Identification of 2,4-Di-tert-butylphenol as a Novel Agonist for Insect Odorant Receptors
Abstract
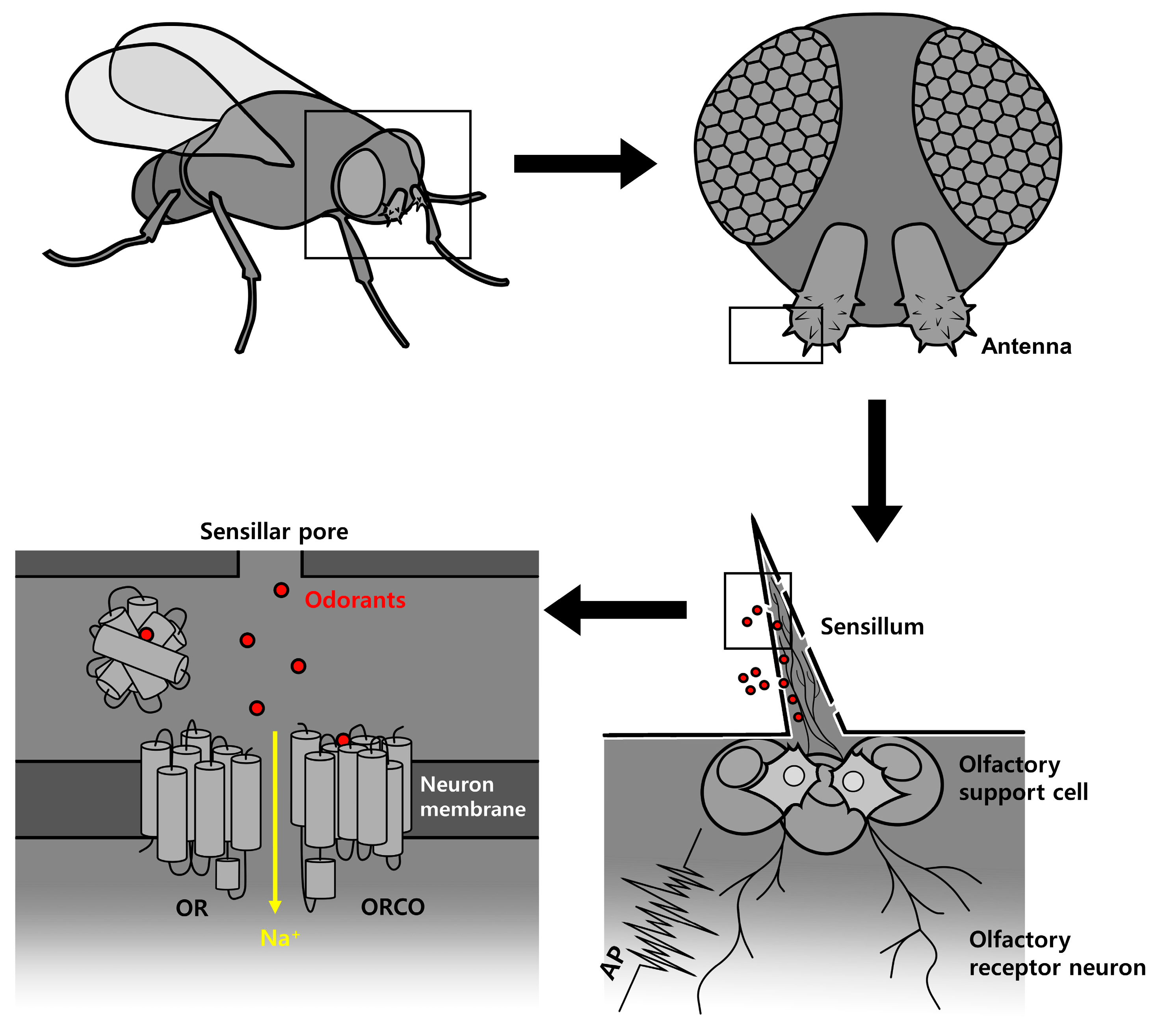
:1. Introduction
2. Results
2.1. Results of Applying the Odorants and DTBP to Various ORs
2.2. Confirmation of the DTBP Activation Mechanism on Heteromeric and Homomeric Receptors
2.3. Confirmation of Interaction between DTBP and Orco Homomeric Receptor
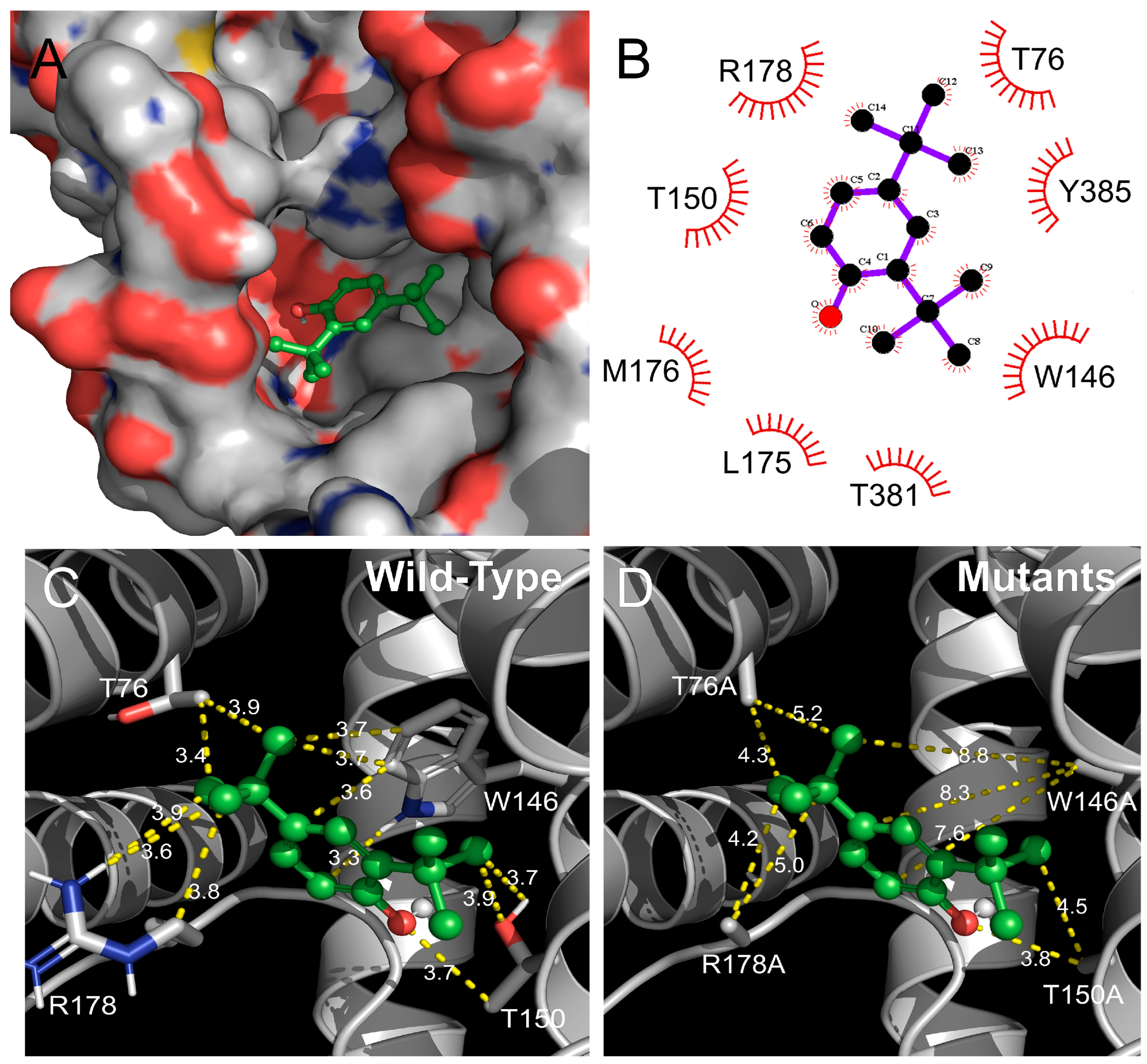
2.4. Identification of the Binding Site in the Orco Homomeric Receptor of DTBP through Point Mutations
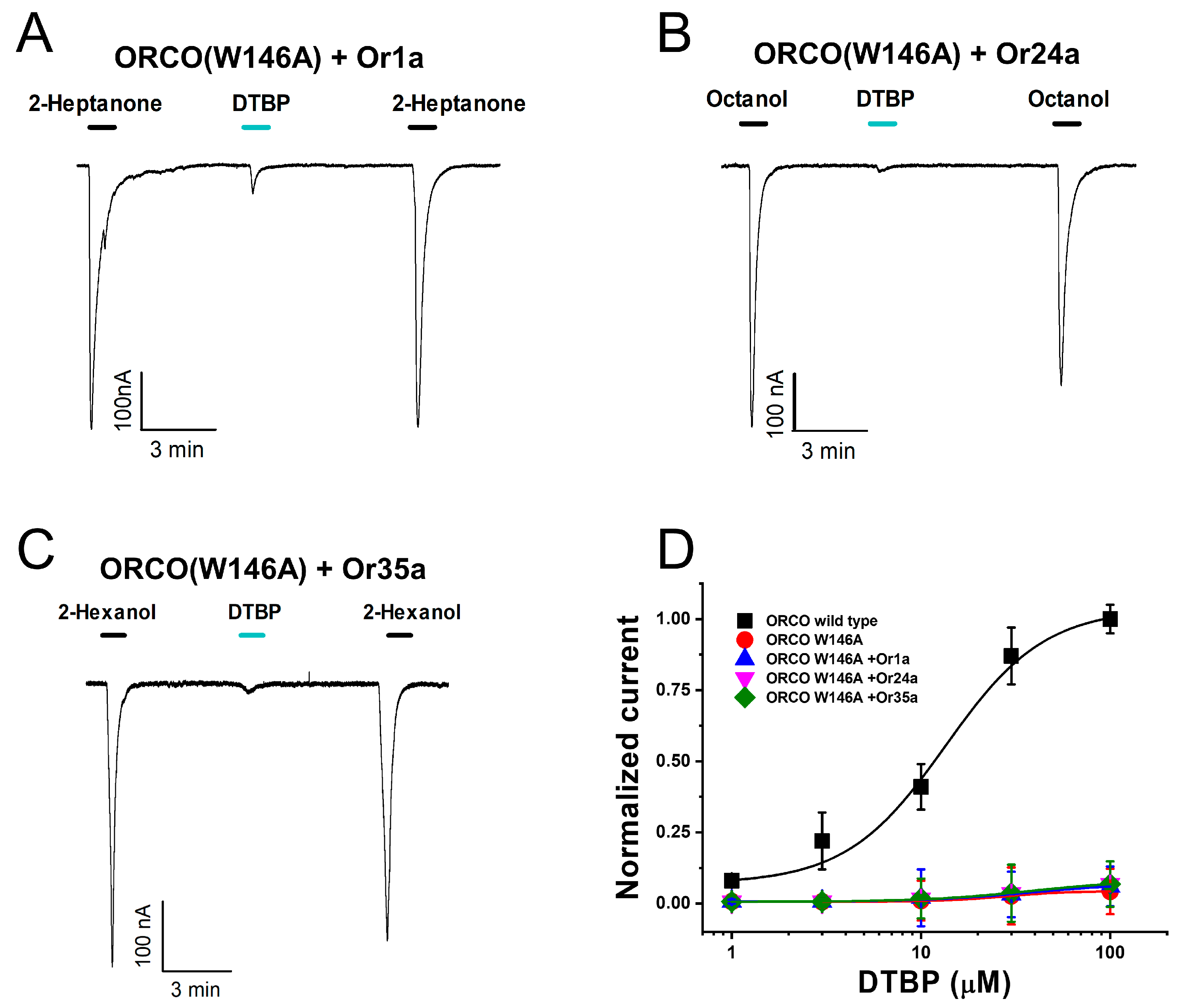
2.5. Confirmation of Changes in DTBP Activity in Mutant-Type Receptor
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. In Vitro Transcription
4.3. Xenopus Oocyte Preparation and mRNA Injection
4.4. Electrophysiological Recording Using a Two-Electrode Voltage Clamp
4.5. Site-Directed Mutagenesis to Verify Interaction Sites
4.6. 3D Protein Structure Composition and Molecular Interaction Confirmation
4.7. Data Statistics and Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ha, T.S.; Smith, D.P. Recent Insights into Insect Olfactory Receptors and Odorant-Binding Proteins. Insects 2022, 13, 926. [Google Scholar] [CrossRef] [PubMed]
- Butterwick, J.A.; Del Mármol, J.; Kim, K.H.; Kahlson, M.A.; Rogow, J.A.; Walz, T.; Ruta, V. Cryo-EM structure of the insect olfactory receptor Orco. Nature 2018, 560, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Karner, T.; Kellner, I.; Schultze, A.; Breer, H.; Krieger, J. Co-expression of six tightly clustered odorant receptor genes in the antenna of the malaria mosquito Anopheles gambiae. Front. Ecol. Evol. 2015, 3, 26. