Expression Dynamics Indicate Potential Roles of KIF17 for Nuclear Reshaping and Tail Formation during Spermiogenesis in Phascolosoma esculenta
Abstract
:1. Introduction
2. Results
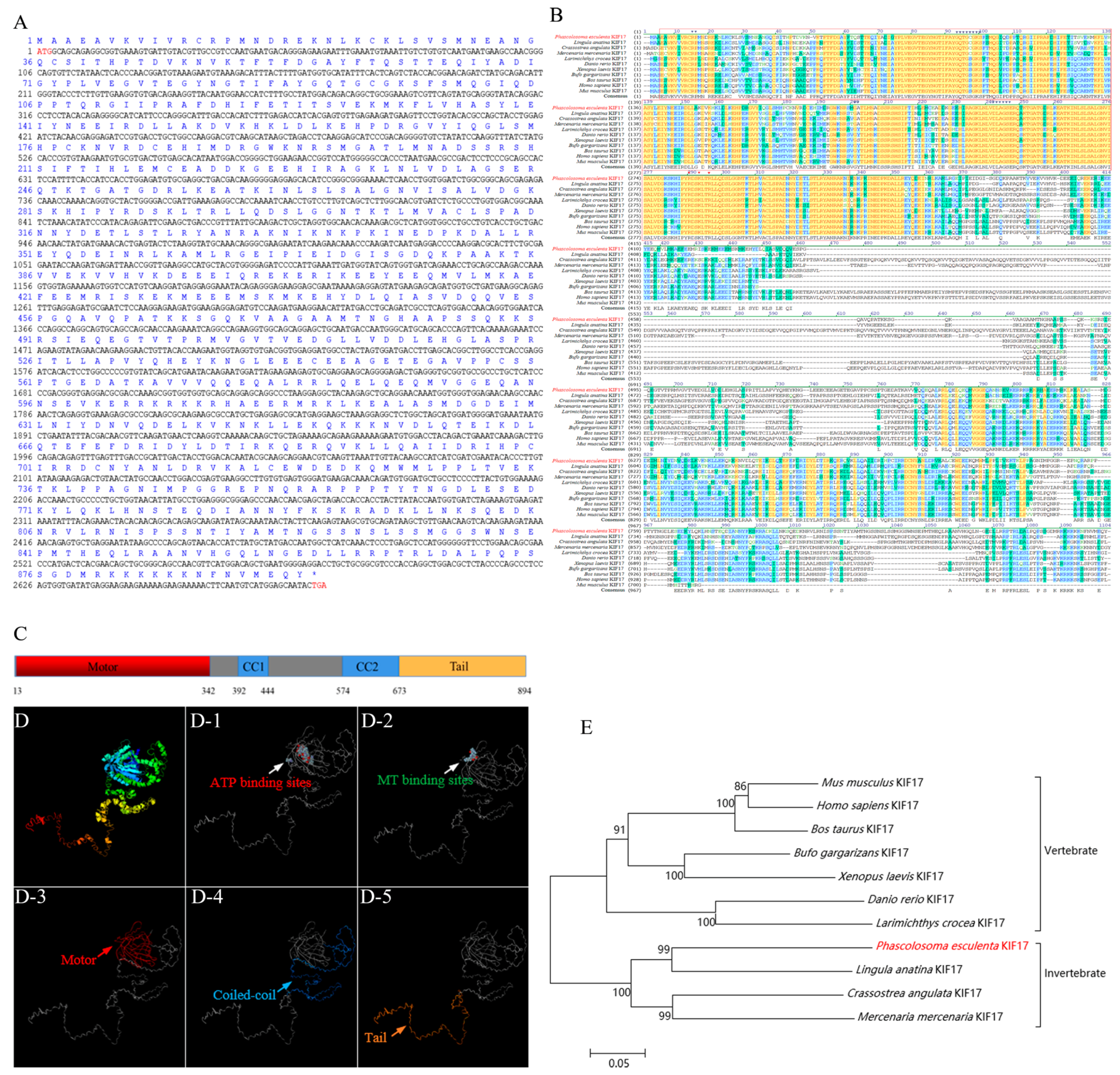
2.1. Bioinformatic Analysis of the Pe-kif17 Gene
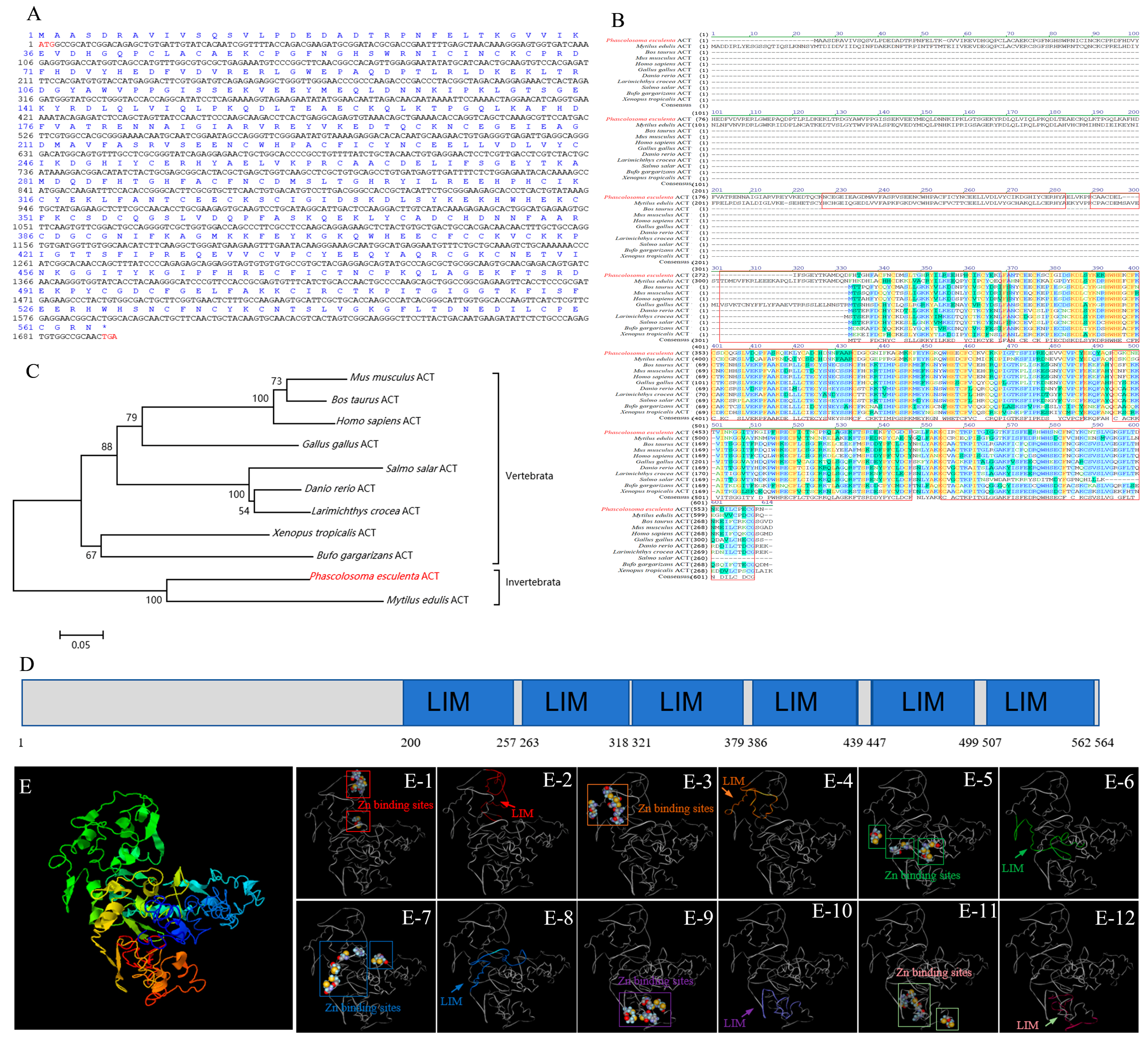
2.2. Bioinformatic Analysis of Pe-act Gene
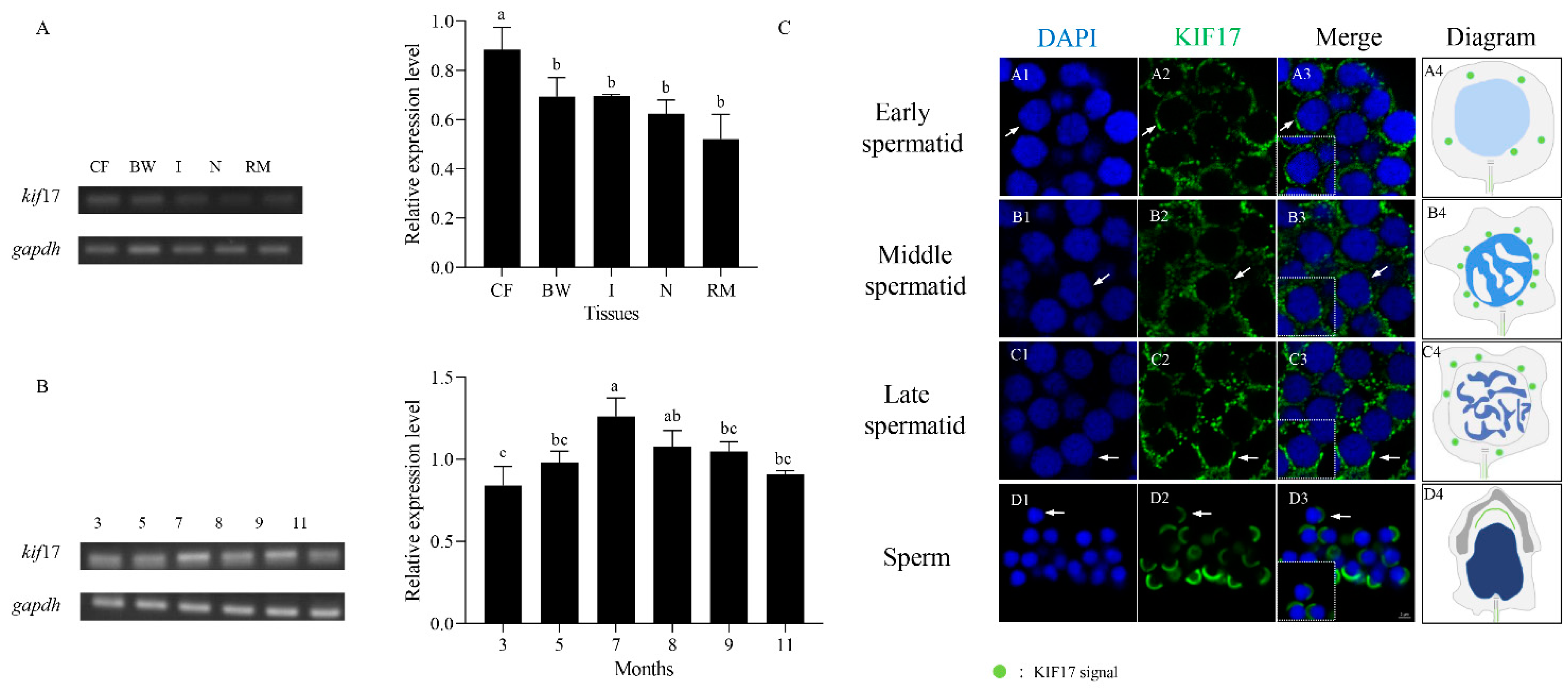
2.3. Expression Analysis of Pe-kif17 mRNA
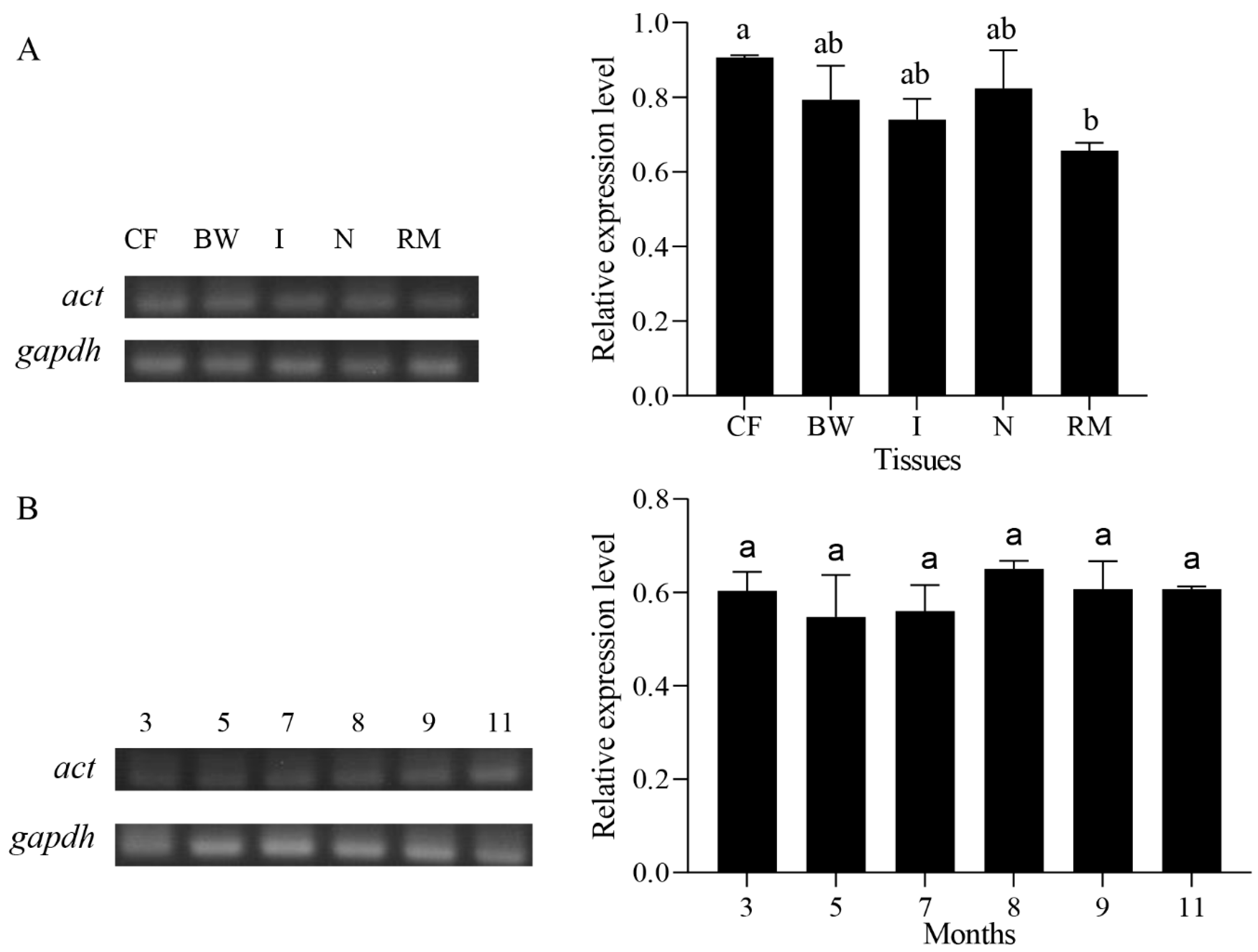
2.4. Expression Analysis of Pe-act mRNA
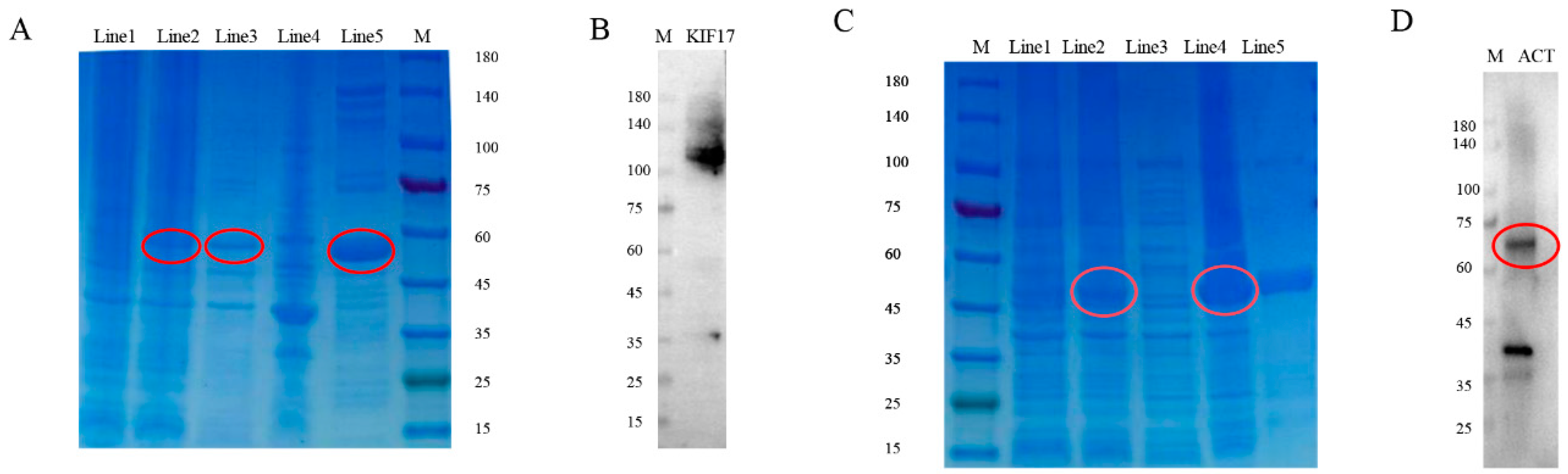
2.5. Preparation and Specificity of Pe-KIF17/ACT Antibodies
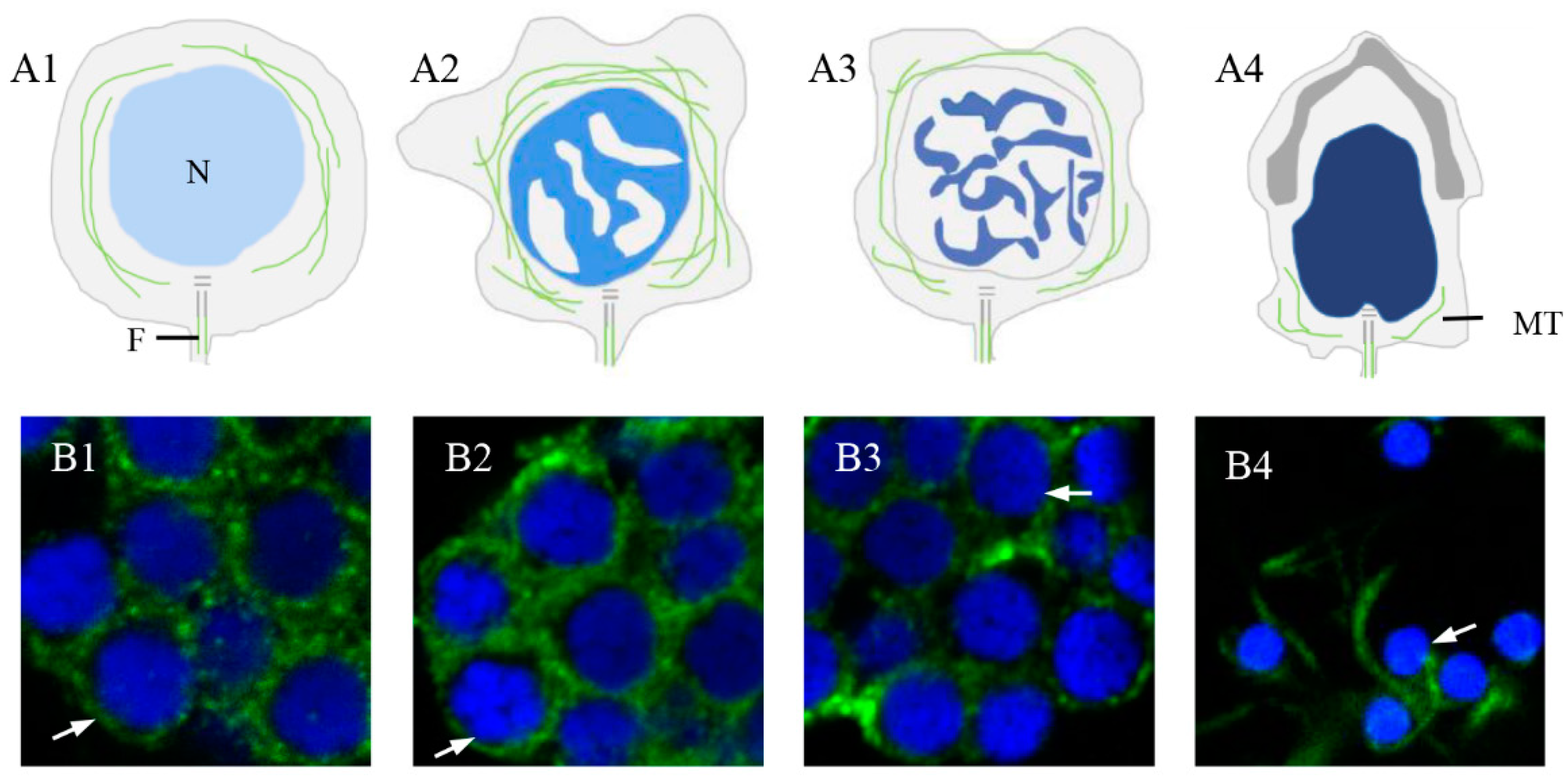
2.6. Expression and Colocalization Characteristics of Pe-KIF17 with Tubulin during Spermiogenesis
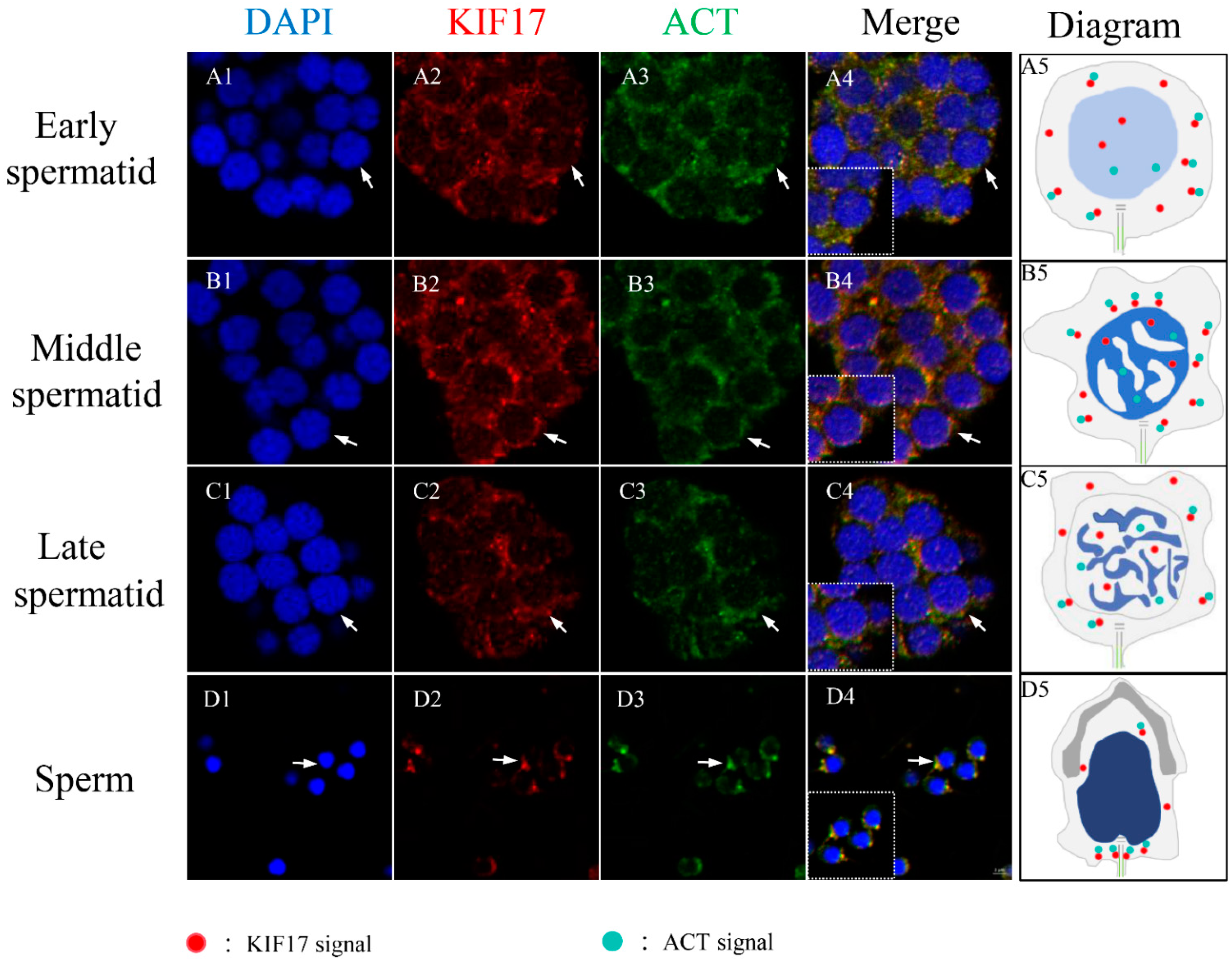
2.7. Colocalization between Pe-KIF17 and Pe-ACT in Spermiogenesis
3. Discussion
3.1. Structural Features of KIF17 and ACT
3.2. Expression Characteristics of Pe-kif17/act mRNA
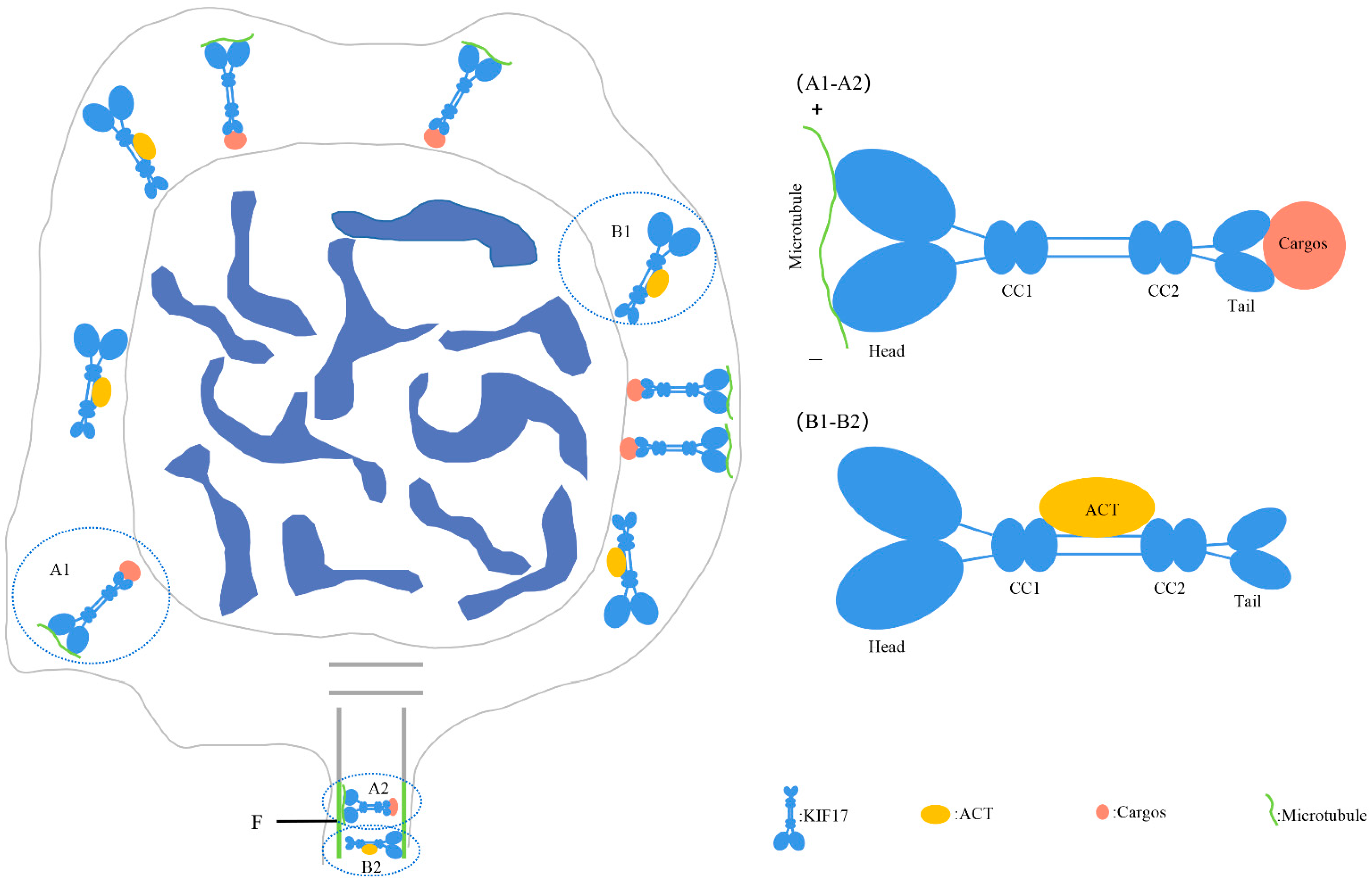
3.3. Pe-KIF17 May Be Involved in Nuclear Reshaping and Tail Formation in a Microtubule-Dependent Manner
3.4. Pe-KIF17 and Pe-ACT May Participate in Nuclear Reshaping and Tail Formation Independently of Microtubules
4. Materials and Methods
4.1. Tissue Sampling
4.2. RNA and Protein Extraction
4.3. Cloning of kif17/act (ORF)
4.4. Bioinformatics Analysis of KIF17/ACT
4.5. Semiquantitative RT-PCR Analysis of kif17/act mRNA Expression
4.6. FISH
4.7. Antibodies
4.8. Western Blotting
4.9. IF
