Downregulation of Salt-Inducible Kinase 3 Enhances CCL24 Activation in the Placental Environment with Preeclampsia
Abstract
:1. Introduction
2. Results
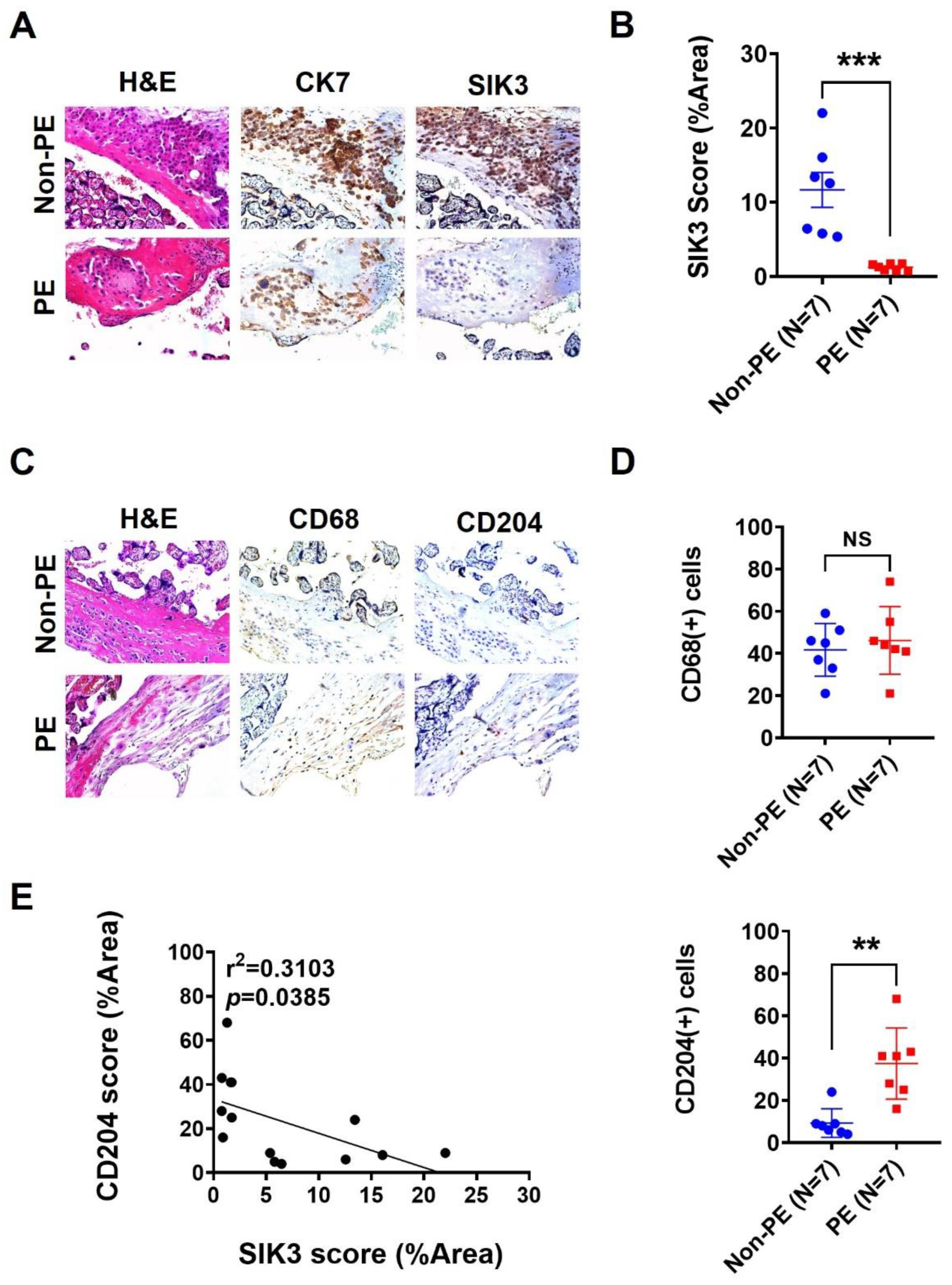
2.1. SIK3 Downregulation in Trophoblasts Is Associated