Molecular Characteristics, Functional Definitions, and Regulatory Mechanisms for Cross-Presentation Mediated by the Major Histocompatibility Complex: A Comprehensive Review
Abstract
:1. Introduction
2. Discovery of MHC
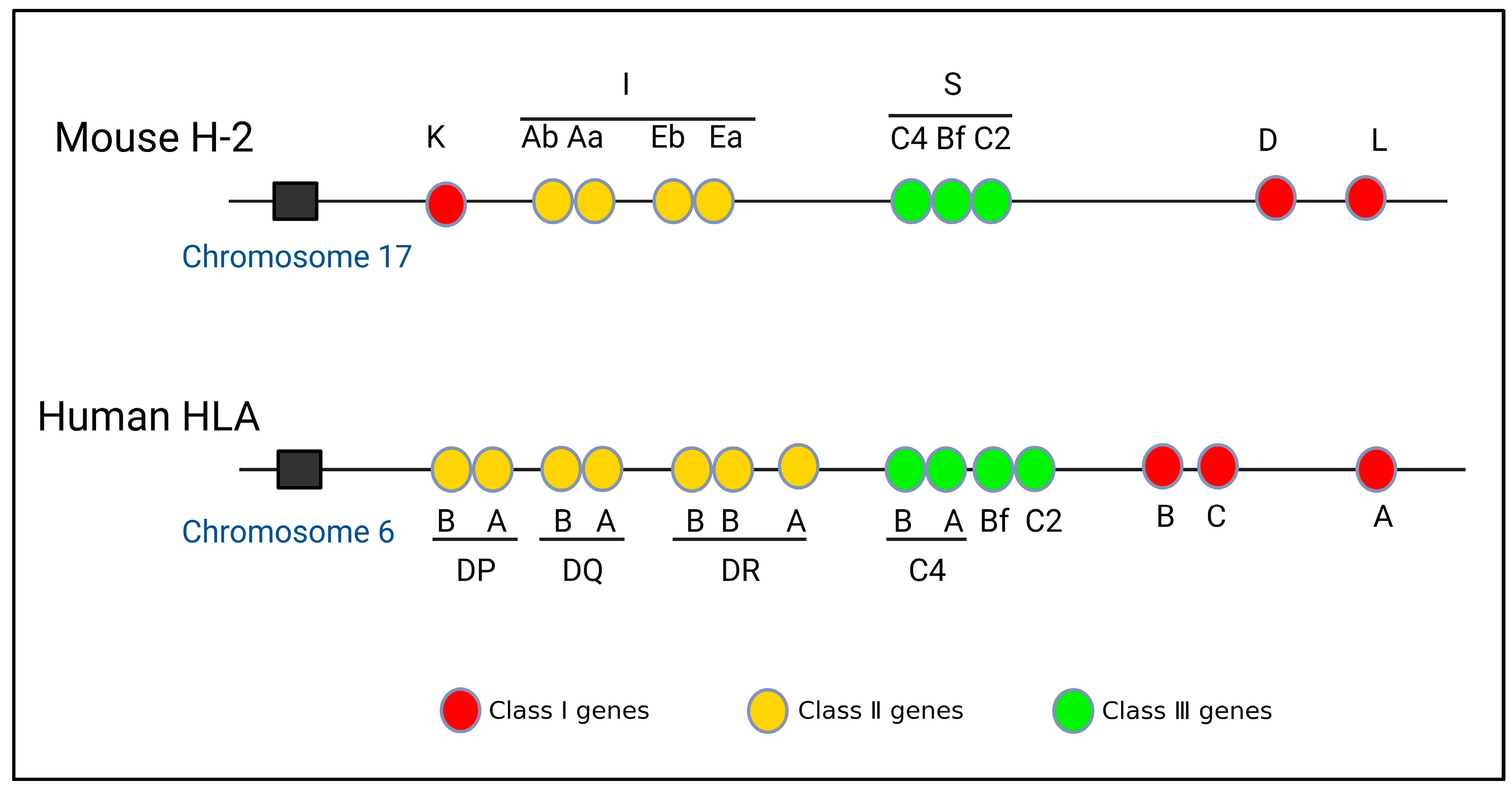
3. Structural Characteristics of MHC Gene
4. Classification, Structure, and Function of MHC
5. Antigen Presentation of MHC Molecules
6. Cross-Presentation of MHC Molecules
6.1. Cytosolic Pathway
6.2. Vesicular Pathway
7. Cells Performing Cross-Presentation
7.1. Dendritic Cells
7.2. Liver Sinusoidal Endothelial Cells
7.3. T Cells
8. Transporting of MHC Molecules
9. Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Heise, E.R.; Cook, D.J.; Schepart, B.S.; Manning, C.H.; McMahan, M.R.; Chedid, M.; Keever, C.A. The Major Histocompatibility Complex of Primates. Genetica 1987, 73, 53–68. [Google Scholar] [CrossRef]
- Hughes, A.L.; Yeager, M. Natural Selection at Major Histocompatibility Complex Loci of Vertebrates. Annu. Rev. Genet. 1998, 32, 415–435. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.M.; Saxena, V.K.; Tomar, A.; Singh, K.P.; Singh, K.B.; Tiwari, A.K. MHC-B Haplotypes Impact Susceptibility and Resistance to RSV-A Infection. Front. Biosci. 2018, 10, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Role of Cytokines and Major Histocompatibility Complex Restriction in Mouse Resistance to Infection with a Natural Recombinant Strain (Type I-III) of Toxoplasma Gondii. Available online: https://journals.asm.org/doi/epdf/10.1128/iai.71.11.6392-6401.2003 (accessed on 13 December 2023).
