Co-Loading of Black Phosphorus Nanoflakes and Doxorubicin in Lysolipid Temperature-Sensitive Liposomes for Combination Therapy in Prostate Cancer
Abstract
:1. Introduction
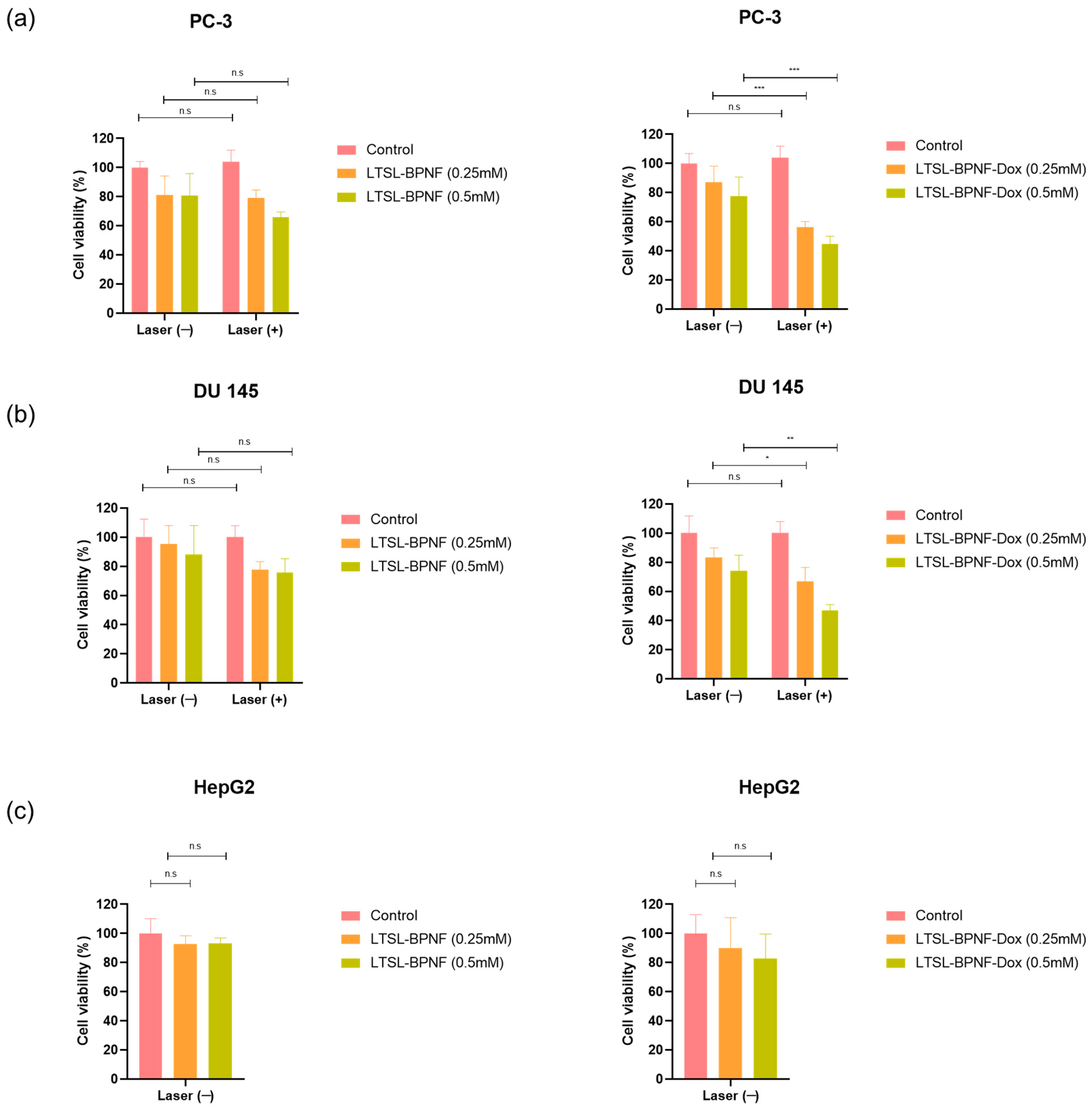
