Abstract
Male reproduction depends on hormonally driven behaviors and numerous genes for testis development and spermatogenesis. Neuroplastin-deficient (Nptn−/−) male mice cannot sire offspring. By immunohistochemistry, we characterized neuroplastin expression in the testis. Breeding, mating behavior, hormonal regulation, testicular development, and spermatogenesis were analyzed in cell-type specific neuroplastin mutant mice. Leydig, Sertoli, peritubular myoid, and germ cells express Np, but spermatogenesis and sperm number are not affected in Nptn−/− males. Neuroplastin lack from CNS neurons or restricted to spermatogonia or Sertoli cells permitted reproduction. Normal luteinizing hormone (LH) and follicle-stimulating hormone (FSH) blood levels in Nptn−/− males support undisturbed hormonal regulation in the brain. However, Nptn−/− males lack mounting behavior accompanied by low testosterone blood levels. Testosterone rise from juvenile to adult blood levels is absent in Nptn−/− males. LH-receptor stimulation raising intracellular Ca2+ in Leydig cells triggers testosterone production. Reduced Plasma Membrane Ca2+ ATPase 1 (PMCA1) in Nptn−/− Leydig cells suggests that Nptn−/− Leydig cells produce sufficient testosterone for testis and sperm development, but a lack of PMCA-Np complexes prevents the increase from reaching adult blood levels. Behavioral immaturity with low testosterone blood levels underlies infertility of Nptn−/− males, revealing that Np is essential for reproduction.
1. Introduction
Infertility is a complex multifactorial condition and can result from both genetic and environmental causes. Genetic defects in humans, accounting for 15–30% of male infertility, majorly elicit malfunctioning of sex determination and/or development, sperm production and/or function, and a plethora of other physiological abnormalities, including endocrinopathies (for review, see [1,2,3]). Previous reports have identified several autosomal gene mutations and polymorphisms that affect male fertility, e.g., the cystic fibrosis transmembrane conductance regulator (CFTR) gene encoding a glycosylated transmembrane chloride channel affecting the vas deferens (for review, see [4]), the SHBG gene encoding the glycoprotein sex hormone-binding globulin (SHBG), or genes associated with spermatogenic failure (for review, see [5]). However, approximately 25% of the cases of clinical infertility are idiopathic, reflecting our relatively insufficient understanding of the molecular mechanisms underlying it (for review, see [1,3]).
Recently, we reported that the constitutive lack of neuroplastin (Np) in mice, in addition to learning and memory deficits, also affects the endocrine system [6]. We observed elevated corticosterone levels, decreased levels of corticotropin-releasing hormone mRNA, and behavioral symptoms associated with stress, anxiety, and/or depression, pathological conditions that are usually neuroendocrine in nature [6]. Furthermore, neuroplastin-deficient (Nptn−/−) male mice do not sire offspring, demonstrating a role of Np in reproduction [6].
Np is a phylogenetically conserved type I glycoprotein cell adhesion molecule belonging to the immunoglobulin (Ig) superfamily [7] (for a recent review, see [8]). The major isoforms Np55 and Np65 consist of two or three Ig-like extracellular domains, a single transmembrane domain, and a short cytoplasmic tail [7]. The shorter isoform Np55 is widely expressed in most organs, whereas the 3 Ig-isoform Np65 is expressed only in neurons. Polymorphisms in the regulatory region of the human NPTN gene correlated with cortical thickness and intellectual abilities in adolescents [9] and were detected in individuals suffering from schizophrenia [10]. Recently, the human NPTN gene has been associated with neuropsychiatric diseases (for review, see [11]), heart rate [12], lung cancer [13], and Nptn in mice with hearing deficits [11,14,15] and T-cell activation [16]. Np undergoes multiple interactions with protein binding partners (for review, see [8]). Importantly, Np promotes the expression of Plasma Membrane Calcium ATPases (PMCA) and forms functional complexes [6,17,18] for extrusion of intracellular Ca2+ related Np to termination and control of Ca2+-mediated signal transduction (for review, see [19]). Np is a close paralog of CD147 (basigin/EMMPRIN) [20]. However, basigin-deficient mice of both sexes are severely affected and do not reproduce [21,22], whereas Nptn−/− females are fully fertile [6].
In this study, we investigated the essential role of neuroplastin in male fertility. We analyzed Np expression in the mouse testis and studied Nptn−/− male mice using histological, hormonal, and behavioral analyses. The production of morphologically normal sperm and testis development in the absence of Np is contrasted by the complete absence of mating behavior of adult Nptn−/− males. We show that the testosterone levels in the blood of adult Nptn−/− males do not reach adult wild-type concentrations but remain at juvenile levels, being insufficient for expression of normal mating behavior. We suggest that LH-signaling triggering testosterone production by adult Leydig cells is compromised caused by insufficient Ca2+ extrusion via Np-associated PMCA1.
2. Results
2.1. Nptn−/− Male Mice Do Not Produce Offspring
Confirming our previous observations, Nptn−/− male mice did not sire any offspring, nor was any pregnancy detected (see Materials and Methods). In contrast, when Nptn+/− males were paired with Nptn+/− females or with C57BL/6NCrl females, the size and frequency of litters produced were similar to C57BL/6NCrl breedings.
2.1.1. Nptn−/− Males Display Abnormal Mating Behavior
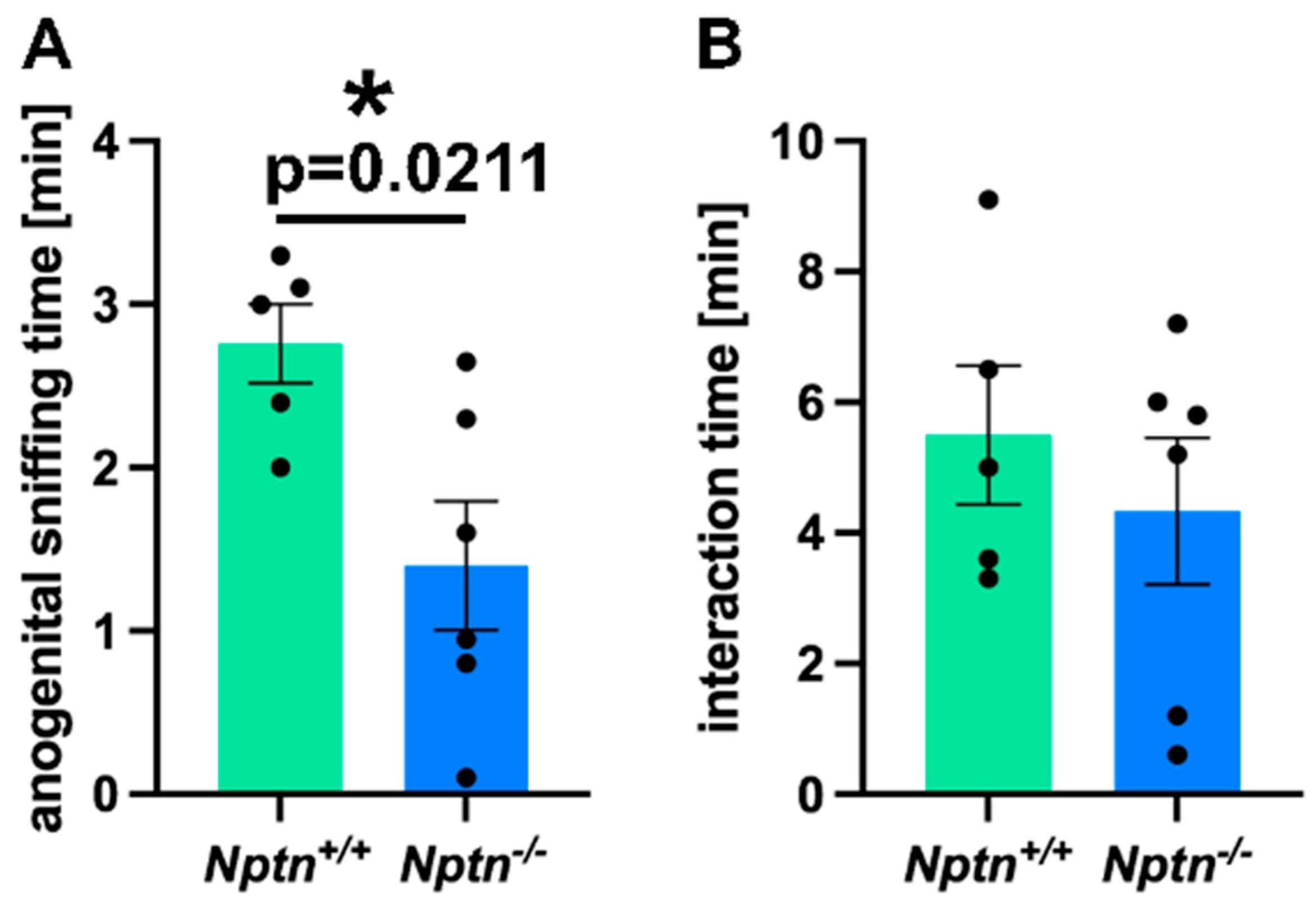
Therefore, we investigated the mating behavior of Nptn−/− male mice in detail. On exposure to a socially naive female in their home cage, Nptn−/− males did not attempt to mount the females, resulting in a complete absence of mounts with thrusts compared to Nptn+/+ littermate males, which all mounted within short latencies (12.4 ± 1.21 s latency to first mount; 4 ± 1.18 mounts/30 min). Additionally, the Nptn−/− male mice displayed aberrant investigatory behavior by spending significantly reduced time in anogenital sniffing (84 ± 24 s) as compared to the wild-type Nptn+/+ (168 ± 12 s) counterparts (Figure 1A, one-way ANOVA F(1,9) = 7.778, p = 0.0211). The total interaction time (=body sniffing + anogenital sniffing + direct contact) revealed no difference between the genotypes (Figure 1B, Nptn+/+ 5.4 min ± 1.09; Nptn−/− 4.3 min ± 1.12).
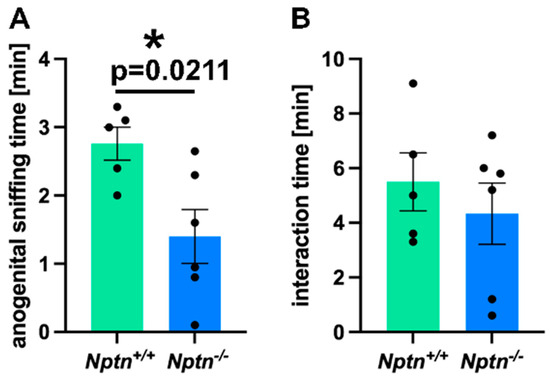
Figure 1.
Mating behavior of Nptn−/− male mice. (A) The time spent with anogenital sniffing at socially naive wild-type females was significantly shorter (p = 0.0211) for Nptn−/− (n = 6) compared to Nptn+/+ (n = 5) adult, socially naive male mice during 30 min exposure (one-way ANOVA, * p < 0.05). (B) The interaction time with socially naive wild-type females was similar for Nptn+/+ (n = 5) and Nptn−/− (n = 6) adult, socially naive male mice during 30 min exposure (one-way ANOVA).