- Madden, D.R. The Three-Dimensional Structure of Peptide-MHC Complexes. Annu. Rev. Immunol. 1995, 13, 587–622. [Google Scholar] [CrossRef] [PubMed]
- González, E.R. Immunogenetics Recognized by Nobel Prize. JAMA 1980, 244, 2396–2400. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, Y.; Zhao, H.; Wang, J.; Gao, X.; Chen, J.; Fu, B.; Shen, Y.; Miao, F.; Zhang, J.; et al. Non-Invasive Monitoring of CNS MHC-I Molecules in Ischemic Stroke Mice. Theranostics 2017, 7, 2837–2848. [Google Scholar] [CrossRef]
- Malviya, G.; de Vries, E.F.J.; Dierckx, R.A.; Signore, A. Synthesis and Evaluation of 99mTc-Labelled Monoclonal Antibody 1D09C3 for Molecular Imaging of Major Histocompatibility Complex Class II Protein Expression. Mol. Imaging Biol. 2011, 13, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Szolek, A.; Schubert, B.; Mohr, C.; Sturm, M.; Feldhahn, M.; Kohlbacher, O. OptiType: Precision HLA Typing from next-Generation Sequencing Data. Bioinformatics 2014, 30, 3310–3316. [Google Scholar] [CrossRef]
- Kamiya, T.; O’Dwyer, K.; Westerdahl, H.; Senior, A.; Nakagawa, S. A Quantitative Review of MHC-Based Mating Preference: The Role of Diversity and Dissimilarity. Mol. Ecol. 2014, 23, 5151–5163. [Google Scholar] [CrossRef]
- Nanaware, P.P.; Jurewicz, M.M.; Leszyk, J.D.; Shaffer, S.A.; Stern, L.J. HLA-DO Modulates the Diversity of the MHC-II Self-Peptidome*[S]. Mol. Cell. Proteom. 2019, 18, 490–503. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Lin, Q.; Fang, W.; Chen, X. Diversity and Selection of MHC Class I Genes in the Vulnerable Chinese Egret (Egretta Eulophotes). PLoS ONE 2017, 12, e0176671. [Google Scholar] [CrossRef] [PubMed]
- Dirscherl, H.; McConnell, S.C.; Yoder, J.A.; de Jong, J.L.O. The MHC Class I Genes of Zebrafish. Dev. Comp. Immunol. 2014, 46, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Reimann, J.; Miller, R.G. Polymorphism and MHC Gene Function. Dev. Comp. Immunol. 1983, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Radwan, J.; Babik, W.; Kaufman, J.; Lenz, T.L.; Winternitz, J. Advances in the Evolutionary Understanding of MHC Polymorphism. Trends Genet. 2020, 36, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, K.; Zhao, H. Haplotype-Association Analysis. Adv. Genet. 2008, 60, 335–405. [Google Scholar] [CrossRef] [PubMed]
- Good, B.H. Linkage Disequilibrium between Rare Mutations. Genetics 2022, 220, iyac004. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.H.; Tay, M.Z.; Wang, B.; Xiao, Z.; Ren, E.C. Intrahaplotypic Variants Differentiate Complex Linkage Disequilibrium within Human MHC Haplotypes. Sci. Rep. 2015, 5, 16972. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, R.H.; Saito, P.K.; Gelmini, G.F.; da Silva, J.S.; Bicalho, M.D.G.; Borelli, S.D. MICA Diversity and Linkage Disequilibrium with HLA-B Alleles in Renal-Transplant Candidates in Southern Brazil. PLoS ONE 2017, 12, e0176072. [Google Scholar] [CrossRef]
- Ivy-Israel, N.M.D.; Moore, C.E.; Schwartz, T.S.; Ditchkoff, S.S. Characterization of Two MHC II Genes (DOB, DRB) in White-Tailed Deer (Odocoileus Virginianus). BMC Genet. 2020, 21, 83. [Google Scholar] [CrossRef]
- Accolla, R.S.; Jotterand-Bellomo, M.; Scarpellino, L.; Maffei, A.; Carra, G.; Guardiola, J. aIr-1, a Newly Found Locus on Mouse Chromosome 16 Encoding a Trans-Acting Activator Factor for MHC Class II Gene Expression. J. Exp. Med. 1986, 164, 369–374. [Google Scholar] [CrossRef]
- Villadangos, J.A. Presentation of Antigens by MHC Class II Molecules: Getting the Most out of Them. Mol. Immunol. 2001, 38, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Shiina, T.; Blancher, A.; Inoko, H.; Kulski, J.K. Comparative Genomics of the Human, Macaque and Mouse Major Histocompatibility Complex. Immunology 2017, 150, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Dijkstra, J.M.; Tsukamoto, K.; Grimholt, U.; Wiegertjes, G.F.; Kondow, A.; Yamaguchi, H.; Hashimoto, K. Discovery of an Ancient MHC Category with Both Class I and Class II Features. Proc. Natl. Acad. Sci. USA 2021, 118, e2108104118. [Google Scholar] [CrossRef] [PubMed]
