± 0.072 | 99.96 ± 0.017 |
| TPW-S-M | 220 | 101.33 ± 14.19 ** | 101.07 ± 15.68 ** | 2.12 ± 0.51 | 0.4 ± 0.14 | 100.00 ± 0.00 |
| TPW-H-F | TPW-S-F | TPW-TH-F | TPW-HT-F | TPW-S-M |
|---|---|---|---|---|
| Wolbachia 99.01 ± 2.30 | Wolbachia 38.49 ± 14.44 ** | Wolbachia 20.12 ± 2.31 ** | Wolbachia 96.43 ± 1.48 | Wolbachia 0.28 ± 0.27 ** |
| Acinetobacter 0.72 ± 0.31 | Acinetobacter 40.59 ± 6.27 ** | Unclassified 13.06 ± 4.01 | Unclassified 0.59 ± 0.31 | Acinetobacter 63.25 ± 17.99 ** |
| Pseudomonas 0.04 ± 0.06 | Pseudomonas 1.22 ± 0.28 | Enhydrobacter 12.16 ± 3.49 | Acinetobacter 0.39 ± 0.16 | Pseudomonas 2.95 ± 1.72 |
| Pelomonas 0.02 ± 0.018 | Pelomonas 1.56 ± 0.88 | Burkholderia 7.60 ± 1.52 | Burkholderia 0.33 ± 0.09 | Pelomonas 1.86 ± 0.64 |
| Prevotella 0.18 ± 0.31 | Prevotella 1.23 ± 1.68 | Bradyrhizobium 5.78 ± 1.94 | Others (<0.5%) 0.30 ± 0.06 | Prevotella 1.46 ± 2.35 |
| Megasphaera - | Megasphaera 1.85 ± 3.21 | Acinetobacter 4.90 ± 1.11 ** | Bradyrhizobium 0.30 ± 0.07 | Megasphaera 0.75 ± 1.29 |
| Enhydrobacter 0.03 ± 0.05 | Enhydrobacter 0.63 ± 0.24 | Sphingomonas 4.14 ± 1.08 | Serratia 0.27 ± 0.11 | Enhydrobacter 1.88 ± 0.80 |
| Coprococcus - | Coprococcus 0.76 ± 0.85 | Others (<0.5%) 3.78 ± 1.07 | Sphingomonas 0.14 ± 0.02 | Coprococcus 1.50 ± 1.73 |
| Ruminococcus - | Ruminococcus 0.38 ± 0.62 | Methylobacterium 3.61 ± 1.01 | Prevotella 0.12 ± 0.06 | Ruminococcus 1.75 ± 2.92 |
| Butyricicoccus - | Butyricicoccus 0.81 ± 1.29 | Prevotella 2.51 ± 0.61 | Staphylococcus 0.10 ± 0.009 | Butyricicoccus 1.27 ± 2.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Zhang, M.; Lin, L.; Zeng, C.; Zhang, Y.; He, X. Bacterial Community Survey of Wolbachia-Infected Parthenogenetic Parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) Treated with Antibiotics and High Temperature. Int. J. Mol. Sci. 2023, 24, 8448. https://doi.org/10.3390/ijms24098448
Guo W, Zhang M, Lin L, Zeng C, Zhang Y, He X. Bacterial Community Survey of Wolbachia-Infected Parthenogenetic Parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) Treated with Antibiotics and High Temperature. International Journal of Molecular Sciences. 2023; 24(9):8448. https://doi.org/10.3390/ijms24098448
Chicago/Turabian StyleGuo, Wei, Meijiao Zhang, Liangguan Lin, Chenxu Zeng, Yuping Zhang, and Xiaofang He. 2023. "Bacterial Community Survey of Wolbachia-Infected Parthenogenetic Parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) Treated with Antibiotics and High Temperature" International Journal of Molecular Sciences 24, no. 9: 8448. https://doi.org/10.3390/ijms24098448
APA StyleGuo, W., Zhang, M., Lin, L., Zeng, C., Zhang, Y., & He, X. (2023). Bacterial Community Survey of Wolbachia-Infected Parthenogenetic Parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) Treated with Antibiotics and High Temperature. International Journal of Molecular Sciences, 24(9), 8448. https://doi.org/10.3390/ijms24098448