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, D.D.; Wang, D.H.; Yang, W.X. Kinesins in Spermatogenesis. Biol. Reprod. 2017, 96, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Mochida, K.; Tres, L.L.; Kierszenbaum, A.L. Structural and Biochemical Features of Fractionated Spermatid Manchettes and Sperm Axonemes of theAzh/Azh Mutant Mouse. Mol. Reprod. Dev. 1999, 52, 434–444. [Google Scholar] [CrossRef]
- Lehti, M.S.; Sironen, A. Formation and Function of the Manchette and Flagellum during Spermatogenesis. Reproduction 2016, 151, R43–R54. [Google Scholar] [CrossRef] [PubMed]
- Dooher, G.B.; Bennett, D. Spermiogenesis and Spermatozoa in Sterile Mice Carrying Different Lethal T/t Locus Haplotypes: A Transmission and Scanning Electron Microscopic Study. Biol. Reprod. 1977, 17, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.S.; Eveleth, J.; Jiang, C.; Redenbach, D.M.; Boekelheide, K. Distribution of the Microtubule-Dependent Motors Cytoplasmic Dynein and Kinesin in Rat Testis 1. Biol. Reprod. 1992, 46, 817–828. [Google Scholar] [CrossRef]
- Kierszenbaum, A.L. Spermatid Manchette: Plugging Proteins to Zero into the Sperm Tail. Mol. Reprod. Dev. 2001, 59, 347–349. [Google Scholar] [CrossRef]
- Russell, L.D.; Russell, J.A.; MacGregor, G.R.; Meistrich, M.L. Linkage of Manchette Microtubules to the Nuclear Envelope and Observations of the Role of the Manchette in Nuclear Shaping during Spermiogenesis in Rodents. Am. J. Anat. 1991, 192, 97–120. [Google Scholar] [CrossRef]
- Brady, S.T. A Novel Brain ATPase with Properties Expected for the Fast Axonal Transport Motor. Nature 1985, 317, 73–75. [Google Scholar] [CrossRef]