- Downs, I.; Vijayan, S.; Sidiq, T.; Kobayashi, K.S. CITA/NLRC5: A Critical Transcriptional Regulator of MHC Class I Gene Expression. Biofactors 2016, 42, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Scavuzzi, B.M.; van Drongelen, V.; Holoshitz, J. HLA-G and the MHC Cusp Theory. Front. Immunol. 2022, 13, 814967. [Google Scholar] [CrossRef]
- Piertney, S.B.; Oliver, M.K. The Evolutionary Ecology of the Major Histocompatibility Complex. Heredity 2006, 96, 7–21. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The Ins and Outs of MHC Class II-Mediated Antigen Processing and Presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S. The Cell Biology of Antigen Presentation in Dendritic Cells. Curr. Opin. Immunol. 2001, 13, 45–51. [Google Scholar] [CrossRef]
- Wang, F.; Ullah, A.; Fan, X.; Xu, Z.; Zong, R.; Wang, X.; Chen, G. Delivery of Nanoparticle Antigens to Antigen-Presenting Cells: From Extracellular Specific Targeting to Intracellular Responsive Presentation. J. Control. Release 2021, 333, 107–128. [Google Scholar] [CrossRef]
- Kashem, S.W.; Haniffa, M.; Kaplan, D.H. Antigen-Presenting Cells in the Skin. Annu. Rev. Immunol. 2017, 35, 469–499. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a Systems Understanding of MHC Class I and MHC Class II Antigen Presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Blanchong, C.A.; Chung, E.K.; Rupert, K.L.; Yang, Y.; Yang, Z.; Zhou, B.; Moulds, J.M.; Yu, C.Y. Genetic, Structural and Functional Diversities of Human Complement Components C4A and C4B and Their Mouse Homologues, Slp and C4. Int. Immunopharmacol. 2001, 1, 365–392. [Google Scholar] [CrossRef] [PubMed]
- Gruen, J.R.; Weissman, S.M. Human MHC Class III and IV Genes and Disease Associations. Front. Biosci. 2001, 6, D960–D972. [Google Scholar] [CrossRef] [PubMed]
- Kedzierska, K.; Koutsakos, M. The ABC of Major Histocompatibility Complexes and T Cell Receptors in Health and Disease. Viral Immunol. 2020, 33, 160–178. [Google Scholar] [CrossRef] [PubMed]
- Serçinoğlu, O.; Ozbek, P. Sequence-Structure-Function Relationships in Class I MHC: A Local Frustration Perspective. PLoS ONE 2020, 15, e0232849. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Hua, J.C. Production of Soluble HLA-Class-I Molecules by IFN-Gamma-Induced Colon-Adenocarcinoma Cells. Int. J. Cancer 1995, 60, 576–581. [Google Scholar] [CrossRef]
- Olson, R.; Huey-Tubman, K.E.; Dulac, C.; Bjorkman, P.J. Structure of a Pheromone Receptor-Associated MHC Molecule with an Open and Empty Groove. PLoS Biol. 2005, 3, e257. [Google Scholar] [CrossRef]
- Bui, H.-H.; Schiewe, A.J.; von Grafenstein, H.; Haworth, I.S. Structural Prediction of Peptides Binding to MHC Class I Molecules. Proteins 2006, 63, 43–52. [Google Scholar] [CrossRef]
- Ohta, Y.; Shiina, T.; Lohr, R.L.; Hosomichi, K.; Pollin, T.I.; Heist, E.J.; Suzuki, S.; Inoko, H.; Flajnik, M.F. Primordial Linkage of Β2-Microglobulin to the MHC. J. Immunol. 2011, 186, 3563–3571. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Zhang, X.; Jia, K.; Deng, J.; Zhou, C.; He, Y. Major Histocompatibility Complex Class II Molecule in Non-Small Cell Lung Cancer Diagnosis, Prognosis and Treatment. OncoTargets Ther. 2019, 12, 7281–7288. [Google Scholar] [CrossRef]
- Jensen, K.K.; Andreatta, M.; Marcatili, P.; Buus, S.; Greenbaum, J.A.; Yan, Z.; Sette, A.; Peters, B.; Nielsen, M. Improved Methods for Predicting Peptide Binding Affinity to MHC Class II Molecules. Immunology 2018, 154, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Three Es of Cancer Immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Hamilos, D.L. Antigen Presenting Cells. Immunol. Res. 1989, 8, 98–117. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E.; Benacerraf, B. Functional Specificity of Thymus- Dependent Lymphocytes. Science 1977, 195, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Gaczynska, M.; Rock, K.L.; Goldberg, A.L. Role of Proteasomes in Antigen Presentation. Enzym. Protein 1993, 47, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I. Dendritic Cells: Master Regulators of the Immune Response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.; Lobos, C.A.; Nguyen, A.T.; Gras, S. TCR Recognition of Peptide–MHC-I: Rule Makers and Breakers. Int. J. Mol. Sci. 2020, 22, 68. [Google Scholar] [CrossRef]