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
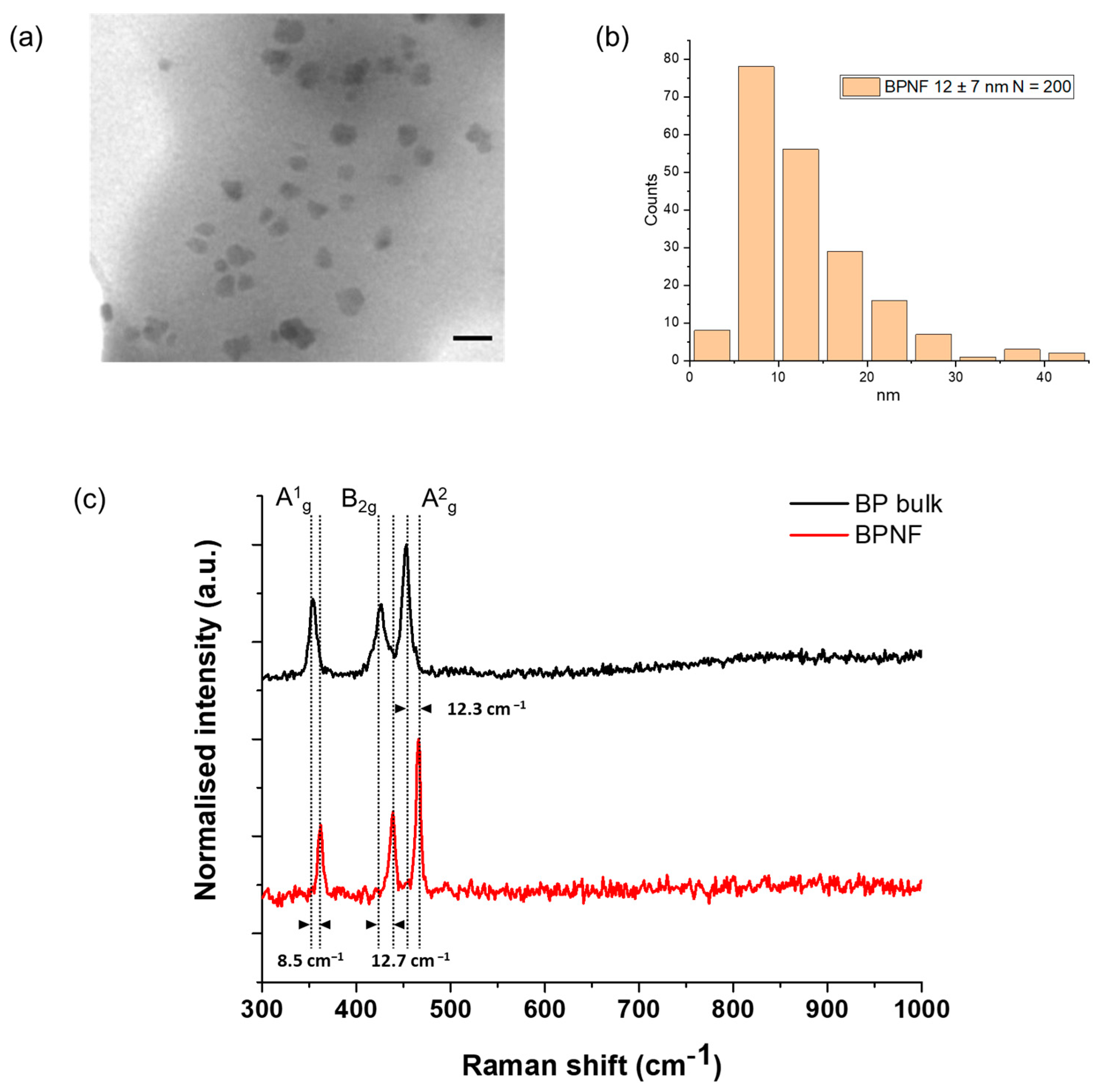
3.2.1. Synthesis of BPNFs
3.2.2. Preparation of LTSL-BPNFs
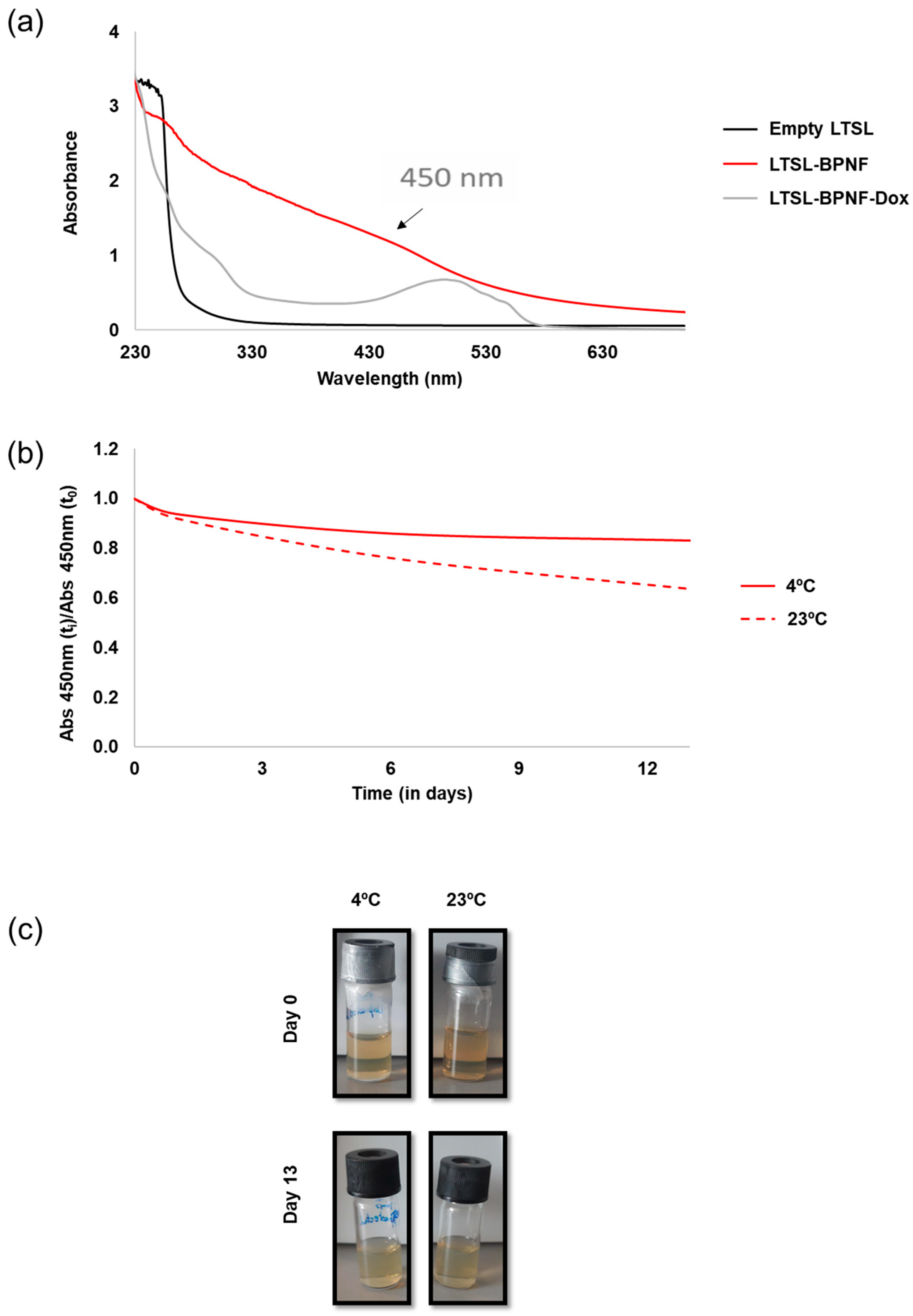