with an Increase in CD204(+) Cells in the Decidua
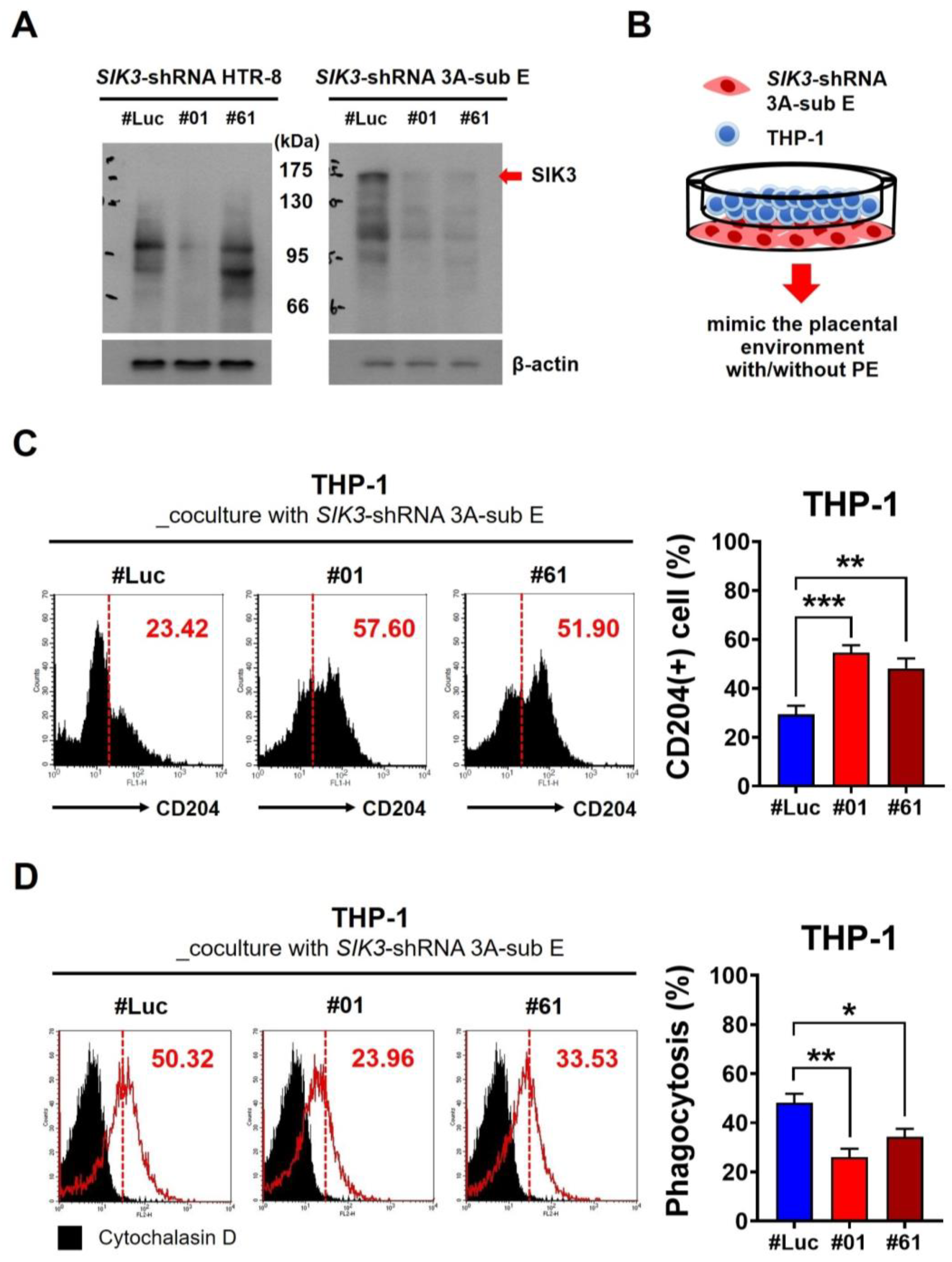
2.2. Low SIK3 Expression at the Maternal–Fetal Interface of the Placenta Contributed to M2 Skewing and Reduced Phagocytosis in the In Vitro Model