2.1.2. External Genitalia, Testis, and Sperm in Nptn−/− Male Mice
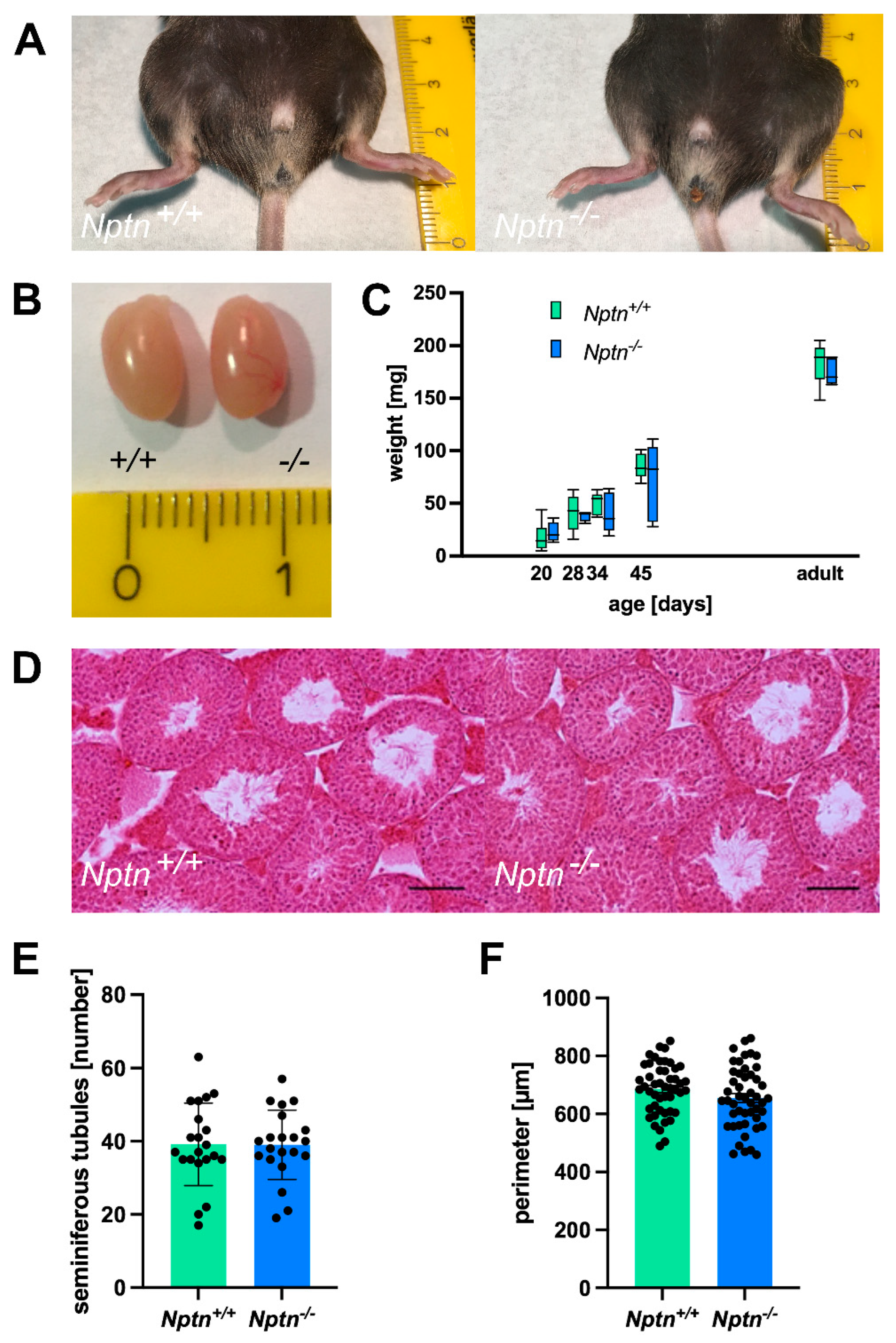
In 4–5-month-old adult Nptn−/− males, the appearance of the external genitalia, the weight, and the morphology of the testis were comparable to their wild-type Nptn+/+ littermates (Figure 2). Additionally, the testis weight during male development and of adult Nptn−/− males and wild-type littermates was similar (Figure 2C). The testicular histology did not reveal significant differences with respect to the size and morphological appearance of cross sections observed between adult Nptn−/− and Nptn+/+ males (Figure 2D). The average number of seminiferous tubules and their perimeter were quantified per transverse section obtained from the testis and found to be comparable between the genotypes (Figure 2F). Furthermore, the amount and concentration of sperm isolated from the cauda of the epididymis from adult Nptn−/− (140 days old, n = 6, 18.25 ± 0.78 × 106/cauda) were similar to the sperm derived from wild-type littermate males (n = 6, 15.08 ± 1.30 × 106/cauda). Isolated sperm from Nptn+/+ and Nptn−/− male mice showed comparable beat frequencies, which were similarly increased after activation with 15 mM bicarbonate (Figure 3A). The spermatogenetic activity assessed by the ploidy status of testicular cell nuclei at 20, 28, 34, and 45 days of age revealed that the proportion of haploid (1c), diploid (2c), and tetraploid (4c) cells from Nptn+/+ and Nptn−/− male mice did not differ (Figure 3B).
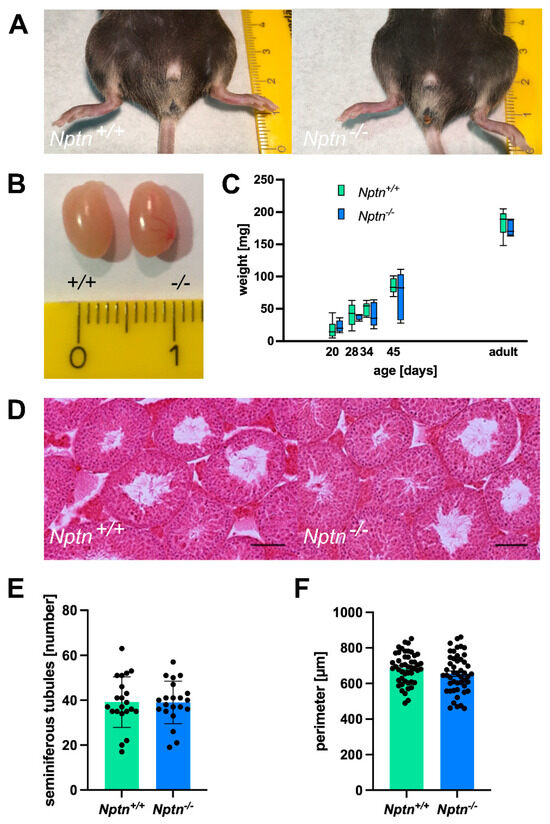
Figure 2.
Normal appearance of genitalia and testes of Nptn−/− male mice. (A) Outer genitalia of Nptn−/− male mice are normally developed (ruler in A and B: cm). (B) The testes of Nptn−/− male mice have normal morphology and size. (C) The average weights of testes from Nptn−/− and Nptn+/+ male littermates are similar (one-way ANOVA, n = 5, F (1,8) = 0.726, p = 0.419). Boxes indicate minimum-to-maximum range of data; for individual data points, see supplement. (D) The gross morphology of Nptn+/+ and Nptn−/− testes was found to be comparable, as revealed by standard Haematoxylin & Eosin staining (scale bar = 500 μm). (E) The average number of fully formed and identifiable seminiferous tubules counted per testis transverse section was not significantly different between the genotypes (7 random sections from each animal; n = 3 mice for each genotype were selected for counting). (F) The average perimeter of seminiferous tubules was similar in Nptn+/+ and Nptn−/− male mice.
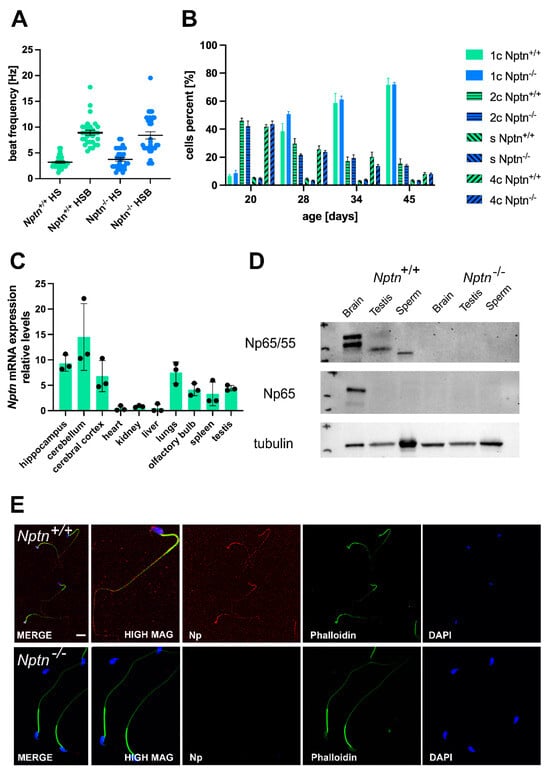
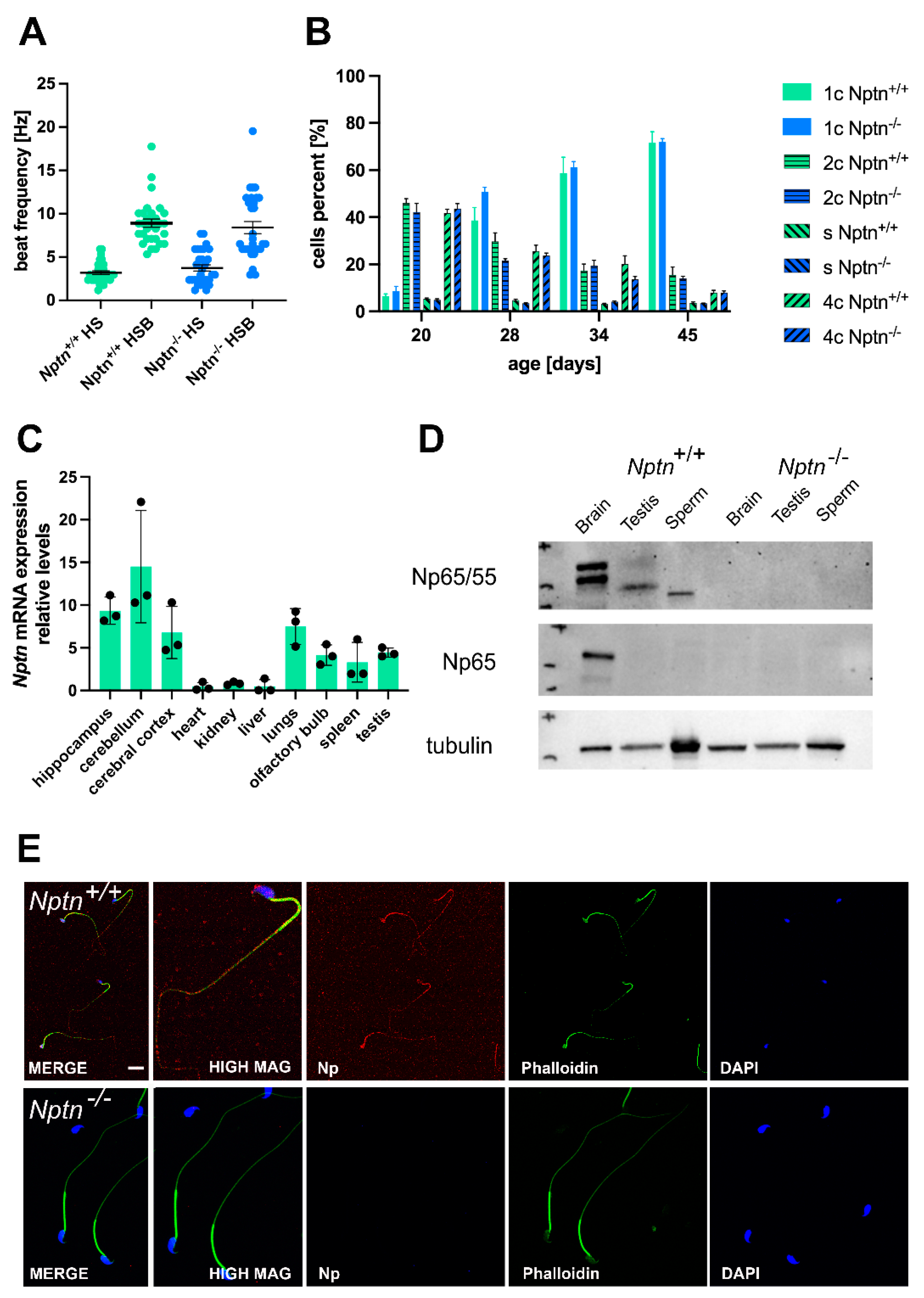
Figure 3.
Neuroplastin expression in testis. (A) The beat frequency of sperm isolated from adult Nptn+/+ and Nptn−/− male mice was similar and could be increased by bicarbonate activation. (B) The ploidy status of testicular cells from male mice at different ages during maturation was examined. At 20, 28, 34, and 45 days of age, the proportion of haploid (1c), diploid (2c), and tetraploid (4c) cells from Nptn+/+ (n = 6 for each age) and Nptn−/− (n = 4 for 20 and 28 days each; n = 6 for 34 and 45 days each) male mice did not differ. For individual data points, see supplement. (C) RT-PCR revealed that Nptn mRNA is expressed predominantly in the central nervous system but also in other organs, including the testis of Nptn+/+ male mice. (D) By Western blot analysis, Np expression was detected in testis and sperm of Nptn+/+ but not in Nptn−/− male mice. The lower molecular weight reflects different glycosylation of Np in testis (50 kDa) and sperm (45 kDa) compared to brain (55 and 65 kDa). The neuron-specific isoform Np65 could not be detected in testis or sperm of Nptn+/+ male mice (for original blots, see supplement). (E) Np (red) is expressed on isolated mature sperm heads and tails of Nptn+/+ male mice (upper panel), as detected by immunofluorescence (scale bar = 20 μm). The lower panel confirms lack of Np on sperm from Nptn−/− male mice. Counterstaining was performed by phalloidin (green) and DAPI (blue).