- Kamal, S.; Kerndt, C.C.; Lappin, S.L. Genetics, Histocompatibility Antigen. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Veerappan Ganesan, A.P.; Eisenlohr, L.C. The Elucidation of Non-Classical MHC Class II Antigen Processing through the Study of Viral Antigens. Curr. Opin. Virol. 2017, 22, 71–76. [Google Scholar] [CrossRef]
- Shen, H.; Ackerman, A.L.; Cody, V.; Giodini, A.; Hinson, E.R.; Cresswell, P.; Edelson, R.L.; Saltzman, W.M.; Hanlon, D.J. Enhanced and Prolonged Cross-Presentation Following Endosomal Escape of Exogenous Antigens Encapsulated in Biodegradable Nanoparticles. Immunology 2006, 117, 78–88. [Google Scholar] [CrossRef]
- Blees, A.; Januliene, D.; Hofmann, T.; Koller, N.; Schmidt, C.; Trowitzsch, S.; Moeller, A.; Tampé, R. Structure of the Human MHC-I Peptide-Loading Complex. Nature 2017, 551, 525–528. [Google Scholar] [CrossRef]
- van de Weijer, M.L.; Luteijn, R.D.; Wiertz, E.J.H.J. Viral Immune Evasion: Lessons in MHC Class I Antigen Presentation. Semin. Immunol. 2015, 27, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Watts, C. The Exogenous Pathway for Antigen Presentation on Major Histocompatibility Complex Class II and CD1 Molecules. Nat. Immunol. 2004, 5, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Zachova, K.; Krupka, M.; Raska, M. Antigen Cross-Presentation and Heat Shock Protein-Based Vaccines. Arch. Immunol. Et Ther. Exp. 2016, 64, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Lepenies, B. Glycans as Vaccine Antigens and Adjuvants: Immunological Considerations. Methods Mol. Biol. 2015, 1331, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.B.; Klaeger, S.; Clauser, K.R.; Sarkizova, S.; Weingarten-Gabbay, S.; Graham, D.B.; Carr, S.A.; Abelin, J.G. MS-Based HLA-II Peptidomics Combined with Multiomics Will Aid the Development of Future Immunotherapies. Mol. Cell Proteom. 2021, 20, 100116. [Google Scholar] [CrossRef]
- van den Hoorn, T.; Paul, P.; Jongsma, M.L.M.; Neefjes, J. Routes to Manipulate MHC Class II Antigen Presentation. Curr. Opin. Immunol. 2011, 23, 88–95. [Google Scholar] [CrossRef]
- Goldberg, A.C.; Rizzo, L.V. MHC Structure and Function—Antigen Presentation. Part 1. Einstein (Sao Paulo) 2015, 13, 153–156. [Google Scholar] [CrossRef]
- Colbert, J.D.; Cruz, F.M.; Rock, K.L. Cross-Presentation of Exogenous Antigens on MHC I Molecules. Curr. Opin. Immunol. 2020, 64, 1–8. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, J.; Chu, C.-C. Enhanced MHC-I Antigen Presentation from the Delivery of Ovalbumin by Light-Facilitated Biodegradable Poly(Ester Amide)s Nanoparticles. J. Mater. Chem. B 2018, 6, 1930–1942. [Google Scholar] [CrossRef]
- Gutiérrez-Martínez, E.; Planès, R.; Anselmi, G.; Reynolds, M.; Menezes, S.; Adiko, A.C.; Saveanu, L.; Guermonprez, P. Cross-Presentation of Cell-Associated Antigens by MHC Class I in Dendritic Cell Subsets. Front. Immunol. 2015, 6, 363. [Google Scholar] [CrossRef]
- Grotzke, J.E.; Sengupta, D.; Lu, Q.; Cresswell, P. The Ongoing Saga of the Mechanism(s) of MHC Class I-Restricted Cross-Presentation. Curr. Opin. Immunol. 2017, 46, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M. Regulation of the Cell Biology of Antigen Cross-Presentation. Annu. Rev. Immunol. 2018, 36, 717–753. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.M.; Chan, A.; Rock, K.L. Pathways of MHC I Cross-Presentation of Exogenous Antigens. Semin. Immunol. 2023, 66, 101729. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.M.; Colbert, J.D.; Merino, E.; Kriegsman, B.A.; Rock, K.L. The Biology and Underlying Mechanisms of Cross-Presentation of Exogenous Antigens on MHC-I Molecules. Annu. Rev. Immunol. 2017, 35, 149–176. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, P.J. Presentation of Antigenic Peptides by Products of the Major Histocompatibility Complex. J. Pept. Sci. 1998, 4, 182–194. [Google Scholar] [CrossRef]