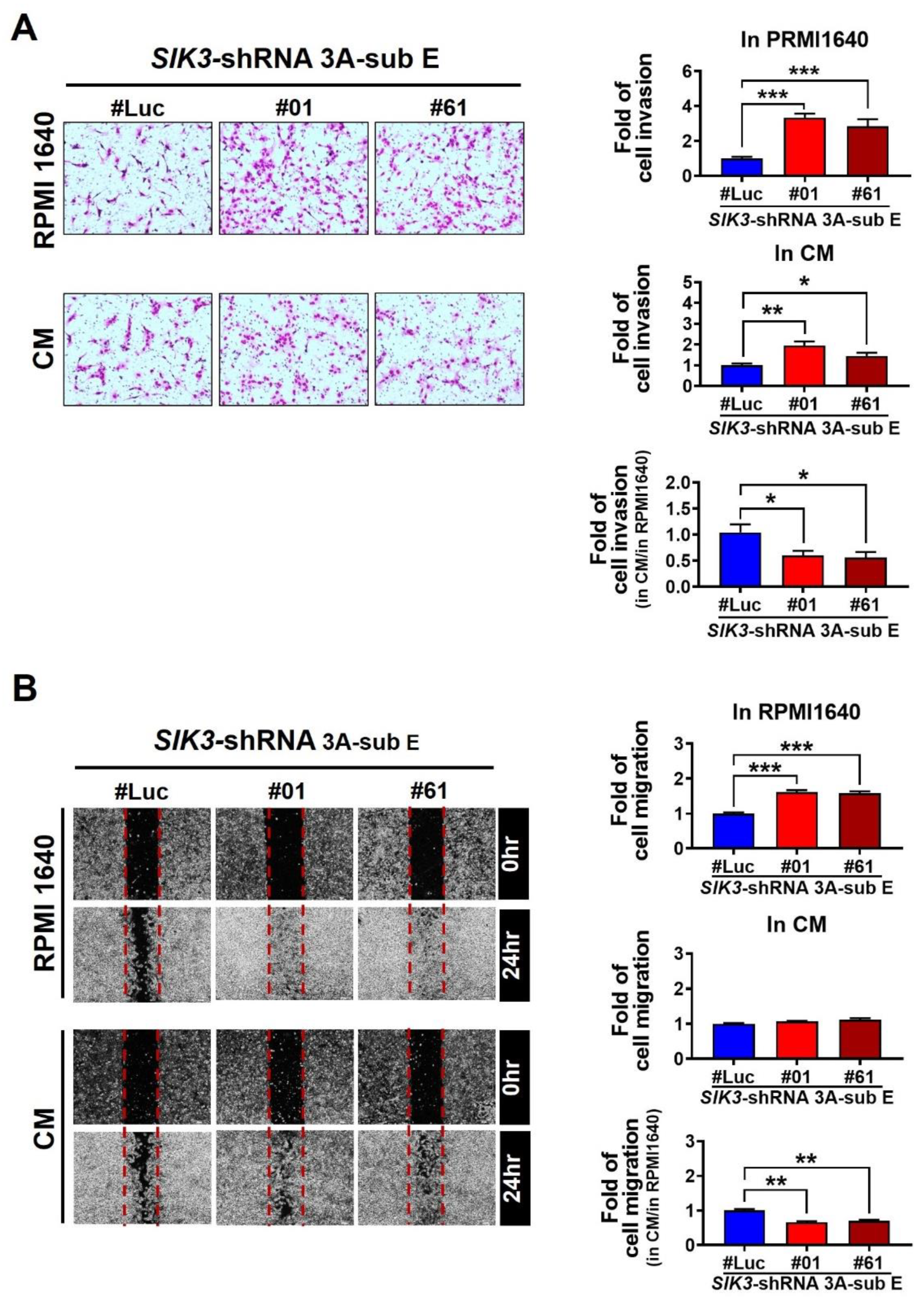
2.3. Low SIK3 Expression at the Maternal–Fetal Interface of the Placenta Reduced the Invasion and Migration Ability of Trophoblast Cells
2.4. Trophoblast-Derived CCL24 Positively Affected Macrophage