2.1.3. Neuroplastin Is Expressed in Testis and Present on Sperm of Male Mice
By RT-PCR, we confirmed the expression of neuroplastin mRNA in the testis of adult male mice (Figure 3C), consistent with the expression of Np in rat testis [23] and single-cell RNA sequencing data by Green et al. [24], who reported Nptn mRNA expression by several cell types in the testis, e.g., 5x higher expression in peritubular myoid cells compared to spermatogonia, Leydig, and Sertoli cells and expressed also in spermatocytes and spermatids of male mice. At the protein level, polyclonal antibodies against Np reveal two bands at 55 and 65 kDa in brain and a single band of 50 kDa in testis and of 45 kDa in sperm from Nptn+/+ male mice on Western blots (Figure 3D). The brain-specific Np65 isoform is detected by Np65-specific antibodies in the brain but not in testis tissue or in sperm. Cell type-specific glycosylation of the 28 kDa core protein, which is expressed in most tissues [7,23], leads to the observed band size around 50 kDa in testis and 45 kDa in sperm. On isolated wild-type sperm, Np was detectable as punctate surface staining on sperm heads and tails (Figure 3E) but could not be detected in sperm of Nptn−/− male mice.
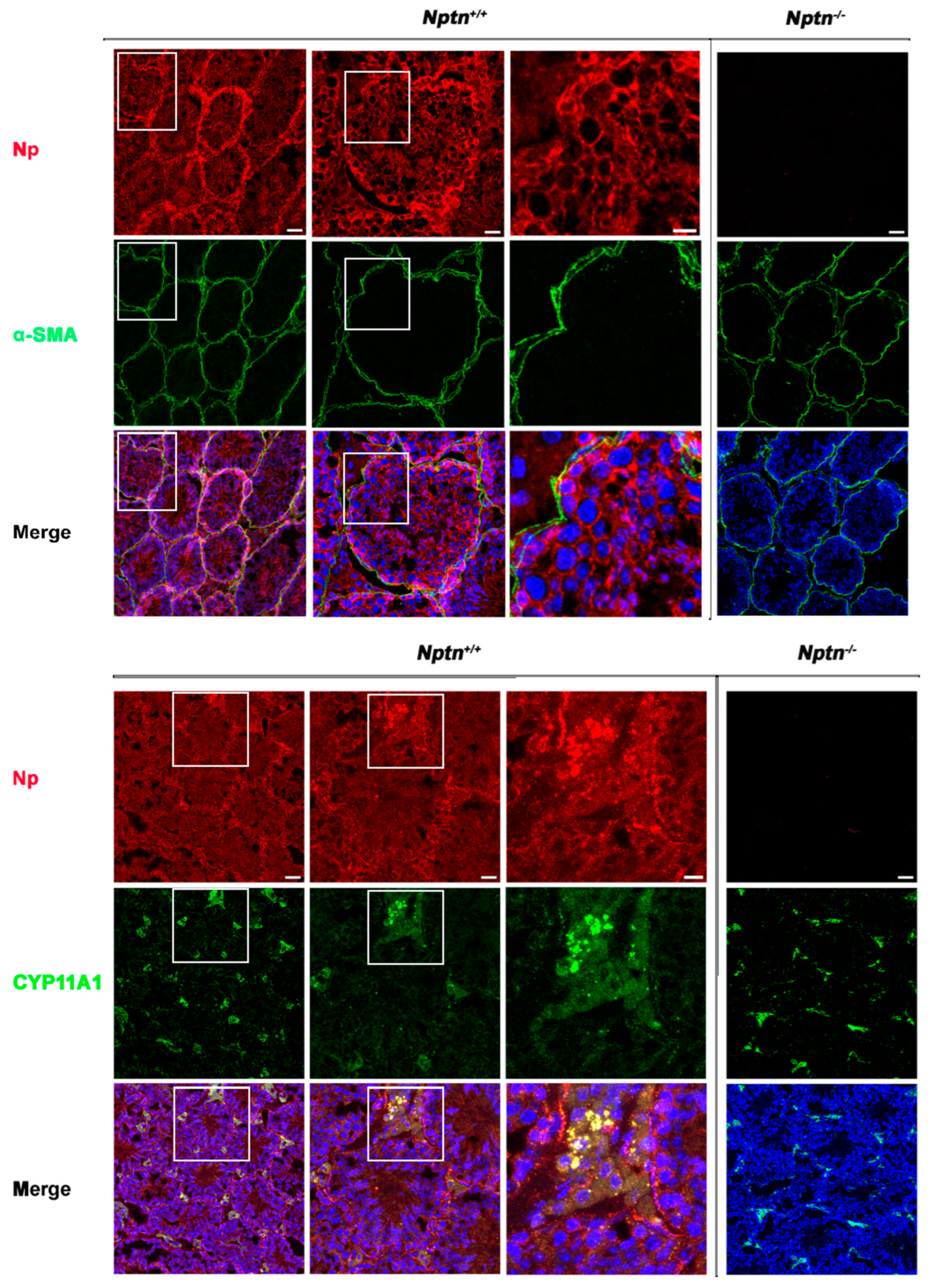
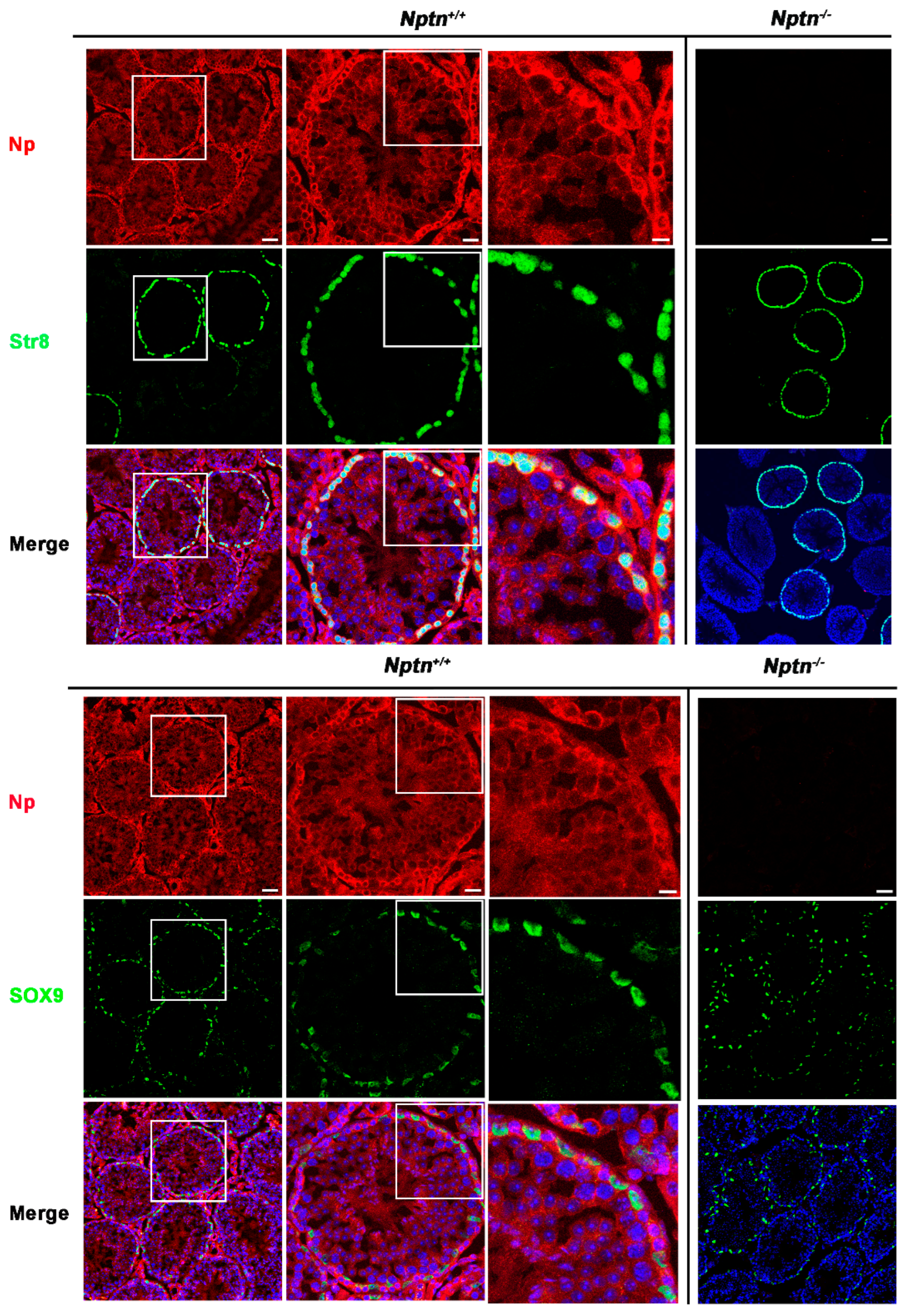
Expression of Np in mouse testis was detected by immunohistochemistry in the seminiferous tubules of Nptn+/+ males, in highest amounts in the outermost layer of the tubules, whereas Np could not be detected in the testis from Nptn−/− males (Figure 4 and Figure 5). In adult male Nptn+/+ mice, Np expression was detected in myoid cells, Leydig cells, spermatogonia, and spermatocytes, as well as in Sertoli cells. Noteworthy, the expression patterns revealed by antibodies detecting alpha-smooth muscle actin (myoid cells), CYP11A (Cytochrome P450 Family 11 Subfamily A Member 1, Leydig cells) (Figure 4), Stra8 (spermatogonia and spermatocytes), and Sox9 (Sertoli cells) (Figure 5) were undistinguishable between Nptn+/+ and Nptn−/−, indicating the normal development and maturation of the testis in Nptn−/− male mice.
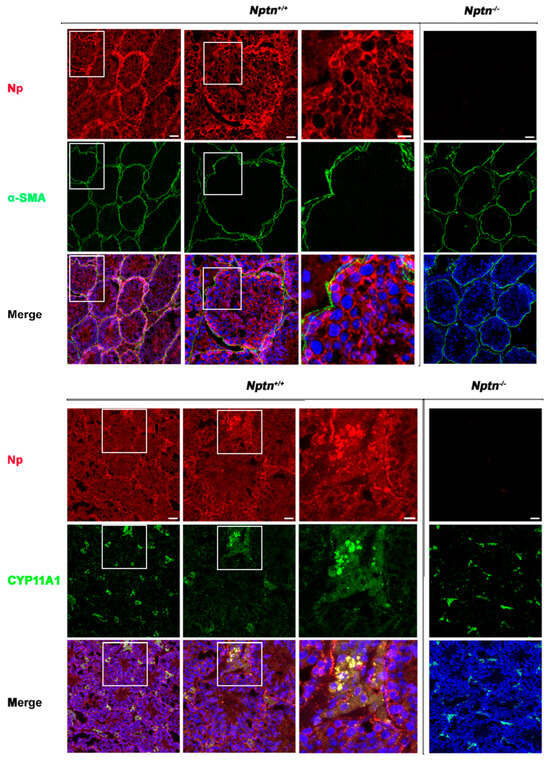
Figure 4.
Neuroplastin expression by Leydig and peritubular myoid cells. Neuroplastin expression in the testis is clearly revealed by antibodies recognizing all neuroplastin isoforms (Np, red). In Nptn−/− testes, Np could not be detected. Antibodies against alpha-smooth muscle actin (⍺-SMA, green upper panel) identified peritubular myoid cells and demonstrated that Np was expressed by myoid cells in Nptn+/+ (see also Figure 6 Amh+Stra8Cre) but not in Nptn−/−. Antibodies against CPY11A (green lower panel) identified Leydig cells and demonstrated that Np was expressed by Leydig cells in Nptn+/+ but not in Nptn−/−. Note that expression of alpha-smooth muscle actin and of CPY11A are not altered in Nptn−/− (for Nptn+/+ from left to right increasing magnification of boxed area; scale bar left = 50 µm; middle = 20 µm; right = 10 µm; for Nptn−/−, scale bar = 50 µm). In Merge, nuclei are labeled by DAPI (blue).