3.2.3. Dox Loading into LTSL-BPNFs
3.2.4. Characterisation of BPNFs
3.2.5. Colloidal Properties
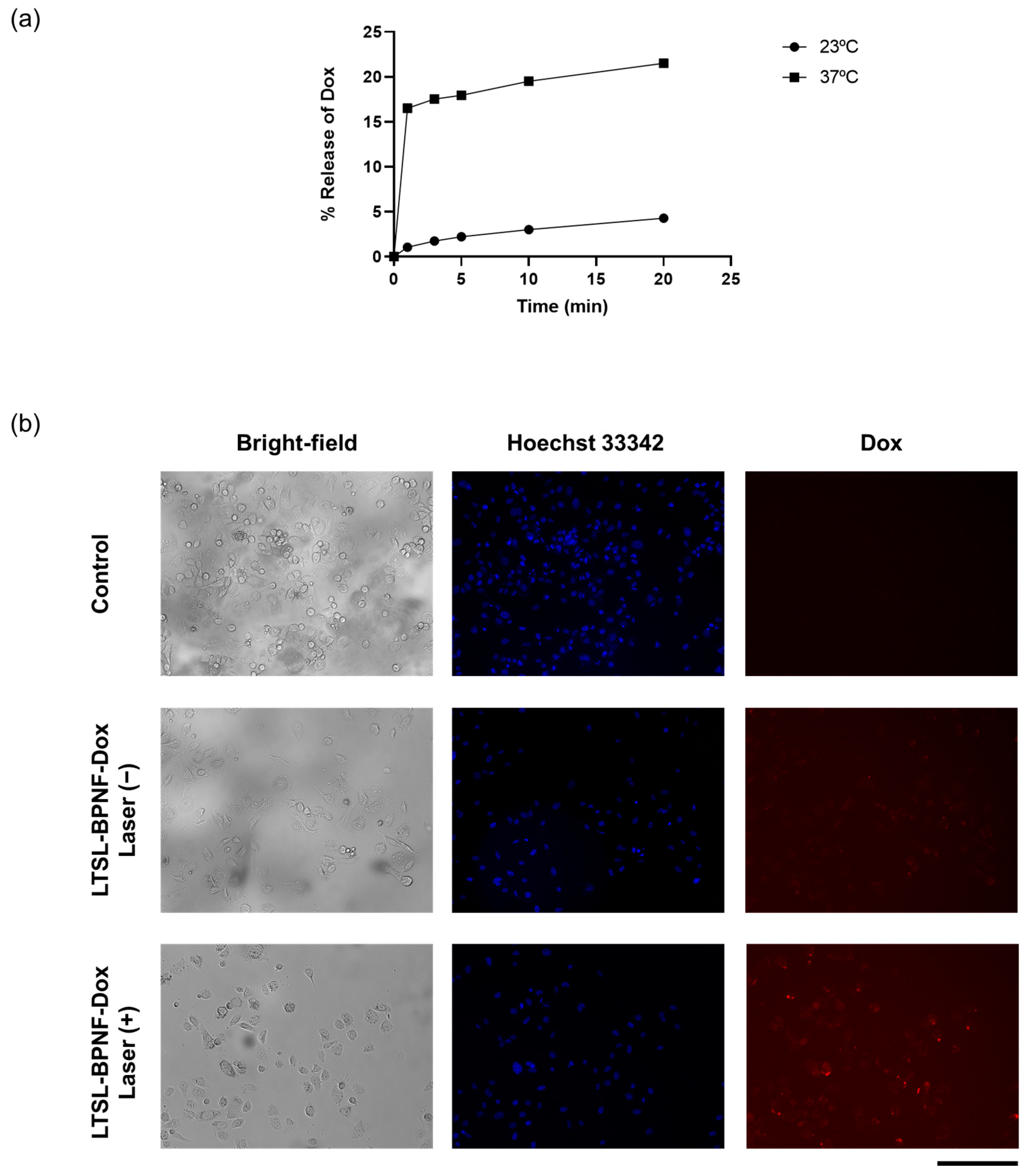
3.2.6. Near-Infrared (808 nm) Laser-Induced Dox Release