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, T.; Guo, H.-W.; Liang, X.-F.; Cheng, Y.-Q. Ultrastructure of the olfactory sensilla across the antennae and maxillary palps of Bactrocera dorsalis (Diptera: Tephritidae). Insects 2021, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Komarov, N.; Sprecher, S.G. The chemosensory system of the Drosophila larva: An overview of current understanding. Fly 2022, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell. Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Benton, R. Molecular mechanisms of olfactory detection in insects: Beyond receptors. Open Biol. 2020, 10, 200252. [Google Scholar] [CrossRef]
- Zhang, R.B.; Liu, Y.; Yan, S.C.; Wang, G.R. Identification and functional characterization of an odorant receptor in pea aphid, Acyrthosiphon pisum. Insect Sci. 2019, 26, 58–67. [Google Scholar] [CrossRef]
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218. [Google Scholar] [CrossRef]
- Ruta, V. Sensing Scents: Structural Insights into Insect Olfactory Receptors. Biophys. J. 2020, 118, 171a. [Google Scholar] [CrossRef]
- Morinaga, S.; Nagata, K.; Ihara, S.; Yumita, T.; Niimura, Y.; Sato, K.; Touhara, K. Structural model for ligand binding and channel opening of an insect gustatory receptor. J. Biol. Chem. 2022, 298, 102573. [Google Scholar] [CrossRef] [PubMed]