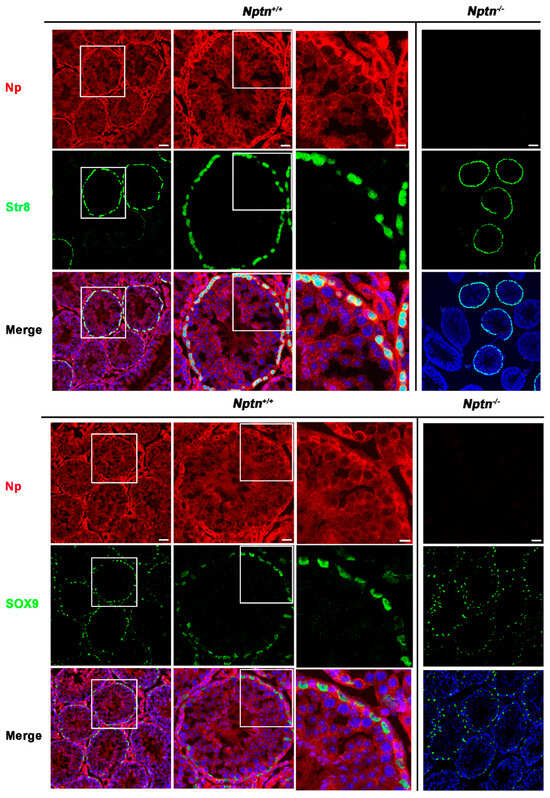
Figure 5.
Neuroplastin expression by spermatogonia, spermatocytes, and Sertoli cells. Antibodies against Stra8 (green upper panel) identified spermatogonia and spermatocytes and demonstrated that Np (red) is expressed by spermatogonia and spermatocytes in Nptn+/+ but not Nptn−/−. Antibodies against Sox9 (green lower panel) identified Sertoli cells and demonstrated that Np was expressed by Sertoli cells in Nptn+/+ but not in Nptn−/−. Note that expressions of Stra8 and of Sox9 are not altered in Nptn−/− (for Nptn+/+ from left to right increasing magnification of boxed area; scale bar left = 50 µm; middle = 20 µm; right = 10 µm; for Nptn−/−, scale bar = 50 µm). In Merge, nuclei are labeled by DAPI (blue).
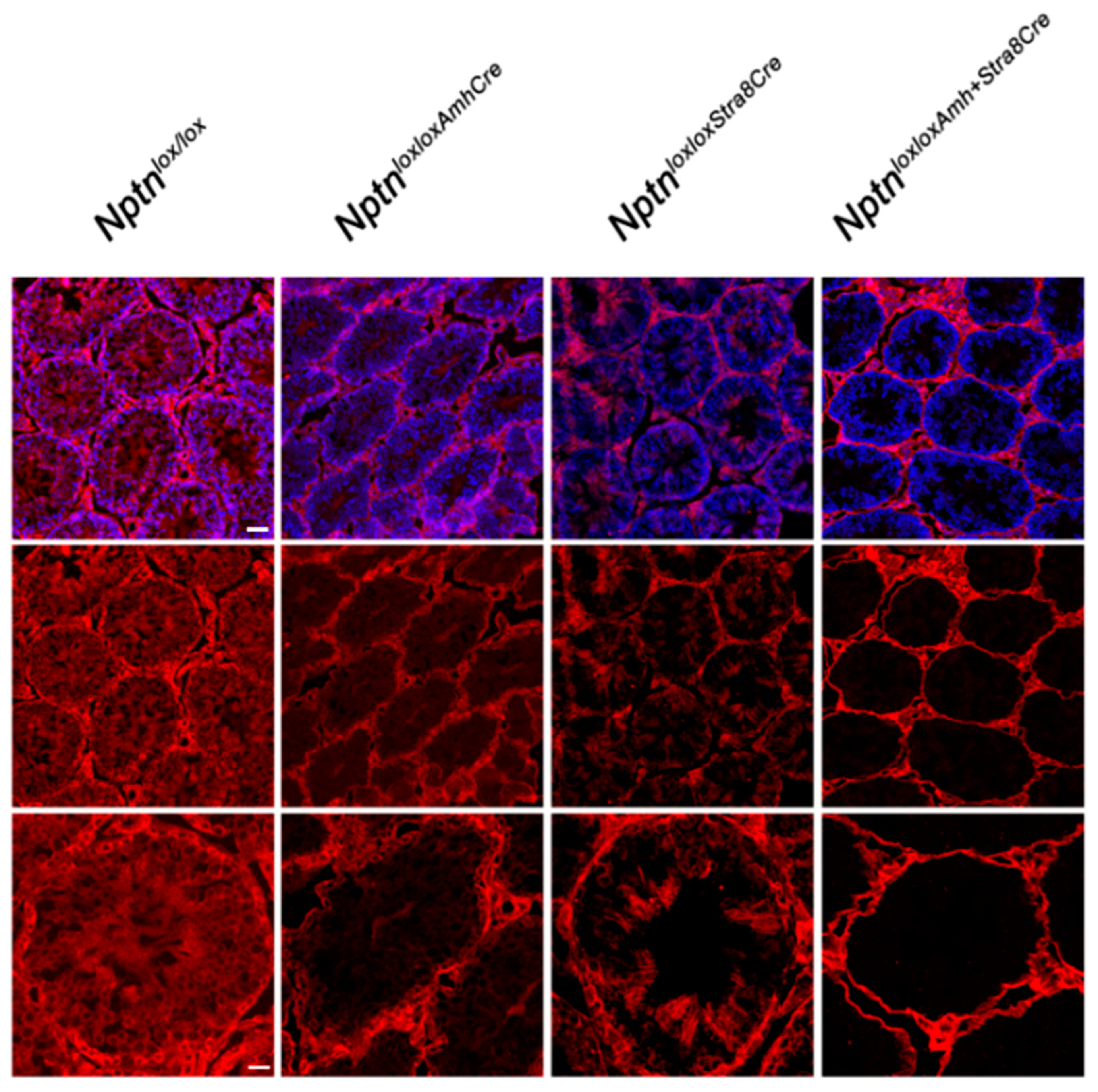
2.2. Ablation of Nptn-Expression in Spermatogonia, Sertoli-Cells, or Central Nervous System
Directing expression of Cre recombinase by the Stra8 promoter in NptnloxloxStra8Cre mice, Np expression was no longer detectable in spermatogonia and spermatocytes (Figure 6). In NptnloxloxAmhCre mice, Nptn expression was ablated in Sertoli cells (Figure 6). In NptnloxloxAmhCre+Stra8Cre, Np was completely absent from spermatocytes, spermatogonia, and Sertoli cells but still expressed by Leydig and myoid cells (Figure 6). The cell-type specific ablation of Nptn confirmed the neuroplastin expression revealed by our immunohistochemistry. NptnloxloxStra8Cre, NptnloxloxAmhCre, and NptnloxloxAmhCre+Stra8Cre males sired offspring successfully, showing that Np expression by spermatocytes, spermatogonia, and Sertoli cells in the testis is not required for fertility or reproduction.
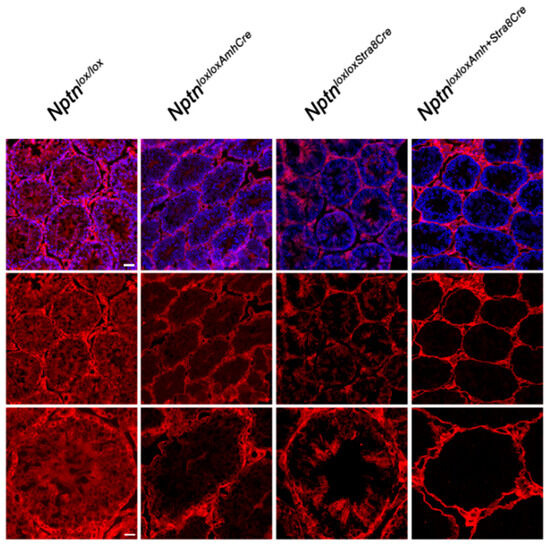
Figure 6.
Conditional inactivation of Nptn. Cell type-restricted inactivation of the floxed Nptn gene (Nptnloxlox) was achieved using Amh-promoter (Anti-Mullerian Hormone, Sertoli cells) (NptnloxloxAmhCre) or Stra8-promoter (NptnloxloxStra8Cre) driven Cre, and double Cre (NptnloxloxAmhCre+Stra8Cre) mice. Np was detected by antibodies recognizing all isoforms (red), and sections were counterstained with DAPI (blue; top panel; scale bar = 50 µm). Bottom panel shows higher magnification (scale bar = 20 µm).
NptnloxloxEmx1Cre males lacking Np expression, specifically in glutamatergic neurons [25], sired offspring similarly to wild-type males, excluding Np expression by glutamatergic neurons as essential for male fertility and mounting behavior. Similarly, male NptnloxloxPrCreERT mice [6] that sired offspring before induction by tamoxifen injection continued to sire offspring for more than 2 months after inactivation of the Nptn gene, ruling out a role of neuronal Np expression for fertility and mounting behavior after completed development and maturation.
2.3. Hormonal Regulation in Nptn−/− Male Mice
2.3.1. Levels of Luteinizing Hormone (LH) and Follicle Stimulating Hormone (FSH) in Nptn−/− Male Mice
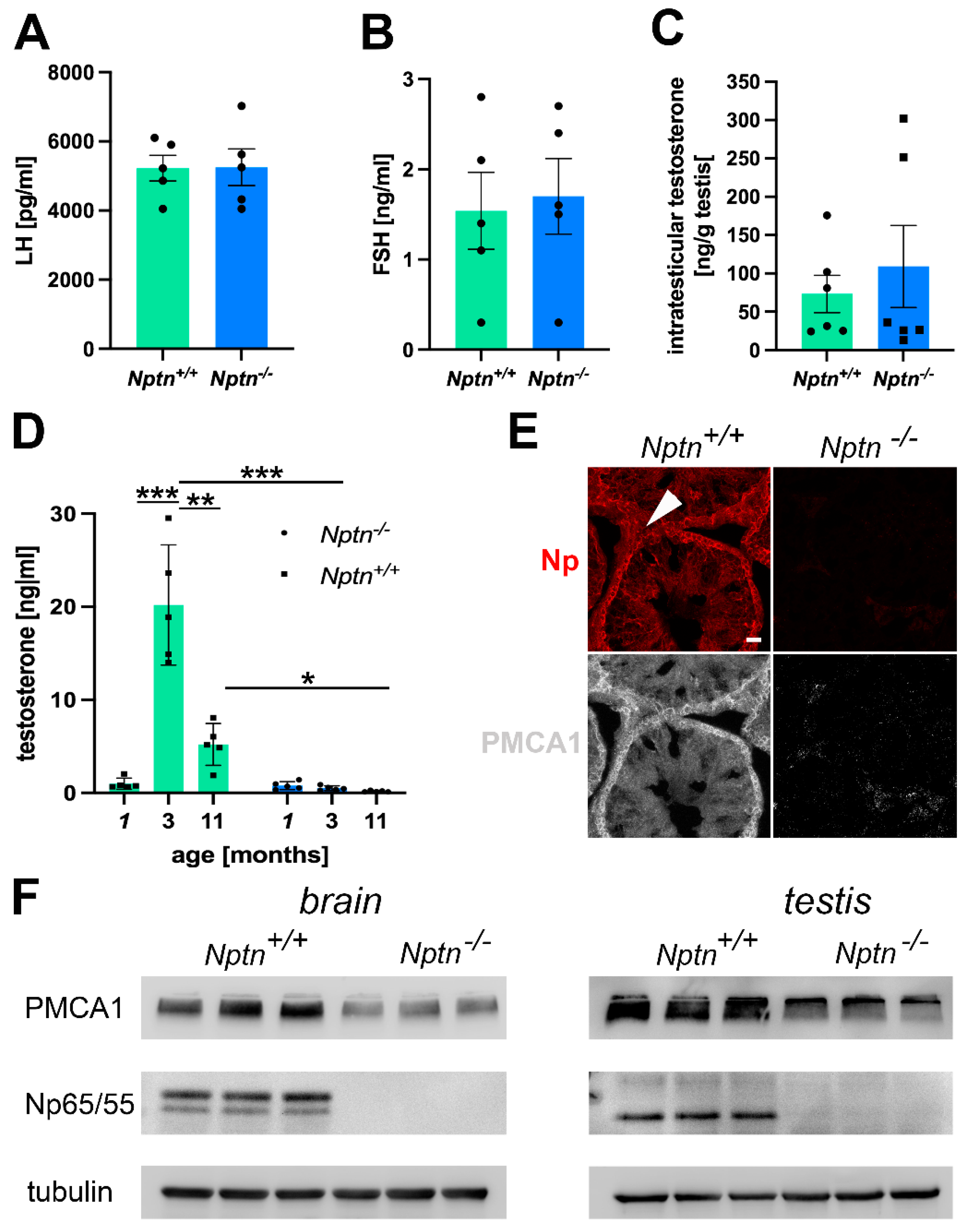
Elevated corticosterone levels observed in Nptn−/− mice [6] may inhibit the secretion of LH [26], which triggers the production of testosterone in the Leydig cells of the testis [27]. We determined LH and FSH levels in the blood but did not detect significant differences in the levels of LH (Figure 7A) and FSH (Figure 7B) in adult Nptn−/− male mice when compared to their wild-type Nptn+/+ littermates.