3.2.7. Cell Culture
3.2.8. Dox Uptake Study
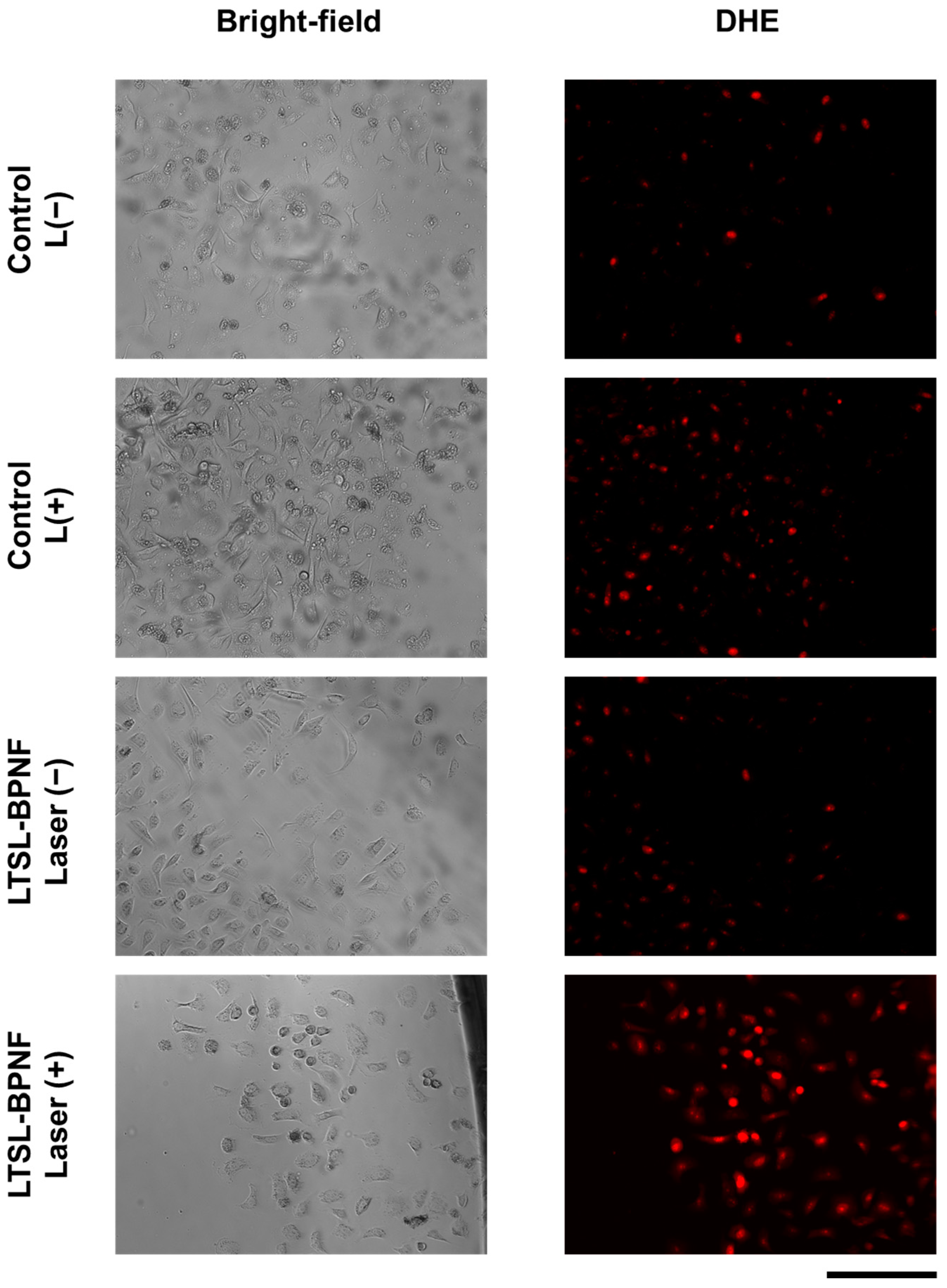
3.2.9. ROS Production
3.2.10. In Vitro Cancer Therapy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in Nanomaterials for Photodynamic Therapy Applications: Status and Challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Home|Beta ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 17 December 2023).