- Iatrou, K.; Schulz, S.; Kythreoti, G.; Sdralia, N.; Tsitoura, P.; Michaelakis, A.; Papachristos, D.P. Identification of Modifiers of Odor-Triggered Mosquito Behaviors Acting through Binding to the ORco Subunit of Odorant Receptor Heteromers. FASEB J. 2019, 33, 471–478. [Google Scholar] [CrossRef]
- Montague, S.A.; Mathew, D.; Carlson, J.R. Similar odorants elicit different behavioral and physiological responses, some supersustained. J. Neurosci. 2011, 31, 7891–7899. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-B.; Mo, B.-T.; Li, G.-C.; Huang, L.-Q.; Guo, H.; Gong, X.-L.; Wang, C.-Z. Mutagenesis of the odorant receptor co-receptor (Orco) reveals severe olfactory defects in the crop pest moth Helicoverpa armigera. BMC Biol. 2022, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Jing, W.; Liu, M.; Liu, J. The global trends and regional differences in incidence of dengue infection from 1990 to 2019: An analysis from the global burden of disease study 2019. Infect. Dis. Ther. 2021, 10, 1625–1643. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.; Vezilier, J.; Weill, M.; Read, A.F.; Gandon, S. Insecticide control of vector-borne diseases: When is insecticide resistance a problem? PLoS Pathog. 2010, 6, e1001000. [Google Scholar] [CrossRef]
- Sparks, T.C.; Storer, N.; Porter, A.; Slater, R.; Nauen, R. Insecticide resistance management and industry: The origins and evolution of the I nsecticide R esistance A ction C ommittee (IRAC) and the mode of action classification scheme. Pest Manag. Sci. 2021, 77, 2609–2619. [Google Scholar] [CrossRef]