- Koch, J.; Tampé, R. The Macromolecular Peptide-Loading Complex in MHC Class I-Dependent Antigen Presentation. Cell Mol. Life Sci. 2006, 63, 653–662. [Google Scholar] [CrossRef]
- Alloatti, A.; Kotsias, F.; Magalhaes, J.G.; Amigorena, S. Dendritic Cell Maturation and Cross-Presentation: Timing Matters! Immunol. Rev. 2016, 272, 97–108. [Google Scholar] [CrossRef]
- Dixon, A.M.; Roy, S. Role of Membrane Environment and Membrane-Spanning Protein Regions in Assembly and Function of the Class II Major Histocompatibility Complex. Hum. Immunol. 2019, 80, 5–14. [Google Scholar] [CrossRef]
- Muraille, E.; Gounon, P.; Cazareth, J.; Hoebeke, J.; Lippuner, C.; Davalos-Misslitz, A.; Aebischer, T.; Muller, S.; Glaichenhaus, N.; Mougneau, E. Direct Visualization of Peptide/MHC Complexes at the Surface and in the Intracellular Compartments of Cells Infected in Vivo by Leishmania Major. PLoS Pathog. 2010, 6, e1001154. [Google Scholar] [CrossRef]
- Schurich, A.; Berg, M.; Stabenow, D.; Böttcher, J.; Kern, M.; Schild, H.-J.; Kurts, C.; Schuette, V.; Burgdorf, S.; Diehl, L.; et al. Dynamic Regulation of CD8 T Cell Tolerance Induction by Liver Sinusoidal Endothelial Cells. J. Immunol. 2010, 184, 4107–4114. [Google Scholar] [CrossRef]
- Imai, J.; Otani, M.; Sakai, T.; Hatta, S. Purification of the Membrane Compartment for Endoplasmic Reticulum-Associated Degradation of Exogenous Antigens in Cross-Presentation. J. Vis. Exp. 2017, 21, e55949. [Google Scholar] [CrossRef]
- Imai, K.; Hao, F.; Fujita, N.; Tsuji, Y.; Oe, Y.; Araki, Y.; Hamasaki, M.; Noda, T.; Yoshimori, T. Atg9A Trafficking through the Recycling Endosomes Is Required for Autophagosome Formation. J. Cell Sci. 2016, 129, 3781–3791. [Google Scholar] [CrossRef] [PubMed]
- Dingjan, I.; Verboogen, D.R.; Paardekooper, L.M.; Revelo, N.H.; Sittig, S.P.; Visser, L.J.; von Mollard, G.F.; Henriet, S.S.; Figdor, C.G.; Ter Beest, M.; et al. Lipid Peroxidation Causes Endosomal Antigen Release for Cross-Presentation. Sci. Rep. 2016, 6, 22064. [Google Scholar] [CrossRef]
- Jancic, C.; Savina, A.; Wasmeier, C.; Tolmachova, T.; El-Benna, J.; Dang, P.M.-C.; Pascolo, S.; Gougerot-Pocidalo, M.-A.; Raposo, G.; Seabra, M.C.; et al. Rab27a Regulates Phagosomal pH and NADPH Oxidase Recruitment to Dendritic Cell Phagosomes. Nat. Cell Biol. 2007, 9, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, A.R.; Savina, A.; Vermeulen, M.; Pérez, L.; Geffner, J.; Hermine, O.; Rosenzweig, S.D.; Faure, F.; Amigorena, S. NADPH Oxidase Controls Phagosomal pH and Antigen Cross-Presentation in Human Dendritic Cells. Blood 2008, 112, 4712–4722. [Google Scholar] [CrossRef] [PubMed]
- York, I.A.; Brehm, M.A.; Zendzian, S.; Towne, C.F.; Rock, K.L. Endoplasmic Reticulum Aminopeptidase 1 (ERAP1) Trims MHC Class I-Presented Peptides in Vivo and Plays an Important Role in Immunodominance. Proc. Natl. Acad. Sci. USA 2006, 103, 9202–9207. [Google Scholar] [CrossRef] [PubMed]
- Canton, J. Phagosome Maturation in Polarized Macrophages. J. Leukoc. Biol. 2014, 96, 729–738. [Google Scholar] [CrossRef]
- Adiko, A.C.; Babdor, J.; Gutiérrez-Martínez, E.; Guermonprez, P.; Saveanu, L. Intracellular Transport Routes for MHC I and Their Relevance for Antigen Cross-Presentation. Front. Immunol. 2015, 6, 335. [Google Scholar] [CrossRef]
- Montealegre, S.; Abramova, A.; Manceau, V.; de Kanter, A.-F.; van Endert, P. The Role of MHC Class I Recycling and Arf6 in Cross-Presentation by Murine Dendritic Cells. Life Sci. Alliance 2019, 2, e201900464. [Google Scholar] [CrossRef]