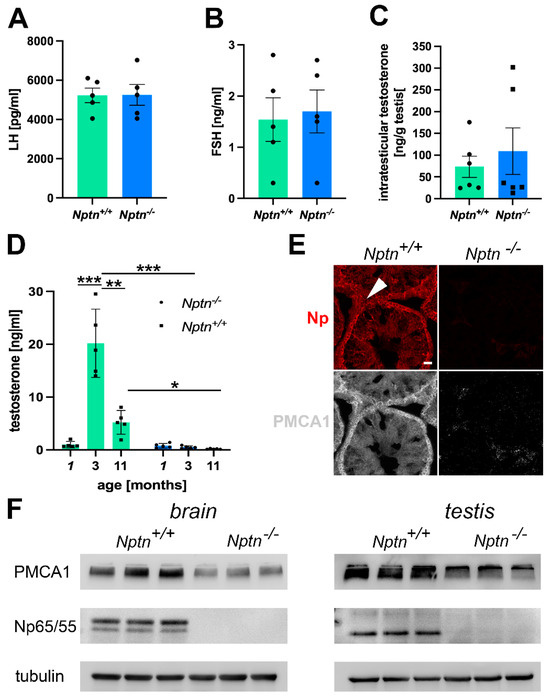
Figure 7.
Hormonal status in Nptn−/− male mice. (A) LH levels, as determined by ELISA in the blood of adult Nptn+/+ (n = 5) and Nptn−/− (n = 5) male mice, were similar (one-way ANOVA). (B) FSH levels, as determined by ELISA in the blood of adult Nptn+/+ (n = 5) and Nptn−/− (n = 5) male mice, were similar (one-way ANOVA). (C) Intratesticular testosterone levels determined by ELISA in adult Nptn+/+ (n = 6) and Nptn−/− (n = 6) male mice were similar (one-way ANOVA). (D) Testosterone levels, as determined by ELISA in the blood of juvenile (1-month-old; n = 5), adult (3-month-old; n = 5), and aged (11-month-old; n = 5) Nptn+/+ male mice differed significantly, showing the strong increase after puberty and decline with age. In Nptn−/− male mice, low juvenile (n = 5) testosterone levels did not increase with age and were significantly lower in adult (n = 5) and aged (n = 5) males compared to Nptn+/+ (* p < 0.05, ** p < 0.01, *** p < 0.001; one-way ANOVA). (E) Reduced expression of PMCA1 in testes of Nptn−/− compared to Nptn+/+ male mice was revealed by immunohistochemistry (arrowhead points to a Leydig cell; scale bar = 20 µm). (F) Reduced expression of PMCA1 in brain (46% decrease) and testis (39% decrease) of adult Nptn−/− (n = 3) compared to Nptn+/+ (n = 3) male mice was revealed by Western blot analysis (one-way ANOVA) (for original blots, see supplement).
2.3.2. Nptn−/− Male Mice Do Not Attain Adult Levels of Testosterone
The intra-testicular levels of testosterone in adult Nptn−/− (n = 6) and littermate Nptn+/+ (n = 6) male mice did not differ (Figure 7C), conforming to the observed normal development of testis and sperm in Nptn−/− males. Testosterone levels in the blood drastically increase at puberty and decline with age [28]. At 3 months of age, testosterone levels in Nptn+/+ male mice were approximately 19-fold higher compared to juvenile 1-month-old Nptn+/+ males (Figure 7D; n = 5; one-way ANOVA; F(1,6) = 173.202; p < 0.0001). At the age of 11 months, Nptn+/+ males displayed approximately three-fold lower levels of testosterone compared to the 3-month-old male mice (n = 3; one-way ANOVA; F(1,4) = 30.797; p = 0.0052). In contrast, testosterone levels of Nptn−/− males examined at 1, 3, or 11 months of age were not significantly different from each other. When compared to their wild-type littermates, the levels of testosterone in the Nptn−/− male mice were found to be approximately 19-fold lower at 3 months (one-way ANOVA, F(1,4) = 101.895, p = 0.0005) and approximately 6-fold lower at 11 months of age (one-way ANOVA, F(1,4) = 7.936, p = 0.048).
2.3.3. Reduced PMCA1 Expression in Nptn−/− Leydig Cells
Np is crucial for the expression of PMCAs [16,17,25], regulating Ca2+-efflux and potentially interfering with elevation of the intracellular Ca2+ level accompanied with LH-mediated stimulation of testosterone production by Leydig cells. Using immunohistochemistry, we observed a decrease in PMCA1 expression in the testis, particularly in the Leydig cells of Nptn−/− males compared to Nptn+/+ littermates (Figure 7E). The decrease in PMCA1 expression in the testis could be confirmed by Western blot analysis (Figure 7F; testis Nptn+/+ 3.013 ± 0.051, Nptn−/− 1.84 ± 0.314 relative units, 39% decrease, one-way ANOVA, F(1,4) = 13.588, p = 0.0211; brain Nptn+/+ 1.145 ± 0.11, Nptn−/− 0.617 ± 0.01 relative units, 46% decrease, one-way ANOVA, F(1,4) = 22.716, p = 0.0089).
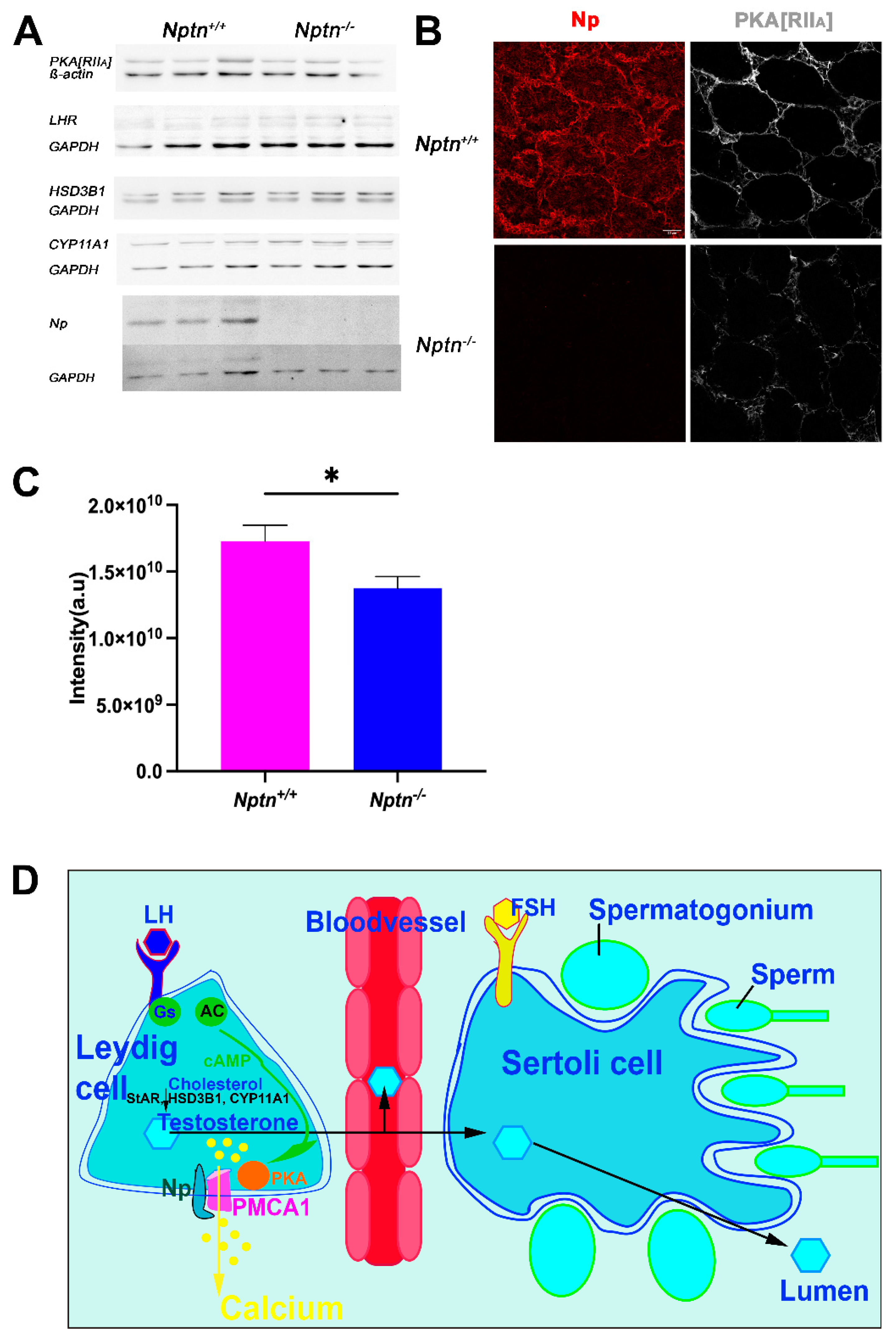
LH-receptor and PKA are critical components of LH signaling, and HSD3B1 (3ß-hydroxysteroid dehydrogenase/isomerase type 1) and CYP11A1 are the critical enzymes for testosterone synthesis. In adult testis homogenates, significant differences in expression of the regulatory subunit IIa of PKA (PKA[RIIa]), LH-receptor, HSD3B1, and CYP11A1 (Figure 8A) were not detected. However, the subcellular localization of PKA[RIIa] was altered from more membrane-associated in Nptn+/+ to more cytoplasmic localization in Nptn−/− (Figure 8B) accompanied by a significant reduction in PKA[RIIa] staining intensity in testis from Nptn−/− compared to Nptn+/+ male mice (Figure 8C; one-way ANOVA, Nptn−/− n = 14 sections (132 tubules), Nptn+/+ n = 9 sections (100 tubules), F(1,21) = 5.795, p = 0.0254). This finding may be particularly interesting because A-kinase anchoring protein (AKAP)-mediated multiprotein complex formation and the tethering of PKA holoenzyme via their regulatory subunit II dimers to defined cellular sites determines the efficiency and specificity of cAMP signaling (for review, see [29]).
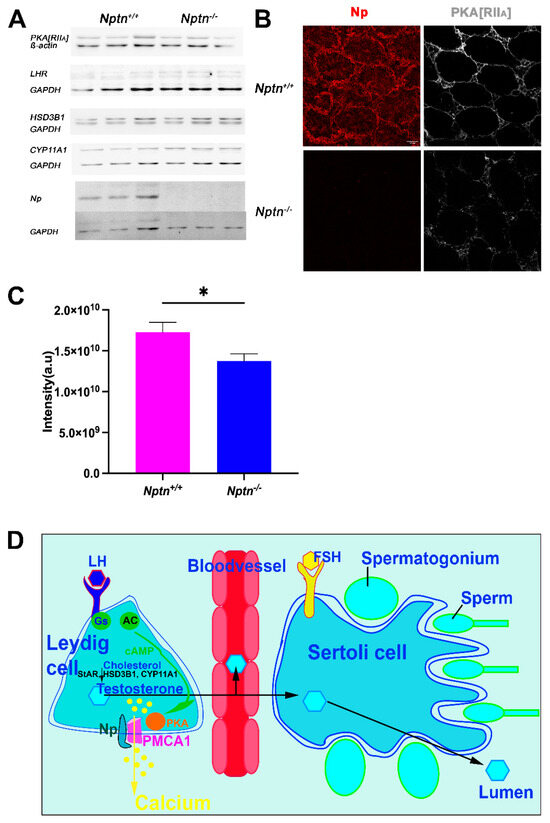
Figure 8.