- Yang, W.-X.; Sperry, A.O. C-Terminal Kinesin Motor KIFC1 Participates in Acrosome Biogenesis and Vesicle Transport1. Biol. Reprod. 2003, 69, 1719–1729. [Google Scholar] [CrossRef]
- Kierszenbaum, A.L. Intramanchette Transport (IMT): Managing the Making of the Spermatid Head, Centrosome, and Tail. Mol. Reprod. Dev. 2002, 63, 1–4. [Google Scholar] [CrossRef]
- Navolanic, P.M.; Sperry, A.O. Identification of Isoforms of a Mitotic Motor in Mammalian Spermatogenesis1. Biol. Reprod. 2000, 62, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Lehti, M.S.; Kotaja, N.; Sironen, A. KIF3A Is Essential for Sperm Tail Formation and Manchette Function. Mol. Cell. Endocrinol. 2013, 377, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.P.; Guzik-Lendrum, S.; Rayment, I. Kinesin-2 Motors: Kinetics and Biophysics. J. Biol. Chem. 2018, 293, 4510–4518. [Google Scholar] [CrossRef] [PubMed]
- Setou, M.; Nakagawa, T.; Seog, D.-H.; Hirokawa, N. Kinesin Superfamily Motor Protein KIF17 and mLin-10 in NMDA Receptor-Containing Vesicle Transport. Science 2000, 288, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Feng, X.; Takei, Y.; Hirokawa, N. Regulation of NMDA Receptor Transport: A KIF17–Cargo Binding/Releasing Underlies Synaptic Plasticity and Memory In Vivo. J. Neurosci. 2012, 32, 5486–5499. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Okada, Y.; Hirokawa, N. Analysis of the Kinesin Superfamily: Insights into Structure and Function. Trends Cell Biol. 2005, 15, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, X.; Zheng, X.; Du, C.; Hou, C.; Xie, Q.; Lou, B.; Liu, F.; Jin, S.; Zhu, J. Evidence for the Role of KIF17 in Fish Spermatid Reshaping: Expression Pattern of KIF17 in Larimichthys polyactis Spermiogenesis. J. Ocean. Limnol. 