- Moore, C.M.; Pendse, D.; Emberton, M. Photodynamic Therapy for Prostate Cancer—A Review of Current Status and Future Promise. Nat. Clin. Pract. Urol. 2009, 6, 18–30. [Google Scholar] [CrossRef]
- Ruiz, A.; Martín, C.; Reina, G. Does Black Phosphorus Hold Potential to Overcome Graphene Oxide? A Comparative Review of Their Promising Application for Cancer Therapy. Nanoscale Adv. 2021, 3, 4029–4036. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal Therapy and Photoacoustic Imaging via Nanotheranostics in Fighting Cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B. Doxorubicin Induces Cardiotoxicity through Upregulation of Death Receptors Mediated Apoptosis in Cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef]
- Chang, H.I.; Yeh, M.K. Clinical Development of Liposome-Based Drugs: Formulation, Characterization, and Therapeutic Efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar]
- Ruiz, A.; Ma, G.; Seitsonen, J.; Pereira, S.G.T.; Ruokolainen, J.; Al-Jamal, W.T. Encapsulated Doxorubicin Crystals Influence Lysolipid Temperature-Sensitive Liposomes Release and Therapeutic Efficacy In Vitro and In Vivo. J. Control. Release 2020, 328, 665–678. [Google Scholar] [CrossRef]
- Santos, M.A.; Goertz, D.E.; Hynynen, K. Focused Ultrasound Hyperthermia Mediated Drug Delivery Using Thermosensitive Liposomes and Visualized with In Vivo Two-Photon Microscopy. Theranostics 2017, 7, 2718. [Google Scholar] [CrossRef]
- Ma, G.; Kostevšek, N.; Monaco, I.; Ruiz, A.; Markelc, B.; Cheung, C.C.L.; Hudoklin, S.; Kreft, M.E.; Hassan, H.A.F.M.; Barker, M.; et al. PD1 Blockade Potentiates the Therapeutic Efficacy of Photothermally-Activated and MRI-Guided Low Temperature-Sensitive Magnetoliposomes. J. Control. Release 2021, 332, 419–433. [Google Scholar] [CrossRef]
- Geng, S.; Li, Z.; Zhang, R.; Zhou, W.; Luo, G.; Chu, P.K.; Yu, X.F. Complete Ablation of Resistant Tumors with Photosensitive Black Phosphorus Quantum Dots-Based Lipid Nanocapsules. Chem. Eng. J. 2021, 421, 127879. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.F.X.-F.; Guo, Z.; Shao, J.; Zhang, H.; Huang, H.; Wang, H.; Chu, P.K.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef]
- Karki, B.; Freelon, B.; Rajapakse, M.; Musa, R.; Riyadh, S.M.S.; Morris, B.; Abu, U.; Yu, M.; Sumanasekera, G.; Jasinski, J.B. Strain-Induced Vibrational Properties of Few Layer Black Phosphorus and MoTe2 via Raman Spectroscopy. Nanotechnology 2020, 31, 425707. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Liu, Z.; Tan, C.; Luo, Z.; Li, H.; Lin, J.; Sun, L.; Chen, W.; Xu, Z.; et al. Black Phosphorus Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 3653–3657. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, L.; Wang, P.; Fang, Y. Intense, Stable and Excitation Wavelength-Independent Photoluminescence Emission in the Blue-Violet Region from Phosphorene Quantum Dots. Sci. Rep. 2016, 6, 27307. [Google Scholar] [CrossRef]