- Dusfour, I.; Vontas, J.; David, J.-P.; Weetman, D.; Fonseca, D.M.; Corbel, V.; Raghavendra, K.; Coulibaly, M.B.; Martins, A.J.; Kasai, S. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl. Trop. Dis. 2019, 13, e0007615. [Google Scholar] [CrossRef]
- Sparks, T.C.; Bryant, R.J. Innovation in insecticide discovery: Approaches to the discovery of new classes of insecticides. Pest Manag. Sci. 2022, 78, 3226–3247. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Ren, J.; Hou, Y.; Han, Z.; Xiao, J.; Li, Y. Exposure level of neonicotinoid insecticides in the food chain and the evaluation of their human health impact and environmental risk: An overview. Sustainability 2020, 12, 7523. [Google Scholar] [CrossRef]
- David, M.D. The potential of pro-insecticides for resistance management. Pest Manag. Sci. 2021, 77, 3631–3636. [Google Scholar] [CrossRef] [PubMed]
- Dharni, S.; Sanchita; Maurya, A.; Samad, A.; Srivastava, S.K.; Sharma, A.; Patra, D.D. Purification, characterization, and in vitro activity of 2,4-di-tert-butylphenol from Pseudomonas monteilii PsF84: Conformational and molecular docking studies. J. Agric. Food Chem. 2014, 62, 6138–6146. [Google Scholar] [CrossRef] [PubMed]
- Belghit, S.; Driche, E.H.; Bijani, C.; Zitouni, A.; Sabaou, N.; Badji, B.; Mathieu, F. Activity of 2,4-Di-tert-butylphenol produced by a strain of Streptomyces mutabilis isolated from a Saharan soil against Candida albicans and other pathogenic fungi. J. Mycol. Medicale 2016, 26, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural sources and bioactivities of 2,4-di-tert-butylphenol