2021, 39, 2322–2335. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Gao, X.; Du, C.; Hou, C.; Tang, D.; Lou, B.; Shen, W.; Zhu, J. The Potential Function of KIF17 in Large Yellow Croaker (Larimichthys crocea) Spermatid Remodeling: Molecular Characterization and Expression Pattern during Spermiogenesis. Fish Physiol. Biochem. 2022, 48, 603–616. [Google Scholar] [CrossRef]
- Saade, M.; Irla, M.; Govin, J.; Victorero, G.; Samson, M.; Nguyen, C. Dynamic Distribution of Spatial during Mouse Spermatogenesis and Its Interaction with the Kinesin KIF17b. Exp. Cell Res. 2007, 313, 614–626. [Google Scholar] [CrossRef]
- Macho, B.; Brancorsini, S.; Fimia, G.M.; Setou, M.; Hirokawa, N.; Sassone-Corsi, P. CREM-Dependent Transcription in Male Germ Cells Controlled by a Kinesin. Science 2002, 298, 2388–2390. [Google Scholar] [CrossRef]
- Krausz, C.; Sassone-Corsi, P. Genetic Control of Spermiogenesis: Insights from the CREM Gene and Implications for Human Infertility. Reprod. BioMed. Online 2005, 10, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Steger, K. Expression of Activator of CREM in the Testis (ACT) during Normal and Impaired Spermatogenesis: Correlation with CREM Expression. Mol. Hum. Reprod. 2004, 10, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, K.N.; Sassone-Corsi, P. Regulation of Gene Expression in Post-Meiotic Male Germ Cells: CREM-Signalling Pathways and Male Fertility. Hum. Fertil. 