- López de Castro, J.A. How ERAP1 and ERAP2 Shape the Peptidomes of Disease-Associated MHC-I Proteins. Front. Immunol. 2018, 9, 2463. [Google Scholar] [CrossRef]
- Blander, J.M. Different Routes of MHC-I Delivery to Phagosomes and Their Consequences to CD8 T Cell Immunity. Semin. Immunol. 2023, 66, 101713. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Stroobant, V.; Heirman, C.; Sun, Z.; Thielemans, K.; Mulder, A.; van der Bruggen, P.; Van den Eynde, B.J. The Vacuolar Pathway of Long Peptide Cross-Presentation Can Be TAP Dependent. J. Immunol. 2019, 202, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Lawand, M.; Evnouchidou, I.; Baranek, T.; Montealegre, S.; Tao, S.; Drexler, I.; Saveanu, L.; Si-Tahar, M.; van Endert, P. Impact of the TAP-like Transporter in Antigen Presentation and Phagosome Maturation. Mol. Immunol. 2019, 113, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Weimershaus, M.; Mauvais, F.-X.; Saveanu, L.; Adiko, C.; Babdor, J.; Abramova, A.; Montealegre, S.; Lawand, M.; Evnouchidou, I.; Huber, K.J.; et al. Innate Immune Signals Induce Anterograde Endosome Transport Promoting MHC Class I Cross-Presentation. Cell Rep. 2018, 24, 3568–3581. [Google Scholar] [CrossRef]
- Kacen, A.; Javitt, A.; Kramer, M.P.; Morgenstern, D.; Tsaban, T.; Shmueli, M.D.; Teo, G.C.; da Veiga Leprevost, F.; Barnea, E.; Yu, F.; et al. Post-Translational Modifications Reshape the Antigenic Landscape of the MHC I Immunopeptidome in Tumors. Nat. Biotechnol. 2023, 41, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Hasler, P.; Demaurex, N. The ER Phagosome Connection in the Era of Membrane Contact Sites. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, D.; Weinstein-Marom, H.; Fishman, S.; Yossef, R.; Zuri, D.; Barnea, E.; Admon, A.; Margalit, A.; Gross, G. Efficient Peptide Recovery from Secreted Recombinant MHC-I Molecules Expressed via mRNA Transfection. Immunol. Lett. 2015, 165, 32–38. [Google Scholar] [CrossRef]
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans Cells—Programmed by the Epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef]
- Spel, L.; Luteijn, R.D.; Drijfhout, J.W.; Nierkens, S.; Boes, M.; Wiertz, E.J.H. Endocytosed Soluble Cowpox Virus Protein CPXV012 Inhibits Antigen Cross-Presentation in Human Monocyte-Derived Dendritic Cells. Immunol. Cell Biol. 2018, 96, 137–148. [Google Scholar] [CrossRef]
- Mauvais, F.-X.; van Endert, P. Cross-Presentation by the Others. Semin. Immunol. 2023, 67, 101764. [Google Scholar] [CrossRef]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic Cell Subsets and Locations. Int. Rev. Cell Mol. Biol. 2019, 348, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Sie, C.; Korn, T. Dendritic Cells in Central Nervous System Autoimmunity. Semin. Immunopathol. 2017, 39, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A.; Murphy, K.M.; Briseño, C.G. Development, Diversity, and Function of Dendritic Cells in Mouse and Human. Cold Spring Harb. Perspect. Biol. 2018, 10, a028613. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Paulete, A.R.; Teijeira, Á.; Quetglas, J.I.; Rodríguez-Ruiz, M.E.; Sánchez-Arráez, Á.; Labiano, S.; Etxeberria, I.; Azpilikueta, A.; Bolaños, E.; Ballesteros-Briones, M.C.; et al. Intratumoral Immunotherapy with XCL1 and sFlt3L Encoded in Recombinant Semliki Forest Virus-Derived Vectors Fosters Dendritic Cell-Mediated T-Cell Cross-Priming. Cancer Res. 2018, 78, 6643–6654. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Güttler, S.; Bachem, A.; Hartung, E.; Mora, A.; Jäkel, A.; Hutloff, A.; Henn, V.; Mages, H.W.; Gurka, S.; et al. Ontogenic, Phenotypic, and Functional Characterization of XCR1(+) Dendritic Cells Leads to a Consistent Classification of Intestinal Dendritic Cells Based on the Expression of XCR1 and SIRPα. Front. Immunol. 2014, 5, 326. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yamazaki, C.; Takumi, A.; Ikeno, T.; Hemmi, H.; Takahashi, N.; Shimizu, K.; Fraser, S.E.; Hoshino, K.; Kaisho, T.; et al. Imaging of the Cross-Presenting Dendritic Cell Subsets in the Skin-Draining Lymph Node. Proc. Natl. Acad. Sci. USA 2016, 113, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic Cells, Monocytes and Macrophages: A Unified Nomenclature Based on Ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Tang-Huau, T.-L.; Gueguen, P.; Goudot, C.; Durand, M.; Bohec, M.; Baulande, S.; Pasquier, B.; Amigorena, S.; Segura, E. Human in Vivo-Generated Monocyte-Derived Dendritic Cells and Macrophages Cross-Present Antigens through a Vacuolar Pathway. Nat. Commun. 2018, 9, 2570. [Google Scholar] [CrossRef]
- Wu, L.; Chen, H.; Fu, C.; Xing, M.; Fang, H.; Yang, F.; Yang, Q.; Zhang, Y.; Li, W.; Chen, Z. Midkine Mediates Dysfunction of Liver Sinusoidal Endothelial Cells through Integrin A4 and A6. Vasc. Pharmacol. 2022, 147, 107113. [Google Scholar] [CrossRef]
- Schurich, A.; Böttcher, J.P.; Burgdorf, S.; Penzler, P.; Hegenbarth, S.; Kern, M.; Dolf, A.; Endl, E.; Schultze, J.; Wiertz, E.; et al. Distinct Kinetics and Dynamics of Cross-Presentation in Liver Sinusoidal Endothelial Cells Compared to Dendritic Cells. Hepatology 2009, 50, 909–919. [Google Scholar] [CrossRef]
- Kern, M.; Popov, A.; Scholz, K.; Schumak, B.; Djandji, D.; Limmer, A.; Eggle, D.; Sacher, T.; Zawatzky, R.; Holtappels, R.; et al. Virally Infected Mouse Liver Endothelial Cells Trigger CD8+ T-Cell Immunity. Gastroenterology 2010, 138, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Hayday, A.C. γδ Cells: A Right Time and a Right Place for a Conserved Third Way of Protection. Annu. Rev. Immunol. 2000, 18, 975–1026. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Koyanagi-Aoi, M.; Taniguchi-Ikeda, M.; Yoshida, Y.; Azuma, T.; Aoi, T. The Generation of Human γδT Cell-Derived Induced Pluripotent Stem Cells from Whole Peripheral Blood Mononuclear Cell Culture. Stem Cells Transl. Med. 2018, 7, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, O.; Thurnher, M. Functional Phenotypes of Human Vγ9Vδ2 T Cells in Lymphoid Stress Surveillance. Cells 2020, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Loizon, S.; Tyler, C.J.; Duluc, D.; Moser, B.; Mechain, M.; Duvignaud, A.; Malvy, D.; Troye-Blomberg, M.; Moreau, J.-F.; et al. The Antigen-Presenting Potential of Vγ9Vδ2 T Cells During Plasmodium Falciparum Blood-Stage Infection. J. Infect. Dis. 2017, 215, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Pinzon-Charry, A.; Woodberry, T.; Kienzle, V.; McPhun, V.; Minigo, G.; Lampah, D.A.; Kenangalem, E.; Engwerda, C.; López, J.A.; Anstey, N.M.; et al. Apoptosis and Dysfunction of Blood Dendritic Cells in Patients with Falciparum and Vivax Malaria. J. Exp. Med. 2013, 210, 1635–1646. [Google Scholar] [CrossRef]
- Campana, S.; De Pasquale, C.; Carrega, P.; Ferlazzo, G.; Bonaccorsi, I. Cross-Dressing: An Alternative Mechanism for Antigen Presentation. Immunol. Lett. 2015, 168, 349–354. [Google Scholar] [CrossRef]
- Joly, E.; Hudrisier, D. What Is Trogocytosis and What Is Its Purpose? Nat. Immunol. 2003, 4, 815. [Google Scholar] [CrossRef]
- Huang, J.F.; Yang, Y.; Sepulveda, H.; Shi, W.; Hwang, I.; Peterson, P.A.; Jackson, M.R.; Sprent, J.; Cai, Z. TCR-Mediated Internalization of Peptide-MHC Complexes Acquired by T Cells. Science 1999, 286, 952–954. [Google Scholar] [CrossRef]
- Hwang, I.; Huang, J.F.; Kishimoto, H.; Brunmark, A.; Peterson, P.A.; Jackson, M.R.; Surh, C.D.; Cai, Z.; Sprent, J. T Cells Can Use Either T Cell Receptor or CD28 Receptors to Absorb and Internalize Cell Surface Molecules Derived from Antigen-Presenting Cells. J. Exp. Med. 2000, 191, 1137–1148. [Google Scholar] [CrossRef]