Expression of LH-Receptor, PKA[RIIa], and critical enzymes for testosterone synthesis in Nptn−/− male mice. (A) Expressions of PKA[RIIa], LH-receptor (LHR), HSD3B1, and CYP11A1 in testis of adult Nptn+/+ (n = 3) compared to Nptn−/− (n = 3) male mice was analyzed by Western blot and did not reveal significant quantitative differences with respect to genotype (one-way ANOVA) (for original blots, see supplement). Np and GAPDH served as controls. (B) Altered expression of PKA[RIIa] in testes of Nptn−/− compared to Nptn+/+ male mice was revealed by immunohistochemistry (scale bar = 50 µm). Labeling for PKA[RIIa] in Nptn+/+ Leydig cells was more associated with the cell membrane, whereas in Nptn−/−, it was more diffuse and cytoplasmic. (C) The fluorescence intensity of the immunohistochemical staining for PKA[RIIa] was measured and revealed a significant difference (one-way ANOVA, Nptn−/− n = 14 sections (132 tubules), Nptn+/+ n = 9 sections (100 tubules), F(1,21) = 5.795, p = 0.0254, * p < 0.05) between testis from Nptn−/− compared to Nptn+/+ male mice. (D) Schematic illustration of testosterone release by Leydig cells in the testis. Whereas fetal Leydig cells do not require LH for testosterone production, LH binding to the G-protein (Gs) coupled LH receptor triggers cAMP production by Adenylate cyclase (AC), and an increase in intracellular calcium levels leads to testosterone production in adult Leydig cells. PMCA1 is a target of PKA, which is activated by increased cAMP levels. Calcium is extruded by PMCA1, which is stabilized by Np. Lack of Np might interfere with LH signaling, resulting in insufficient trigger of testosterone synthesis to obtain high levels in blood. Expression of StAR (Steroidogenic acute regulatory protein), HSD3B1, and CYP11A mediating conversion of cholesterol to testosterone are influenced by cAMP/PKA/CREB regulation.
3. Discussion
Genetic abnormalities are estimated to cause or contribute to about 15–30% of male infertility. However, the majority of genetic causes of male infertility are still unknown (for review, see [1,3]). The role of Np in reproduction was only recently suggested by the inability of Np-deficient male mice to sire offspring [6], which adds Nptn to the genes essential for fertility. Mice lacking the Np paralog basigin (EMMPRIN, CD147) display sterility of both sexes [21]. In female basigin-deficient mutants, the ovaries and genital tract are morphologically normal, but the uterus is probably incapable of implantation [21,30]. Basigin-deficient male mice are sterile due to the failure of spermatogenesis and show smaller testes compared to wild-type [21,22,31]. In this study, we addressed the novel role of Np for male fertility in detail. We confirmed that male Nptn−/− mice do not produce any offspring, whereas female Nptn−/− mice appear to be fully fertile. Np expression in the testis was confirmed by RT-PCR, Western blot, and immunohistochemistry, which revealed Np in the adult testis most prominently in Leydig, Sertoli, and myoid cells and in spermatogonia and spermatocytes. On mature isolated sperm, Np was detected on heads and tails. These data are in perfect agreement with the data from single-cell RNA-sequencing studies [24].
In Nptn−/− mice, the morphological development of genitalia and testes was found to be normal with respect to size, weight, and histology. The expression patterns of markers for Leydig cells (CPY11A), Sertoli cells (Sox9), spermatogonia, spermatocytes (Stra8), and myoid cells (alpha-smooth muscle actin) appeared normal in the adult Nptn−/− testis, supporting that a morphologically normal maturation of the testis proceeds also in the absence of Np. Furthermore, the conditional ablation of Np expression in spermatogonia, spermatocytes, and Sertoli cells using NptnloxloxAmhCre+Stra8Cre, NptnloxloxStra8Cre, and NptnloxloxAmhCre mice did not interfere with reproduction, and males remained fully fertile and produced offspring. These results indicate that Np expression is not required for spermatogenesis and that Np expression in Sertoli cells, spermatogonia, and spermatocytes is not needed for reproduction. This is strikingly different from basigin expression, which is required on Sertoli cells for the maturation of spermatogonia to spermatocytes. Noteworthy is the pattern of Np staining on mature sperm, which was punctate, whereas basigin was more uniformly expressed along sperm heads and tails. For Nptn−/− male mice, the analysis of sperm development, maturation, number, and motility did not yield any indication of compromised spermatogenesis. Our observation of fully absent mounting behavior prompted us to consider a neurological base underlying infertility in Nptn−/− males. However, selective lack of Np from glutamatergic neurons, which express approximately 95% of brain Np, or inactivation of Nptn in the adult CNS did not interfere with reproduction of the males, ruling out a role of neuronal Np expression directly or indirectly via hormonal regulation for fertility and mounting behavior. Unfortunately, due to the lack of suitable Cre-deleter mouse strains, the conditional ablation of Nptn specifically only in Leydig or smooth muscle cells was not possible, leaving the possibility that Np expression by these cells is decisive.
Leydig cells present in the interstitial tissue of seminiferous tubules in the testes are the major site of testosterone production in mammals [32]. Testosterone synthesis in adult Leydig cells is triggered by LH, and the proliferation of Leydig cells depends on FSH acting on Sertoli cells; these two essential gonadotropins are secreted by the anterior pituitary gland [26,33]. Furthermore, FSH influences testis size and is required for normal Sertoli and germ cell numbers, and the lack of FSH reduces sperm motility [34,35]. FSH receptor knockout mice produce reduced testosterone levels, show reduced testis size, spermatogenesis, and sperm motility, and display aberrant sperm morphology and reduced fertility [36]. Complete lack of LH signaling as in LH-receptor knockout mice preventing postnatal, but not prenatal, Leydig cell maturation and androgen production leads to developmental and morphological deficits with resulting infertility that can, in part, be overcome by testosterone replacement [37]. Noteworthy, corticosterone at concentrations higher than physiological levels, as observed in Nptn−/− mice [6], can potentially inhibit the production of testosterone in Leydig cells [38]. Our analysis provided no hint for abnormal testis morphology or altered sperm capabilities of Nptn−/− males, arguing against LH- or FSH-deficits. Furthermore, we observed normal concentrations of LH and FSH in the blood of Nptn−/− males supporting a functional HPA axis. Interestingly, Oduwole et al. [39] described a patient with a partial selective defect of LH function caused by a homozygous LHß defect that partially affected LH signaling. The reduced intratesticular testosterone was sufficient to allow for normal spermatogenesis with complete germ cell maturation locally. The intratesticular testosterone concentration of this patient was locally sufficient for spermatogenesis but inadequate to maintain systemic T at levels required to induce virilization because of hormone dilution in blood and extracellular space. In Nptn−/− mice, the testosterone level in the testis itself was unaffected by the lack of Np; however, the expected increase in the blood level from puberty to adulthood was missing in Nptn−/− males. Unlike adult Leydig cells, fetal Leydig cells in the mouse do not require LH to stimulate androgen production and are capable of producing sufficient levels of androgens in the absence of LH stimulation to induce male fetal masculinization [26]. Furthermore, sexual differentiation occurs before maturation, which may explain why the intratesticular testosterone production in Nptn−/− suffices for testis development. Importantly, Leydig cell number and testis size develop normally in Nptn−/− whereas in mice lacking LH or the LH receptor, this is not the case [40,41,42], showing that the Nptn−/− phenotype is not identical to the complete lack of LH-signaling. In Nptn−/−, probably insufficient amounts of testosterone are produced and/or released, not fulfilling the large demand of constant testosterone in the bloodstream during adulthood. According to our measurements, the total amount of testosterone in the blood of male mice is higher than the total amount of testosterone in the testis itself. Furthermore, quick conversion of testosterone in the blood and liver leads to underestimation of testosterone secreted into the blood.
A potential explanation for the low testosterone level in the blood of Nptn−/− male mice could be a partially abnormal response to LH signal processing, which is associated with the elevation of intracellular [Ca2+] [43]. In Leydig cells, LH binding to the G-protein coupled LH receptor activates cAMP, producing adenylate cyclase and PKA and leading to intracellular [Ca2+] elevation [43]. Restoration of normal intracellular Ca2+ levels after signaling is achieved by PMCAs extruding Ca2+ to the extracellular space (for review, see [44]). Complex regulation of PMCAs via the cAMP/PKA/calmodulin signaling pathway has been reviewed [45], and PMCA1 is efficiently phosphorylated by PKA [46]. Np forms complexes with PMCA and is required for normal expression levels of PMCAs [6,11,16,17,25]. Therefore, we investigated PMCA levels in the testis and observed that PMCA1 levels in the testis, particularly in Leydig cells of Nptn−/− males, are lower compared to wild-type males. Furthermore, PKA[RIIa] localization was altered in Leydig cells. Similar to the altered Ca2+ extrusion kinetics that we observed for reduced PMCA in various cell types (e.g., neurons, T-cells [16,25]), reduction in PMCA1 levels in Leydig cells may lead to the alteration of intracellular Ca2+ dynamics and eventually insufficient response to LH signals. With reduced amounts of PMCA1 in the absence of Np, PKA-mediated activation of Ca2+ extrusion is compromised, potentially causing a block in further LH signaling. LH signaling, which increases intracellular Ca2+ to trigger testosterone production by Leydig cells, may be silenced by elevated intracellular Ca2+ levels and altered Ca2+ kinetics caused by reduced PMCA1 in the absence of Np. Therefore, we conclude that Np is required to obtain adult testosterone blood levels and to ensure successful mounting and male mating behavior. It will be interesting to investigate whether testosterone supplementation can rescue the mating behavior and fertility of Nptn−/− male mice.
4. Materials and Methods
4.1. Animals
Neuroplastin-deficient mice Nptn−/− (Nptntm1.2Mtg) and floxed Nptnloxlox mice with neuron-specific inducible PrCreERT (NptnloxloxPrCreERT) or with conditional lack of Np in Emx1-expressing cells (NptnloxloxEmx1Cre) were described [6,25]. Mice lacking Np conditionally in Sertoli cells (NptnloxloxAmhCre) or in spermatogonia (NptnloxloxStra8Cre) were obtained by crossing floxed Nptnloxlox mice with 129S.FVB-Tg(Amh-cre)8815Reb/J (strain 007915, The Jackson Laboratory, Bar Harbor, ME, USA) or B6.FVB-Tg(Stra8-icre)1Reb/LguJ (strain 017490, The Jackson Laboratory), respectively, and further backcrossing to Nptnloxlox mice. Double Cre-mutants (NptnloxloxAmhCre+Stra8Cre) lacking Np in Sertoli cells and in spermatogonia were obtained by intercrossing the single mutants.
Mice were kept with a 12 h light/dark cycle and food and water ad libitum. Animal husbandry and tissue collection for RNA, protein, and histochemical analysis were conducted in accordance with German (Tierschutzgesetz TierSchG) and European legislations (European Communities Council Directive (2010/63/EU) for the care of laboratory animals) and with the respective legal and ethical approval by the legal authorities (Landesverwaltungsamt Halle, Sachsen-Anhalt, Germany).
4.2. Breeding and Mating Analysis
To determine the fertility of males, 6 Nptn−/− males were tested. In the LIN breeding facility, each male 2–3-month-old mouse was paired with 2 female 8-week-old wild-type C57BL6/NCrl (Charles River Laboratories, Sulzfeld, Germany) mice in a standard cage (Greenline IVC, Tecniplast S.p.A., Buguggiate, Italy) for 2 months after which the females were replaced by 2 new 8-week-old females until retirement of the male at ≥1 year age. No offspring from any Nptn−/− male nor any pregnancy were obtained. As controls, Nptn+/− males were paired with Nptn+/− females or with C57BL/6NCrl females and yielded offspring in comparable numbers to wild-type Nptn+/+ males. In each of the 6 breedings, 1 Nptn+/− male was crossed with 2 Nptn+/− females during 6 months, resulting in 45 litters with 272 offspring at weaning age (6 offspring/litter, 138 males, 134 females).
For mating behavior analysis, adult, socially naive male mice (6 Nptn−/− males and 5 Nptn+/+ littermate males) were exposed to socially naive wild-type females for 30 min, and their behavior was recorded using an overhead camera and videotaped. The latency to and the number of mounts, mounts with thrusts, and the sniffing behavior (duration) were scored by inspection of the video records by an investigator unaware of the genotype.
4.3. Quantitative RT-PCR
After the dissection of adult male mice, total RNA was isolated from the fresh frozen tissues indicated in Figure 3, using the RNeasy total RNA prep according to the manufacturer’s instructions (Qiagen, Hilden, Germany) and eluted in a suitable volume of DEPC-treated water. The RNA concentration was determined by OD measurement using nanodrop. Only RNA samples with an A260/280 value greater than 2 were used for the subsequent real-time PCR experiments. Real-time PCR experiments were performed using Taqman gene expression assays. Every 1 μg total RNA was reverse-transcribed into cDNA using the high capacity RNA to cDNA Master mix (Applied Biosystems Thermo Fisher Scientific, Darmstadt, Germany). The PCR amplification was performed with the Applied Biosystems 7300 Real-Time PCR instrument with one-tenth of the first strand reaction using the Taqman gene expression Master mix. GAPDH was chosen as the endogenous control in all reactions. Relative quantification was performed using the 7300 version 1.4 software. Gene expression assays were obtained from Applied Biosystems Thermo Fisher Scientific. The Taqman gene expression assays were chosen to span exon borders (neuroplastin Mm00485993_m1 and Mm00485990_m1).