2006, 9, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Fimia, G.M.; Cesare, D.D.; Sassone-Corsi, P. CBP-Independent Activation of CREM and CREB by the LIM-Only Protein ACT. Nature 1999, 398, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Schmeichel, K. The LIM Domain Is a Modular Protein-Binding Interface. Cell 1994, 79, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Dawid, I.B.; Breen, J.J.; Toyama, R. LIM Domains: Multiple Roles as Adapters and Functional Modifiers in Protein Interactions. Trends Genet. 1998, 14, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kotaja, N.; Macho, B.; Sassone-Corsi, P. Microtubule-Independent and Protein Kinase A-Mediated Function of Kinesin KIF17b Controls the Intracellular Transport of Activator of CREM in Testis (ACT). J. Biol. Chem. 2005, 280, 31739–31745. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Wang, W.; Xu, S.J.; Zeng, H.X. Spermatogenesis and sperm morphology of Phascolosoma esculenta. Acta Zool. Sin. 2007, 53, 733–741. [Google Scholar] [CrossRef]
- Gao, X.M.; Mu, D.L.; Hou, C.C.; Zhu, J.Q.; Jin, S.; Wang, C.L. Expression and Putative Functions of KIFC1 for Nuclear Reshaping and Midpiece Formation during Spermiogenesis of Phascolosoma esculenta. Gene 2019, 683, 169–183. [Google Scholar] [CrossRef]
- Du, C.; Mu, D.; Gao, X.; Luo, S.; Wang, J.; Jin, S.; Zhu, J. Expression Characteristics and Putative Functions of KIF3A/KIF3B During Spermiogenesis of Phascolosoma esculenta. J. Ocean Univ. China 2022, 21, 998–1016. [Google Scholar] [CrossRef]