and its analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Kushveer, J.S.; Khan, M.I.K.; Pagal, S.; Meena, C.K.; Murali, A.; Dhayalan, A.; Venkateswara Sarma, V. 2,4-Di-tert-butylphenol isolated from an endophytic fungus, Daldinia eschscholtzii, reduces virulence and quorum sensing in Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 1668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, P.-P.; Guo, S.-S.; Cao, J.-Q.; Pang, X.; Geng, Z.-F.; Sang, Y.-L.; Du, S.-S. Supercritical carbon dioxide extract of Cinnamomum cassia bark: Toxicity and repellency against two stored-product beetle species. Environ. Sci. Pollut. Res. 2018, 25, 22236–22243. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Yin, J.; Zhong, T.; Cao, Y.; Li, K. Function and immunocytochemical localization of two novel odorant-binding proteins in olfactory sensilla of the scarab beetle Holotrichia oblita Faldermann (Coleoptera: Scarabaeidae). Chem. Senses 2012, 37, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, K.D.; Khan, Z.; Wondwosen, B.; Alsanius, B.; Hill, S.R.; Ignell, R.; Lorenzo, M.G. Odor-mediated response of gravid Aedes aegypti to mosquito-associated symbiotic bacteria. Acta Trop. 2023, 237, 106730. [Google Scholar] [CrossRef]
- Chen, S.; Luetje, C.W. Identification of new agonists and antagonists of the insect odorant receptor co-receptor subunit. PLoS ONE 2012, 7, e36784. [Google Scholar] [CrossRef]
- Kepchia, D.; Moliver, S.; Chohan, K.; Phillips, C.; Luetje, C.W. Inhibition of insect olfactory behavior by an airborne antagonist of the insect odorant receptor co-receptor subunit. PLoS ONE 2017, 12, e0177454. [Google Scholar]