4.4. Western Blotting
The whole brain, testis, and sperm from male mice were homogenized in 50 mM Tris-HCl buffer (pH 8.1) with a protease inhibitor cocktail (Roche, Mannheim, Germany). The crude membranes were extracted by adding lysis buffer (50 mM Tris-HCl, 1% Triton X-100, and protease inhibitor cocktail, pH 8.1). Following incubation for 1 h on ice, the supernatant was collected by centrifugation at 12,000× g for 20 min. Samples were denatured with 2X SDS loading buffer for 5 min at 95 °C, separated on 10 or 15% SDS-PAGE-gels, and transferred by Western blot. Blots were incubated overnight with the first antibody. Primary antibodies used were sheep polyclonal anti-Np detecting Np65 and Np55 pan-Np55/65 and goat polyclonal isoform-specific anti-Np65, R&D systems, Wiesbaden, Germany; rabbit anti-PMCA1, rabbit anti-LH-receptor, and mouse monoclonal anti-ß-actin, Thermo Fisher Scientific, Dreieich, Germany; rabbit anti-alpha tubulin, Synaptic Systems, Goettingen, Germany; mouse monoclonal anti-PKARIIa, BD Transduction Laboratories, Heidelberg, Germany; rabbit anti-HSD3B1, Sigma, St. Louis, MO, USA; rabbit anti-CYP11A1, Abcam, Berlin, Germany; mouse monoclonal anti-GAPDH, Santa Cruz, Dallas, TX, USA. After incubation for 1 h with the secondary antibody (anti-sheep, -goat, -mouse, or -rabbit IgG antibody, Jackson ImmunoResearch Dianova, Hamburg, Germany) and washing with TBS containing 0.5% Tween 20, the membrane was developed with ECL solution (Intas Chemocan ECL Imaging, Göttingen, Germany). Staining for alpha-tubulin, ß-actin, or GAPDH served as loading control.
4.5. Enzyme-Linked Immunosorbent Assays
ELISAs were performed exactly according to the instructions provided by the manufacturer. Briefly, ELISA kits were purchased from TECAN IBL International (Hamburg, Germany) for testosterone (RE52151) and Follicle Stimulating Hormone (FSH, RE52121), and USCN life sciences (Aachen, Germany) for Luteinizing Hormone (LH) (E90441Mu). Testosterone, FSH, and LH were determined in the serum of blood collected by heart puncture. In addition, testosterone was analyzed in testis homogenates. In a well of an antibody-coated microtiter plate, 25 µL sample and 200 µL of enzyme conjugate were mixed and incubated for 60 min at room temperature on an orbital shaker. After discarding the incubation solution, the plate was washed 3 times with 300 µL of diluted wash buffer, and the excess buffer solution was removed. An amount of 100 µL of TMB substrate was placed into each well. After 15 min at room temperature, the subtract reaction was stopped by the addition of 100 µL TMB stop solution. After gentle shaking of the plate, the optical density was measured with a photometer at 450 nm.
4.6. Histological Examination of Testis Morphology
Testes were dissected from transcardially perfused males, fixed in Bouin’s fluid, and paraffin-embedded using a standard protocol. In short, they were subjected to immersion in increasing concentrations of ethanol with 70%, 80%, 90%, and 95%, and three changes of 100% for one hour each, subsequently immersed in four changes of xylene for two hours, and then, three changes of paraffin for three hours. Finally, they were embedded in paraffin and stored at 4 °C. An amount of 10 μm sections were obtained on gelatine-treated slides in a 50 °C water bath by a microtome. Standard Haematoxylin & Eosin staining was performed, and sections were viewed using an Axioplan2 (Zeiss, Jena, Germany) microscope. Brightfield images were obtained using 2.5× magnification, and the number and perimeter of seminiferous tubules per transverse section were registered using ImageJ (Version 2.9.0/1.5.3t).
4.7. Immunofluorescence Staining after Paraffin Embedding
Paraffin-embedded testis sections were deparaffinized and blocked with BSA (5% in phosphate-buffered saline) for 1 h and then incubated with primary antibodies (sheep polyclonal Np detecting Np65 and Np55 (pan-Np55/65; 1:500, R&D systems) overnight at 4 °C in a humidified chamber. Post incubation, the sections were washed with PBS and probed with the secondary antibody (Cy3-conjugated anti-sheep 1:1000, Jackson Immunoresearch) in a sequential manner. Sections were examined by an Axioplan2 (Zeiss), and images were captured by Spot RT-KE camera (Diagnostic Instruments, Marburg, Germany).
4.8. Immunofluorescence Staining after Paraformaldehyde Fixation
Adult mice were anesthetized with isoflurane and transcardially perfused with PBS, followed by 4% PFA. Testes were dissected and post-fixed in 4% PFA overnight. Testis sections were blocked (20% horse serum in PBS, one hour, room temperature) and incubated with primary antibodies in PBS containing 0.3% Triton X-100 and 10% horse serum (overnight, 4 °C). Primary antibodies used were sheep polyclonal anti-Np detecting Np65 and Np55 (pan-Np55/65; 1:300, R&D systems) and rabbit polyclonal anti-Stra8 (Stimulated By Retinoic Acid 8, used as a marker for spermatogonia and spermatocytes, 1:500, Abcam), anti-CPY11A1 (Cytochrome P450 Family 11 Subfamily A Member 1, marker for Leydig cells, 1:500, Abcam), anti-alpha-smooth muscle actin (marker for myoid cells, 1:500, Abcam), and anti-Sox9 (SRY-Box Transcription Factor 9, marker for Sertoli cells, 1:500, EMD Millipore, Darmstadt, Germany) antibodies. The specificity of antibodies was assessed with negative controls using pre-immune sera.
Secondary antibodies were Cy3-conjugated anti-sheep and Cy5-conjugated anti-rabbit (1:1000, Jackson Immunoresearch). After washing with PBS and briefly with water, the sections were mounted on glass slides with fluoromount g DAPI (Southern Biotech, Birmingham, AL, USA) and visualized using a Leica SP5 confocal microscope (Leica, Wetzlar, Germany).
4.9. Epididymal Sperm Motility and Ploidy Stages of Testis Cells
Epididymal sperm was prepared as previously described [47]. Excised and cleaned cauda epididymidis and vas deferens were washed, transferred to 1 mL buffer HS (in mM: 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 20 HEPES, 5 glucose, 10 DL-lactic acid, and 10 pyruvic acid pH 7.4) and incised 3–5 times to allow the sperm to swim out into the medium over a period of 20 min at 37 °C and under 5% CO2. After washing three times in buffer HS, sperm was resuspended in 0.5 mL buffer HS at a final concentration of 1–2 × 107 cells/mL and used for determination of motility. The beat frequency of sperm was measured as described in [47] before and after local perfusion of the chamber with bicarbonate (15 mM; HSB), which activates a soluble adenylate cyclase, increasing the beat frequency.
For the analysis of spermatogenetic activity according to [48], frozen testis parenchyma was thawed, dissociated, and testicular cells were applied to flow cytometric DNA analysis: about 50 mg parenchyma was finely minced in 0.8 mL 100 mM citric acid containing 0.5% (v/v) Tween 20 and agitated for 20 min at room temperature. The DNA of lysed cells was stained by adding 4 mL of a 400 mM Na2HPO4 solution containing 5 µM 4′,6-diamidino-2-phenylindol (DAPI) for 10 min in the dark. Measurements were performed on a PAS III flow cytometer (Sysmex Deutschland GmbH, Norderstedt, Germany) equipped with a UV LED and an appropriate filter set (excitation: 365 nm, emission: 420 nm). Cells were recorded for their fluorescence intensity corresponding to the DNA content of their nuclei, and the histograms were analyzed for the proportions of cells in each peak by the FlowMax version 2.3 software (Sysmex). Haploid signals (1c) come from postmeiotic germ cell stages like spermatids and sperm cells, diploid signals (2c) from spermatogonia, secondary spermatocytes, and somatic testicular cells, tetraploid signals (4c) mainly derive from meiotic primary spermatocytes but also in mitotic spermatogonia. Between 2c and 4c, cells in the S-phase (S) were detected.
4.10. Immunofluorescence Staining of Sperm
Sperm was stained using sheep polyclonal antibodies against Np detecting Np65 and Np55 (pan-Np55/65 1:500, R&D systems). Secondary antibodies were Cy3-conjugated anti-sheep (1:1000, Jackson Immunoresearch). Counterstaining used phalloidin-iFluor 488 green (1:1000, Abcam, Berlin, Germany) and fluoromount g DAPI (Southern Biotech). Immunofluorescence was visualized using a Leica SP5 confocal microscope.
4.11. Statistical Analysis
Statview 5.0.1 (SAS Institute, Inc., Cary, NC, USA) was used for the analysis of variance (ANOVA), post hoc analysis (Scheffé or Fisher’s protected least significant difference), repeated-measures analysis of variance, and t-tests. GraphPad Prism 9.4.1 (San Diego, CA, USA) was used for t-tests and graph design. p < 0.05 was considered significant. For all analyses in this manuscript, all data points were included, and outliers were not removed.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms25010177/s1.
Author Contributions
J.C. and X.L. performed and analyzed immunohistochemical and Western blot experiments; S.B. performed and analyzed histochemical, ELISA, and mating experiments and contributed to the concept and original draft preparation; C.W. conducted sperm motility analysis; G.W. conducted sperm motility analysis and reviewed and edited the draft; K.M. performed the assessment of the ploidy status and reviewed and edited the draft. D.M. generated the mouse lines, analyzed mating behavior, conducted statistical analysis, concept and manuscript writing, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support from LIN, BMBF, DFG, CSC, and OvGU. This work was supported by German Federal Ministry of Education and Research (BMBF grant CONICYT to DM, Eckart D. Gundelfinger, Karl-Heinz Smalla, and Constanze Seidenbecher), Deutsche Forschungsgemeinschaft (DFG WE 2344/9-4 to G.W.), and by the China Scholarship Council (to X.L. CSC No. 201506290028). J.C. is thankful for a fellowship provided by Otto-von-Guericke-University, Magdeburg. The publication of this article was funded by the Open Access Fund of the Leibniz Association.
Institutional Review Board Statement
Animal husbandry and tissue collection were conducted in accordance with German (Tierschutzgesetz TierSchG) and European legislations (European Communities Council Directive (2010/63/EU) for the care of laboratory animals) and with the respective approval by the legal authorities (Landesverwaltungsamt Halle, Sachsen-Anhalt, Germany), including the ethical approval by an independent commission appointed by Landesverwaltungsamt Halle, Sachsen-Anhalt, Germany.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files). Further data may be obtained from the corresponding author upon reasonable request.
Acknowledgments
We thank Karla Sowa for expert technical assistance with the behavioral experiments and Alexandra Weber for help with the determination of the ploidy status.