- Wong-Riley, M.T.T.; Besharse, J.C. The Kinesin Superfamily Protein KIF17: One Protein with Many Functions. BioMol. Concepts 2012, 3, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Takei, Y.; Kido, M.A.; Hirokawa, N. Molecular Motor KIF17 Is Fundamental for Memory and Learning via Differential Support of Synaptic NR2A/2B Levels. Neuron 2011, 70, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.-C.; Setou, M.; Teng, J.; Takei, Y.; Hirokawa, N. Overexpression of Motor Protein KIF17 Enhances Spatial and Working Memory in Transgenic Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 14500–14505. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Chung, E.Y.; Chung, J.S.; Lee, K.Y. Ultrastructural Studies of Spermatogenesis and the Functions of Leydig Cells and Sertoli Cells Associated with Spermatogenesis in Larimichthys polyactis (Teleostei, Perciformes, Sciaenidae). Anim. Cells Syst. 2013, 17, 250–258. [Google Scholar] [CrossRef]
- Chen, H.; Lin, G.W.; Chen, W.; Liu, Z.K.; Wang, X. Observation on the reproductive cycle of Phascolosoma esculenta. Mar. Fish. 2009, 31, 139–145. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, D.; Yan, H. Study on breeding biology of Phascolosoma esculenta. Pop. Sci. Technol. 2015, 17, 114–115. [Google Scholar] [CrossRef]
- Fimia, G.M.; De Cesare, D.; Sassone-Corsi, P. A Family of LIM-Only Transcriptional Coactivators: Tissue-Specific Expression and Selective Activation of CREB and CREM. Mol. Cell. Biol. 2000, 20, 8613–8622. [Google Scholar] [CrossRef] [PubMed]