- Hoare, D.J.; Humble, J.; Jin, D.; Gilding, N.; Petersen, R.; Cobb, M.; McCrohan, C. Modeling Peripheral Olfactory Coding in Drosophila Larvae. PLoS ONE 2011, 6, e22996. [Google Scholar] [CrossRef] [PubMed]
- Soffan, A.; Subandiyah, S.; Makino, H.; Watanabe, T.; Horiike, T. Evolutionary analysis of the highly conserved insect odorant coreceptor (Orco) revealed a positive selection mode, implying functional flexibility. J. Insect Sci. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Zufall, F.; Domingos, A.I. The structure of Orco and its impact on our understanding of olfaction. J. Gen. Physiol. 2018, 150, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazy, H.; Erez, E.; Martz, E.; Pupko, T.; Ben-Tal, N. ConSurf 2010: Calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010, 38, W529–W533. [Google Scholar] [CrossRef] [PubMed]
- Celniker, G.; Nimrod, G.; Ashkenazy, H.; Glaser, F.; Martz, E.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf: Using evolutionary data to raise testable hypotheses about protein function. Isr. J. Chem. 2013, 53, 199–206. [Google Scholar] [CrossRef]
- Ashkenazy, H.; Abadi, S.; Martz, E.; Chay, O.; Mayrose, I.; Pupko, T.; Ben-Tal, N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016, 44, W344–W350. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.A.; Dweck, H.K.; Weiss, B.L.; Carlson, J.R. A volatile sex attractant of tsetse flies. Science 2023, 379, eade1877. [Google Scholar] [CrossRef] [PubMed]