Conflicts of Interest
Author S. B. was employed by the company Iconeus after her participation in this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hwang, K.; Yatsenko, A.N.; Jorgez, C.J.; Mukherjee, S.; Nalam, R.L.; Matzuk, M.M.; Lamb, D.J. Mendelian genetics of male infertility. Ann. N. Y. Acad. Sci. 2011, 1214, E1–E17. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.N.; Matzuk, M.M. Genetics of male fertility. Methods Mol. Biol. 2014, 1154, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Laan, M.; Kasak, L.; Punab, M. Translational aspects of novel findings in genetics of male infertility-status quo 2021. Br. Med. Bull. 2021, 140, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Cuppens, H.; Cassiman, J.J. CFTR mutations and polymorphisms in male infertility. Int. J. Androl. 2004, 27, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Guerri, G.; Maniscalchi, T.; Barati, S.; Busetto, G.M.; Del Giudice, F.; De Berardinis, E.; Cannarella, R.; Calogero, A.E.; Bertelli, M. Non-syndromic monogenic male infertility. Acta Bio Medica Atenei Parm. 2019, 90 (Suppl. 10), 62–67. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Herrera-Molina, R.; Sabanov, V.; Ahmed, T.; Iscru, E.; Stöber, F.; Richter, K.; Fischer, K.D.; Angenstein, F.; Goldschmidt, J.; et al. Genetically-induced retrograde amnesia of associative memories after neuroplastin ablation. Biol. Psychiatry 2017, 81, 124–135. [Google Scholar] [CrossRef]
- Langnaese, K.; Beesley, P.W.; Gundelfinger, E.D. Synaptic membrane glycoproteins gp65 and gp55 are new members of the immunoglobulin superfamily. J. Biol. Chem. 1997, 272, 821–827. [Google Scholar] [CrossRef]
- Lin, X.; Liang, Y.; Herrera-Molina, R.; Montag, D. Neuroplastin in Neuropsychiatric Diseases. Genes 2021, 12, 1507. [Google Scholar] [CrossRef]
- Desrivières, S.; Lourdusamy, A.; Tao, C.; Toro, R.; Jia, T.; Loth, E.; Medina, L.M.; Kepa, A.; Fernandes, A.; Ruggeri, B.; et al. IMAGEN Consortium. Single nucleotide polymorphism in the neuroplastin locus associates with cortical thickness and intellectual ability in adolescents. Mol. Psychiatry 2014, 20, 263–274. [Google Scholar] [CrossRef]
- Saito, A.; Fujikura-Ouchi, Y.; Kuramasu, A.; Shimoda, K.; Akiyama, K.; Matsuoka, H.; Ito, C. Association study of putative promoter polymorphisms in the neuroplastin gene and schizophrenia. Neurosci. Lett. 2007, 411, 168–173. [Google Scholar] [CrossRef]
- Lin, X.; Brunk MG, K.; Yuanxiang, P.; Curran, A.W.; Zhang, E.; Stöber, F.; Goldschmidt, J.; Gundelfinger, E.D.; Vollmer, M.; Happel, M.F.K.; et al. Neuroplastin expression is essential for hearing and hair cell PMCA expression. Brain Struct. Funct. 2021, 226, 1533–1551. [Google Scholar] [CrossRef] [PubMed]
- Choy, M.K.; Javierre, B.M.; Williams, S.G.; Baross, S.L.; Liu, Y.; Wingett, S.W.; Akbarov, A.; Wallace, C.; Freire-Pritchett, P.; Rugg-Gunn, P.J.; et al. Promoter interactome of human embryonic stem cell-derived cardiomyocytes connects GWAS regions to cardiac gene networks. Nat. Commun. 2018, 9, 2526. [Google Scholar] [CrossRef] [PubMed]
- Sumardika, I.W.; Chen, Y.; Tomonobu, N.; Kinoshita, R.; Ruma, I.M.W.; Sato, H.; Kondo, E.; Inoue, Y.; Yamauchi, A.; Murata, H.; et al. Neuroplastin-beta mediates S100A8/A9-induced lung cancer disseminative progression. Mol. Carcinog. 2019, 58, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.Z.; Grillet, N.; Dewey, J.B.; Trouillet, A.; Krey, J.F.; Barr-Gillespie, P.G.; Oghalai, J.S.; Müller, U. Neuroplastin Isoform Np55 Is Expressed in the Stereocilia of Outer Hair Cells and Required for Normal Outer Hair Cell Function. J. Neurosci. 2016, 36, 9201–9216. [Google Scholar] [CrossRef] [PubMed]
- Carrott, L.; Bowl, M.R.; Aguilar, C.; Johnson, S.L.; Chessum, L.; West, M.; Morse, S.; Dorning, J.; Smart, E.; Hardisty-Hughes, R.; et al. Absence of Neuroplastin-65 Affects Synaptogenesis in Mouse Inner Hair Cells and Causes Profound Hearing Loss. J. Neurosci. 2016, 36, 222–234. [Google Scholar] [CrossRef]
- Korthals, M.; Langnaese, K.; Smalla, K.H.; Kähne, T.; Herrera-Molina, R.; Handschuh, J.; Lehmann, A.C.; Mamula, D.; Naumann, M.; Seidenbecher, C.; et al. A complex of Neuroplastin and Plasma Membrane Calcium ATPase controls T cell activation. Sci. Rep. 2017, 7, 8358. [Google Scholar] [CrossRef]
- Schmidt, N.; Kollewe, A.; Constantin, C.E.; Henrich, S.; Ritzau-Jost, A.; Bildl, W.; Saalbach, A.; Hallermann, S.; Kulik, A.; Fakler, B.; et al. Neuroplastin and Basigin Are Essential Auxiliary Subunits of Plasma Membrane Ca2+-ATPases and Key Regulators of Ca2+ Clearance. Neuron 2017, 96, 827–838.e829. [Google Scholar] [CrossRef]
- Gong, D.; Chi, X.; Ren, K.; Huang, G.; Zhou, G.; Yan, N.; Lei, J.; Zhou, Q. Structure of the human plasma membrane Ca(2+)-ATPase 1 in complex with its obligatory subunit neuroplastin. Nat. Commun. 2018, 9, 3623. [Google Scholar] [CrossRef]
- Montag, D. Retrograde amnesia—A question of disturbed Calcium levels? Front. Cell. Neurosci. Sect. Cell. Neurophysiol. 2021, 15, 746198. [Google Scholar] [CrossRef]
- Beesley, P.W.; Herrera-Molina, R.; Smalla, K.H.; Seidenbecher, C. The Neuroplastin adhesion molecules: Key regulators of neuronal plasticity and synaptic function. J. Neurochem. 2014, 131, 268–283. [Google Scholar] [CrossRef]
- Igakura, T.; Kadomatsu, K.; Kaname, T.; Muramatsu, H.; Fan, Q.W.; Miyauchi, T.; Toyama, Y.; Kuno, N.; Yuasa, S.; Takahashi, M.; et al. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev. Biol. 1998, 194, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Li, Y.; Sun, F.; Saalbach, A.; Klein, C.; Miller, D.J.; Hess, R.; Nowak, R.A. Basigin null mutant male mice are sterile and exhibit impaired interactions between germ cells and Sertoli cells. Dev. Biol. 2013, 380, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Langnaese, K.; Mummery, R.; Gundelfinger, E.D.; Beesley, P.W. Immunoglobulin superfamily members gp65 and gp55: Tissue distribution of glycoforms. FEBS Lett. 1998, 429, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Green, C.D.; Ma, Q.; Manske, G.L.; Niederriter Shami, A.; Zheng, X.; Marini, S.; Moritz, L.; Sultan, C.; Gurczynski, S.J.; Moore, B.B.; et al. A Comprehensive Roadmap of Murine Spermatogenesis Defined by Single-Cell RNA-Sequential. Dev. Cell 2018, 46, 651–667.e10. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Molina, R.; Mlinac-Jerkovic, K.; Ilic, K.; Stöber, F.; Vemula, S.K.; Sandoval, M.; Milosevic, N.J.; Simic, G.; Smalla, K.H.; Goldschmidt, J.; et al. Neuroplastin deletion in glutamatergic neurons impairs brain functions and calcium regulation: Implication for cognitive deterioration. Sci. Rep. 2017, 7, 7273. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Edward, J.; Mitchell, J.C.; Shao, B.; Bowes, J.E.; Coen, C.W.; Lightman, S.L.; O’Byrne, K.T. Differential effects of repeated restraint stress on pulsatile lutenizing hormone secretion in female Fischer, Lewis and Wistar rats. J. Neuroendocrinol. 2004, 16, 620–627. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, P.J.; Baker, P.J.; Johnston, H. The foetal Leydig cell—Differentiation, function and regulation. Int. J. Androl. 2006, 29, 90–95. [Google Scholar] [CrossRef]
- Grosman, H.; Rosales, M.; Fabre, B.; Nolazco, C.; Mazza, O.; Berg, G.; Mesch, V. Association between testosterone levels and the metabolic syndrome in adult men. Aging Male 2014, 17, 161–165. [Google Scholar] [CrossRef]
- Johnstone, T.B.; Agarwal, S.R.; Harvey, R.D.; Ostrom, R.S. cAMP Signaling Compartmentation: Adenylyl Cyclases as Anchors of Dynamic Signaling Complexes. Mol. Pharmacol. 2018, 93, 270–276. [Google Scholar] [CrossRef]
- Kuno, N.; Kadomatsu, K.; Fan, Q.W.; Hagihara, M.; Senda, T.; Mizutani, S.; Muramatsu, T. Female sterility in mice lacking the basigin gene, which encodes a transmembrane glycoprotein belonging to the immunoglobulin superfamily. FEBS Lett. 1998, 425, 191–194. [Google Scholar] [CrossRef]
- Toyama, Y.; Maekawa, M.; Kadomatsu, K.; Miyauchi, T.; Muramatsu, T.; Yuasa, S. Histological characterization of defective spermatogenesis in mice lacking the basigin gene. Anat. Histol. Embryol. 1999, 28, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Quigley, C.A.; De Bellis, A.; Marschke, K.B.; el-Awady, M.K.; Wilson, E.M.; French, F.S. Androgen receptor defects: Historical, clinical, and molecular perspectives. Endocrinol. Rev. 1995, 16, 271–321. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, H.; Habert, R.; Saez, J.M. Origin, proliferation and differentiation of Leydig cells. J. Mol. Endocrinol. 1998, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.R.; Wang, Y.; Lu, N.; Matzuk, M.M. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 1997, 15, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Wreford, N.G.; Rajendra Kumar, T.; Matzuk, M.M.; de Kretser, D.M. Analysis of the testicular phenotype of the follicle-stimulating hormone β-subunit knockout and the activin type II receptor knockout mice by stereological analysis. Endocrinology 2001, 142, 2916–2920. [Google Scholar] [CrossRef]
- Krishnamurthy, H.; Danilovich, N.; Morales, C.R.; Sairam, M.R. Qualitative and quantitative decline in spermatogenesis of the follicle-stimulating hormone receptor knockout (FORKO) mouse. Biol. Reprod. 2000, 62, 1146–1159. [Google Scholar] [CrossRef]
- Pakarainen, T.; Zhang, F.; Mäkelä, S.; Poutanen, M.; Huhtaniemi, I. Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology 2005, 146, 596–606. [Google Scholar] [CrossRef]
- Rengarajan, S.; Balasubramanian, K. Corticosterone has direct inhibitory effect on the expression of peptide hormone receptors, 11 beta-HSD and glucose oxidation in cultured adult rat Leydig cells. Mol. Cell Endocrinol. 2007, 279, 52–62. [Google Scholar] [CrossRef]
- Oduwole, O.O.; Huhtaniemi, I.T.; Misrahi, M. The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. Int. J. Mol. Sci. 2021, 22, 12735. [Google Scholar] [CrossRef]
- Ma, X.; Dong, Y.; Matzuk, M.M.; Kumar, T.R. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc. Natl. Acad. Sci. USA 2004, 101, 17294–17299. [Google Scholar] [CrossRef]
- Zhang, F.P.; Poutanen, M.; Wilbertz, J.; Huhtaniemi, I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol. Endocrinol. 2001, 15, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.P.; Pakarainen, T.; Zhu, F.; Poutanen, M.; Huhtaniemi, I. Molecular characterization of postnatal development of testicular steroidogenesis in luteinizing hormone receptor knockout mice. Endocrinology 2004, 145, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.R.; Varanda, W.A.; Franci, C.R. A calcium-induced calcium release mechanism supports luteinizing hormone-induced testosterone secretion in mouse Leydig cells. Am. J. Physiololy Cell Physiol. 2010, 299, C316–C323. [Google Scholar] [CrossRef]
- Lopreiato, R.; Giacomello, M.; Carafoli, E. The plasma membrane calcium pump: New ways to look at an old enzyme. J. Biol. Chem. 2014, 289, 10261–10268. [Google Scholar] [CrossRef]
- Bruce, J.I.; Straub, S.V.; Yule, D.I. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium 2003, 34, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Guerini, D.; Pan, B.; Carafoli, E. Expression, purification, and characterization of isoform 1 of the plasma membrane Ca2+ pump: Focus on calpain sensitivity. J. Biol. Chem. 2003, 278, 38141–38148. [Google Scholar] [CrossRef]
- Wennemuth, G.; Carlson, A.E.; Harper, A.J.; Babcock, D.F. Bicarbonate actions on flagellar and Ca2+-channel responses: Initial events in sperm activation. Development 2003, 130, 1317–1326. [Google Scholar] [CrossRef]
- Blottner, S.; Hingst, O.; Meyer, H.H.D. Seasonal spermatogenesis and testosterone production in roe deer (Capreolus capreolus). J. Reprod. Fertil. 1996, 108, 299–305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).