- Kotaja, N.; De Cesare, D.; Macho, B.; Monaco, L.; Brancorsini, S.; Goossens, E.; Tournaye, H.; Gansmuller, A.; Sassone-Corsi, P. Abnormal Sperm in Mice with Targeted Deletion of the Act (Activator of cAMP-Responsive Element Modulator in Testis) Gene. Proc. Natl. Acad. Sci. USA 2004, 101, 10620–10625. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Mu, D.L.; Wang, D.; Han, Y.L.; Hou, C.-C.; Zhu, J.Q. Analysis of the Function of KIF3A and KIF3B in the Spermatogenesis in Boleophthalmus Pectinirostris. Fish Physiol. Biochem. 2018, 44, 769–788. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wan, J.; Yan, F.; Zhang, X.S.; Cao, W.; Tao, W.; Huang, L.Q. The production and application of Ezrin polyclonal antibody. Biotechnol. Bull. 2013, 1, 130–133. [Google Scholar] [CrossRef]
- Hou, C.C.; Gao, X.M.; Ni, J.; Mu, D.L.; Yang, H.Y.; Liu, C.; Zhu, J.Q. The Expression Pattern and Potential Functions of PHB in the Spermiogenesis of Phascolosoma Esculenta. Gene 2018, 652, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Deng, M.; Feng, S. A feasibility study using primary antibodies from the same species in immunofluorescence double staining. Sichuan J. Anat. 2015, 23, 13–19. [Google Scholar] [CrossRef]

| Primers/Probe | Sequences (5′-3′) | Purpose |
|---|---|---|
| KIF17-F1 | GAATGGTAGGTGTGACGG | PCR |
| KIF17-F2 | AGGAACAAATGGTGGGTG | PCR |
| KIF17-R1 | TGGTCATAGCATAGATGGTGT | PCR |
| KIF17-R2 | TTGCTCTGTGCTGTTGTG | PCR |
| KIF17-RT-F | AGGCCCTAAGGAGGCTACAA | Semiquantitative RT-PCR |
| KIF17-RT-R | CCCATCCATGCTAGCCAGAG | Semiquantitative RT-PCR |
| ACT-F1 | CGGGAAGAGCACCCTCACTGTA | PCR |
| ACT-F2 | CAAGGCTGGGATGAAGAAGTTT | PCR |
| ACT-R1 | CGGGATGCCCTTGTAGGTGATA | PCR |
| ACT-R2 | TCCTCGAACGAGATGAACTTGG | PCR |
| ACT-RT-F | GGCTGGGATGAAGAAGTTT | Semiquantitative RT-PCR |