- Renou, M.; Anton, S. Insect olfactory communication in a complex and changing world. Curr. Opin. Insect Sci. 2020, 42, 1–7. [Google Scholar] [CrossRef]
- Zeng, F.; Xu, P.; Leal, W.S. Odorant receptors from Culex quinquefasciatus and Aedes aegypti sensitive to floral compounds. Insect Biochem. Mol. Biol. 2019, 113, 103213. [Google Scholar] [CrossRef]
- Hill, S.R.; Ghaninia, M.; Ignell, R. Blood meal induced regulation of gene expression in the maxillary palps, a chemosensory organ of the mosquito Aedes aegypti. Front. Ecol. Evol. 2019, 7, 336. [Google Scholar] [CrossRef]
- Gondim, K.C.; Atella, G.C.; Pontes, E.G.; Majerowicz, D. Lipid metabolism in insect disease vectors. Insect Biochem. Mol. Biol. 2018, 101, 108–123. [Google Scholar] [CrossRef] [PubMed]
- DeGennaro, M.; McBride, C.S.; Seeholzer, L.; Nakagawa, T.; Dennis, E.J.; Goldman, C.; Jasinskiene, N.; James, A.A.; Vosshall, L.B. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 2013, 498, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect odorscapes: From plant volatiles to natural olfactory scenes. Front. Physiol. 2019, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kunwar, K.; Smith, D. Multiple channels of DEET repellency in Drosophila. Pest Manag. Sci. 2020, 76, 880–887. [Google Scholar] [CrossRef]
- Eom, S.; Jung, W.; Lee, J.; Yeom, H.D.; Lee, S.; Kim, C.; Park, H.-D.; Lee, J.H. Differential regulation of human serotonin receptor type 3A by chanoclavine and ergonovine. Molecules 2021, 26, 1211. [Google Scholar] [CrossRef]