- Late, D.J. Temperature Dependent Phonon Shifts in Few-Layer Black Phosphorus. ACS Appl. Mater. Interfaces 2015, 7, 5857–5862. [Google Scholar] [CrossRef]
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A New Temperature-Sensitive Liposome for Use with Mild Hyperthermia: Characterization and Testing in a Human Tumor Xenograft Model. Cancer Res. 2000, 60, 1197–1201. [Google Scholar]
- Cheung, B.C.L.; Sun, T.H.T.; Leenhouts, J.M.; Cullis, P.R. Loading of Doxorubicin into Liposomes by Forming Mn2+-Drug Complexes. Biochim. Biophys. Acta Biomembr. 1998, 1414, 205–216. [Google Scholar] [CrossRef]
- Song, Y.Y.; Huang, Z.; Song, Y.Y.; Tian, Q.; Liu, X.; She, Z.; Jiao, J.; Lu, E.; Deng, Y. The Application of EDTA in Drug Delivery Systems: Doxorubicin Liposomes Loaded via NH4EDTA Gradient. Int. J. Nanomed. 2014, 9, 3611–3621. [Google Scholar]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.-F.; Zhao, Y.; Zhang, H.; et al. Biodegradable Black Phosphorus-Based Nanospheres for In Vivo Photothermal Cancer Therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef]
- Anyarambhatla, G.R.R.; Needham, D. Enhancement of the Phase Transition Permeability of DPPC Liposomes by Incorporation of MPPC: A New Temperature-Sensitive Liposome for Use with Mild Hyperthermia. J. Liposome Res. 1999, 9, 491–506. [Google Scholar] [CrossRef]
- Mills, J.K.; Needham, D. Lysolipid Incorporation in Dipalmitoylphosphatidylcholine Bilayer Membranes Enhances the Ion Permeability and Drug Release Rates at the Membrane Phase Transition. Biochim. Biophys. Acta Biomembr. 2005, 1716, 77–96. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Al-Ahmady, Z.S.; Kostarelos, K. Pharmacokinetics & Tissue Distribution of Temperature-Sensitive Liposomal Doxorubicin in Tumor-Bearing Mice Triggered with Mild Hyperthermia. Biomaterials 2012, 33, 4608–4617. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef]
- Geng, S.; Wu, L.; Cui, H.; Tan, W.; Chen, T.; Chu, P.K.; Yu, X.F. Synthesis of Lipid–Black Phosphorus Quantum Dot Bilayer Vesicles for near-Infrared-Controlled Drug Release. Chem. Commun. 2018, 54, 6060–6063. [Google Scholar] [CrossRef]
- Cell Line:905934—Cancerrxgene—Genomics of Drug Sensitivity in Cancer. Available online: https://www.cancerrxgene.org/cellline/JHU-029/905934 (accessed on 17 December 2023).
- Garcia-Diaz, M.; Huang, Y.Y.; Hamblin, M.R. Use of Fluorescent Probes for ROS to Tease Apart Type I and Type II Photochemical Pathways in Photodynamic Therapy. Methods 2016, 109, 158–166. [Google Scholar] [CrossRef]
- Bu, Y.; Xu, T.; Zhu, X.; Zhang, J.; Wang, L.; Yu, Z.; Yu, J.; Wang, A.; Tian, Y.; Zhou, H.; et al. A NIR-I Light-Responsive Superoxide Radical Generator with Cancer Cell Membrane Targeting Ability for Enhanced Imaging-Guided Photodynamic Therapy. Chem. Sci. 2020, 11, 10279–10286. [Google Scholar] [CrossRef]
- Rudolf, H. Cytotoxicity Testing According to EN ISO 10993-5. Available online: https://www.johner-institute.com/articles/product-development/and-more/cytotoxicity-testing-according-to-en-iso-10993-5/ (accessed on 17 December 2023).