| ACT-RT-R | GTTGTGCCGATGGGTTT | Semiquantitative RT-PCR |
| GAPDH-F | CTGGTGAAGTTGGAGAAAAAG | Semiquantitative RT-PCR |
| GAPDH-R | GCTGAAGGAGCAGAGATGAT | Semiquantitative RT-PCR |
| KIF17 Probe sequence | GCTATATCTTGCTCTGTGCTGTTGTGTAGTTT | Fluorescence in situ hybridization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Wang, J.; Gao, X.; Du, C.; Hou, C.; Tang, D.; Zhu, J. Expression Dynamics Indicate Potential Roles of KIF17 for Nuclear Reshaping and Tail Formation during Spermiogenesis in Phascolosoma esculenta. Int. J. Mol. Sci. 2024, 25, 128. https://doi.org/10.3390/ijms25010128
Pan Y, Wang J, Gao X, Du C, Hou C, Tang D, Zhu J. Expression Dynamics Indicate Potential Roles of KIF17 for Nuclear Reshaping and Tail Formation during Spermiogenesis in Phascolosoma esculenta. International Journal of Molecular Sciences. 2024; 25(1):128. https://doi.org/10.3390/ijms25010128
Chicago/Turabian StylePan, Yue, Jingqian Wang, Xinming Gao, Chen Du, Congcong Hou, Daojun Tang, and Junquan Zhu. 2024. "Expression Dynamics Indicate Potential Roles of KIF17 for Nuclear Reshaping and Tail Formation during Spermiogenesis in Phascolosoma esculenta" International Journal of Molecular Sciences 25, no. 1: 128. https://doi.org/10.3390/ijms25010128
APA StylePan, Y., Wang, J., Gao, X., Du, C., Hou, C., Tang, D., & Zhu, J. (2024). Expression Dynamics Indicate Potential Roles of KIF17 for Nuclear Reshaping and Tail Formation during Spermiogenesis in Phascolosoma esculenta. International Journal of Molecular Sciences, 25(1), 128. https://doi.org/10.3390/ijms25010128