- Harshyne, L.A.; Watkins, S.C.; Gambotto, A.; Barratt-Boyes, S.M. Dendritic Cells Acquire Antigens from Live Cells for Cross-Presentation to CTL. J. Immunol. 2001, 166, 3717–3723. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, Biogenesis and Function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Février, B.; Raposo, G. Exosomes: Endosomal-Derived Vesicles Shipping Extracellular Messages. Curr. Opin. Cell Biol. 2004, 16, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sun, H.-T.; Wang, S.; Huang, S.-L.; Zheng, Y.; Wang, C.-Q.; Hu, B.-Y.; Qin, W.; Zou, T.-T.; Fu, Y.; et al. Isolation and Characterization of Exosomes for Cancer Research. J. Hematol. Oncol. 2020, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.-H.; Zheng, J.-Q.; Ding, J.-Y.; Wu, Y.-F.; Liu, L.; Yu, Z.-L.; Chen, G. Exosome-Mediated Immunosuppression in Tumor Microenvironments. Cells 2022, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Potolicchio, I.; Carven, G.J.; Xu, X.; Stipp, C.; Riese, R.J.; Stern, L.J.; Santambrogio, L. Proteomic Analysis of Microglia-Derived Exosomes: Metabolic Role of the Aminopeptidase CD13 in Neuropeptide Catabolism. J. Immunol. 2005, 175, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane Vesicles as Conveyors of Immune Responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Wakim, L.M.; Bevan, M.J. Cross-Dressed Dendritic Cells Drive Memory CD8+ T-Cell Activation after Viral Infection. Nature 2011, 471, 629–632. [Google Scholar] [CrossRef]
- Watkins, S.C.; Salter, R.D. Functional Connectivity between Immune Cells Mediated by Tunneling Nanotubules. Immunity 2005, 23, 309–318. [Google Scholar] [CrossRef]
- Schiller, C.; Huber, J.E.; Diakopoulos, K.N.; Weiss, E.H. Tunneling Nanotubes Enable Intercellular Transfer of MHC Class I Molecules. Hum. Immunol. 2013, 74, 412–416. [Google Scholar] [CrossRef]
- Millet, V.; Naquet, P.; Guinamard, R.R. Intercellular MHC Transfer between Thymic Epithelial and Dendritic Cells. Eur. J. Immunol. 2008, 38, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Kroger, C.J.; Spidale, N.A.; Wang, B.; Tisch, R. Thymic Dendritic Cell Subsets Display Distinct Efficiencies and Mechanisms of Intercellular MHC Transfer. J. Immunol. 2017, 198, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Dolan, B.P.; Gibbs, K.D.; Ostrand-Rosenberg, S. Tumor-Specific CD4+ T Cells Are Activated by “Cross-Dressed” Dendritic Cells Presenting Peptide-MHC Class II Complexes Acquired from Cell-Based Cancer Vaccines. J. Immunol. 2006, 176, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.A.; Afzali, B.; Tsang, J.; Lombardi, G.; Lechler, R.I. Intercellular Transfer of MHC and Immunological Molecules: Molecular Mechanisms and Biological Significance. Am. J. Transpl. Transplant. 2007, 7, 1442–1449. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wei, S.; Sun, Y.; Xu, G.; Zhang, S.; Li, J. Molecular Characteristics, Functional Definitions, and Regulatory Mechanisms for Cross-Presentation Mediated by the Major Histocompatibility Complex: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 196. https://doi.org/10.3390/ijms25010196
Liu S, Wei S, Sun Y, Xu G, Zhang S, Li J. Molecular Characteristics, Functional Definitions, and Regulatory Mechanisms for Cross-Presentation Mediated by the Major Histocompatibility Complex: A Comprehensive Review. International Journal of Molecular Sciences. 2024; 25(1):196. https://doi.org/10.3390/ijms25010196
Chicago/Turabian StyleLiu, Sen, Shaoqiang Wei, Yan Sun, Guowei Xu, Shidong Zhang, and Jianxi Li. 2024. "Molecular Characteristics, Functional Definitions, and Regulatory Mechanisms for Cross-Presentation Mediated by the Major Histocompatibility Complex: A Comprehensive Review" International Journal of Molecular Sciences 25, no. 1: 196. https://doi.org/10.3390/ijms25010196
APA StyleLiu, S., Wei, S., Sun, Y., Xu, G., Zhang, S., & Li, J. (2024). Molecular Characteristics, Functional Definitions, and Regulatory Mechanisms for Cross-Presentation Mediated by the Major Histocompatibility Complex: A Comprehensive Review. International Journal of Molecular Sciences, 25(1), 196. https://doi.org/10.3390/ijms25010196