- Banno, B.; Ickenstein, L.M.; Chiu, G.N.C.; Bally, M.B.; Thewalt, J.; Brief, E.; Wasan, E.K. The Functional Roles of Poly(Ethylene Glycol)-Lipid and Lysolipid in the Drug Retention and Release from Lysolipid-Containing Thermosensitive Liposomes In Vitro and In Vivo. J. Pharm. Sci. 2010, 99, 2295–2308. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, X.; Liu, Y.; Yang, X.; Wang, J.; Liu, Q.; Luo, Q.; Jing, C.; Yu, X.F.; Qu, G.; et al. Surface and Interface Control of Black Phosphorus. Chem 2022, 8, 632–662. [Google Scholar] [CrossRef]
- Kong, N.; Ji, X.; Wang, J.; Sun, X.; Chen, G.; Fan, T.; Liang, W.; Zhang, H.; Xie, A.; Farokhzad, O.C.; et al. Ros-Mediated Selective Killing Effect of Black Phosphorus: Mechanistic Understanding and Its Guidance for Safe Biomedical Applications. Nano Lett. 2020, 20, 3943–3955. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Zhang, Q.; Chen, T.; Zhang, C. BP@Au Undergoes Rapid Degradation and Releases Singlet Oxygen under Dark Conditions: Doping Effect and Detrimental Effects on Superoxide-Producing Marine Algae. J. Hazard. Mater. 2023, 454, 131502. [Google Scholar] [CrossRef]

| Hydrodynamic Size ± SD (nm) | PDI ± SD | Z-Potential ± SD (mV) | |
|---|---|---|---|
| BPNF | 154.2 (±3.31) | 0.225 (±0.02) | - |
| Empty LTSL | 80.75 (±0.58) | 0.266 (±0.01) | −8.08 (±0.92) |
| LTSL-BPNF | 114.1 (±4.01) | 0.465 (±0.01) | −8.79 (±0.734) |
| LTSL-BPNF-Dox | 135.8 (±2.01) | 0.277 (±0.01) | −11.4 (±1.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, C.; Martín, C.; Habermann, S.; Walker, H.R.; Iqbal, J.; Elies, J.; Jones, H.S.; Reina, G.; Ruiz, A. Co-Loading of Black Phosphorus Nanoflakes and Doxorubicin in Lysolipid Temperature-Sensitive Liposomes for Combination Therapy in Prostate Cancer. Int. J. Mol. Sci. 2024, 25, 115. https://doi.org/10.3390/ijms25010115
Das C, Martín C, Habermann S, Walker HR, Iqbal J, Elies J, Jones HS, Reina G, Ruiz A. Co-Loading of Black Phosphorus Nanoflakes and Doxorubicin in Lysolipid Temperature-Sensitive Liposomes for Combination Therapy in Prostate Cancer. International Journal of Molecular Sciences. 2024; 25(1):115. https://doi.org/10.3390/ijms25010115
Chicago/Turabian StyleDas, Chandrima, Cristina Martín, Sebastian Habermann, Harriet Rose Walker, Javed Iqbal, Jacobo Elies, Huw Simon Jones, Giacomo Reina, and Amalia Ruiz. 2024. "Co-Loading of Black Phosphorus Nanoflakes and Doxorubicin in Lysolipid Temperature-Sensitive Liposomes for Combination Therapy in Prostate Cancer" International Journal of Molecular Sciences 25, no. 1: 115. https://doi.org/10.3390/ijms25010115