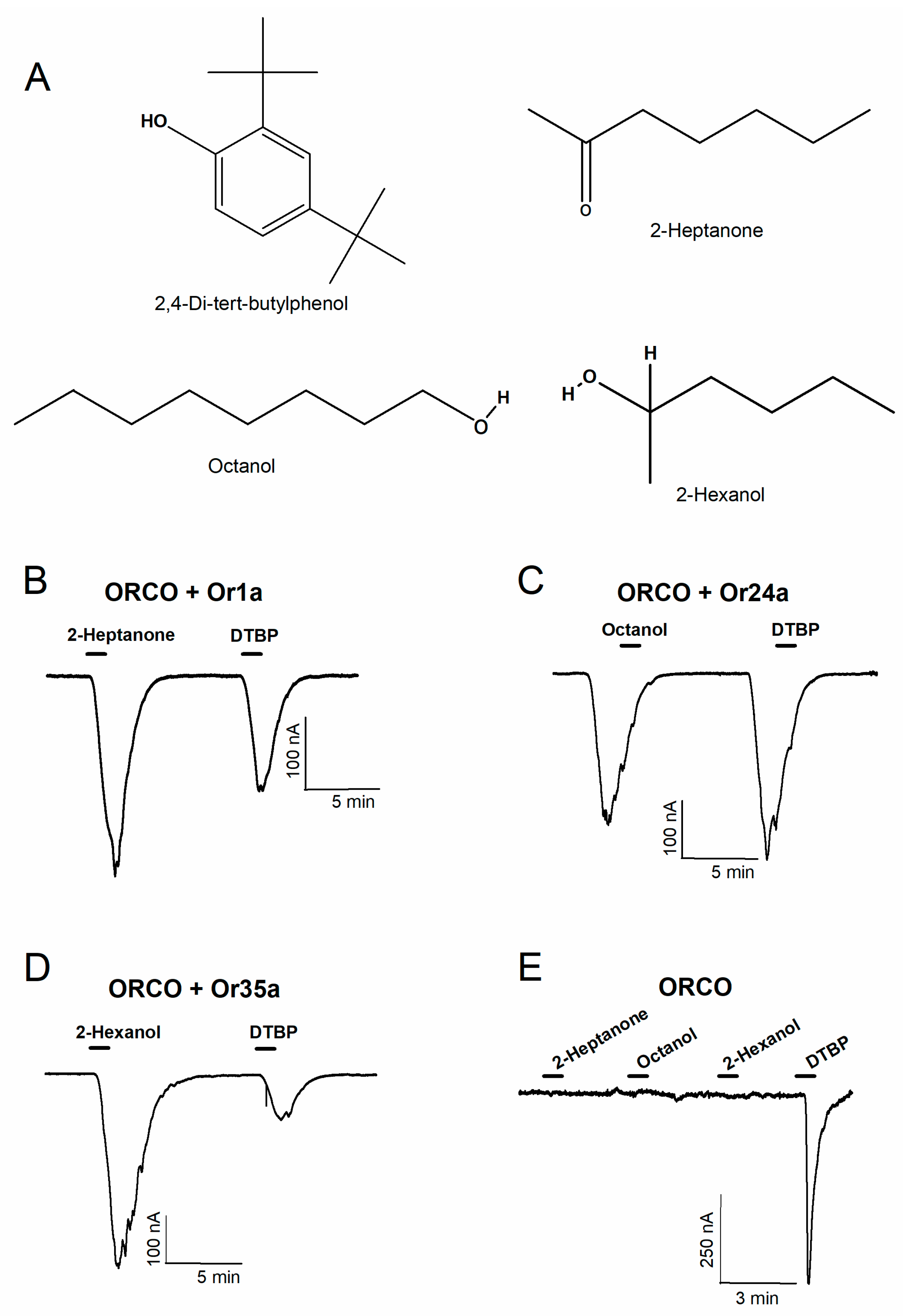
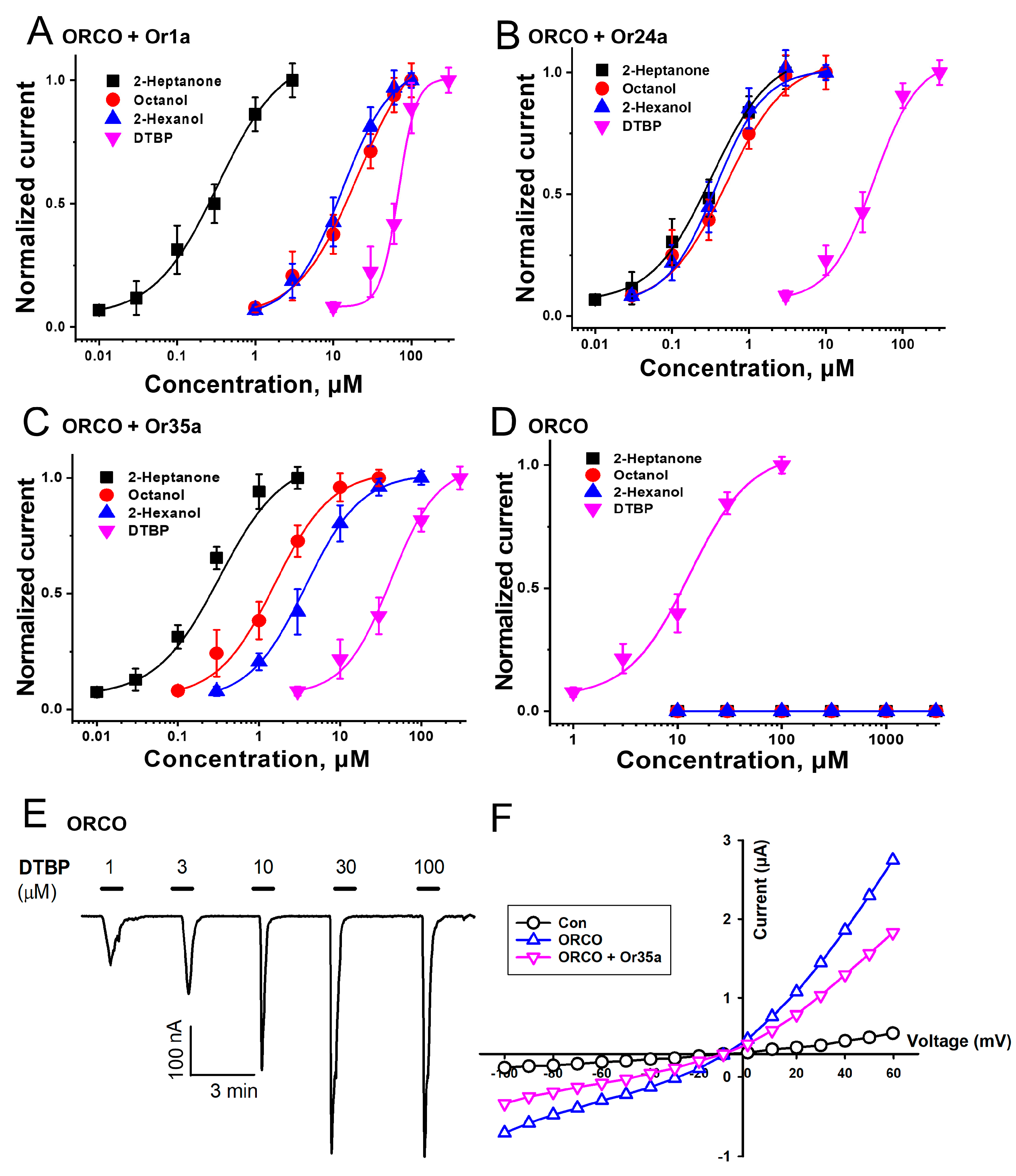
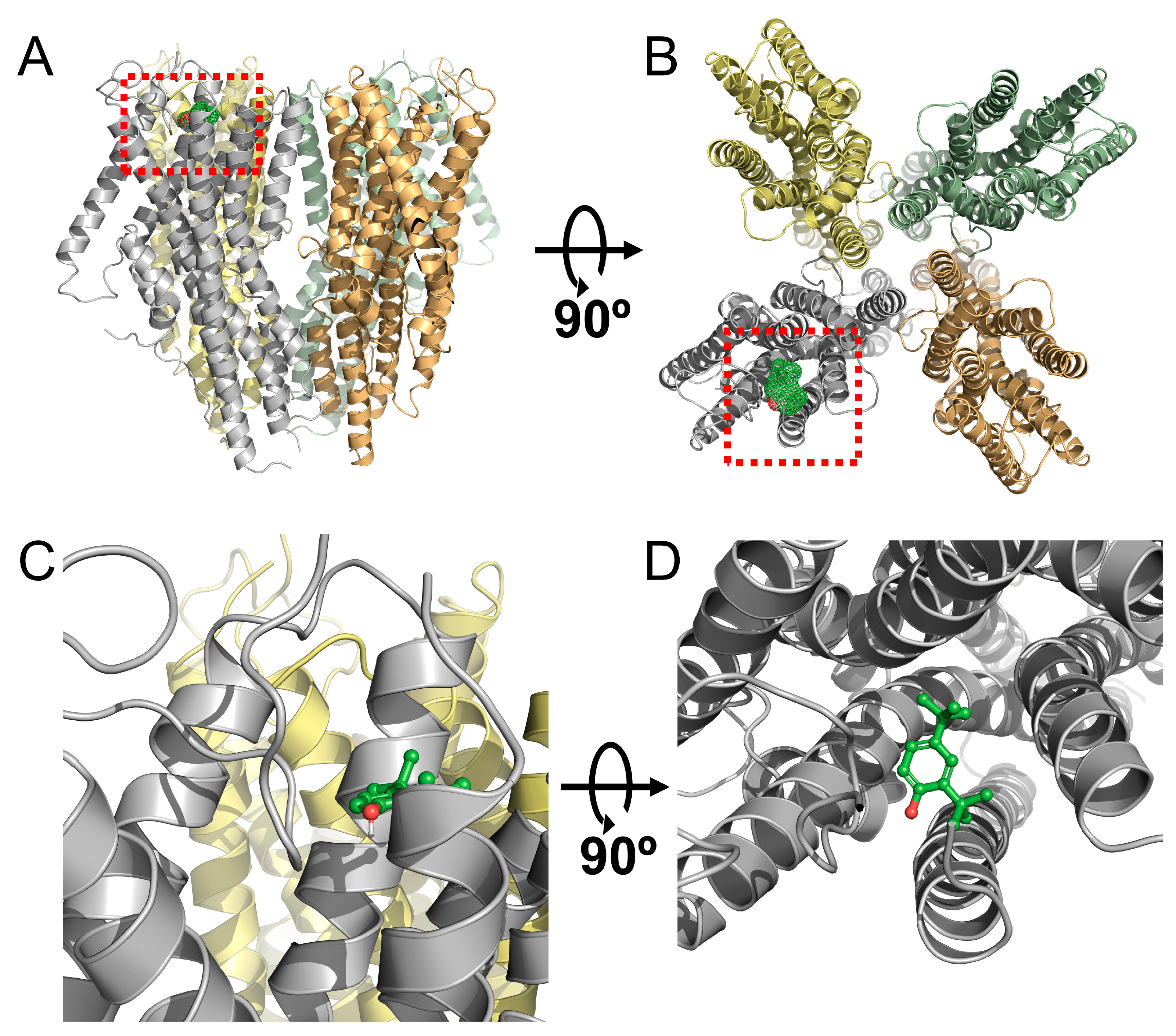
| Type | Emax | EC50 | nH |
|---|---|---|---|
| ORCO + Or1a | 1.1 ± 0.1 | 104.4 ± 27.7 | 1.1 ± 0.2 |
| ORCO + Or24a | 1.0 ± 0.1 | 51.0 ± 8.2 | 1.2 ± 0.3 |
| ORCO + Or35a | 1.0 ± 0.0 | 38.1 ± 3.8 | 1.3 ± 0.2 |
| ORCO | 1.0 ± 0.0 | 13.4 ± 3.0 | 1.7 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Eom, S.; Pyeon, M.; Moon, M.; Yun, J.; Lee, J.; Choi, Y.-S.; Lee, J.H. Identification of 2,4-Di-tert-butylphenol as a Novel Agonist for Insect Odorant Receptors. Int. J. Mol. Sci. 2024, 25, 220. https://doi.org/10.3390/ijms25010220
Lee S, Eom S, Pyeon M, Moon M, Yun J, Lee J, Choi Y-S, Lee JH. Identification of 2,4-Di-tert-butylphenol as a Novel Agonist for Insect Odorant Receptors. International Journal of Molecular Sciences. 2024; 25(1):220. https://doi.org/10.3390/ijms25010220
Chicago/Turabian StyleLee, Shinhui, Sanung Eom, Minsu Pyeon, Myungmi Moon, Jihwon Yun, Jaehyeong Lee, Yong-Seok Choi, and Junho H. Lee. 2024. "Identification of 2,4-Di-tert-butylphenol as a Novel Agonist for Insect Odorant Receptors" International Journal of Molecular Sciences 25, no. 1: 220. https://doi.org/10.3390/ijms25010220
APA StyleLee, S., Eom, S., Pyeon, M., Moon, M., Yun, J., Lee, J., Choi, Y.-S., & Lee, J. H. (2024). Identification of 2,4-Di-tert-butylphenol as a Novel Agonist for Insect Odorant Receptors. International Journal of Molecular Sciences, 25(1), 220. https://doi.org/10.3390/ijms25010220