Recruitment at the Maternal–Fetal Interface of the Placenta and Enhanced M2 Skewing
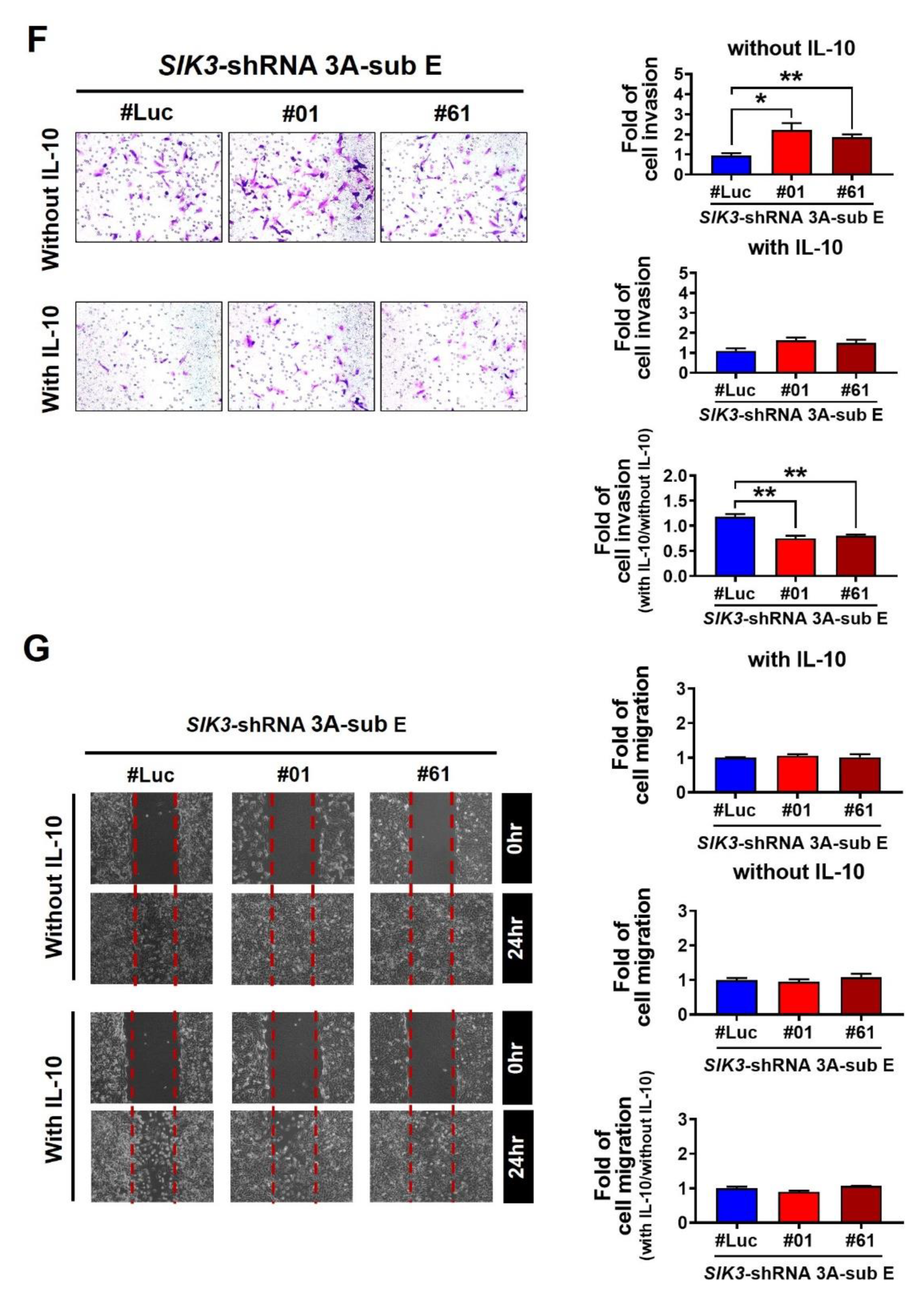
2.5. CCL24/CCR3 Axis Played a Pivotal Role in Polarizing Maternal Macrophages to Produce IL-10, Which Effectively Inhibited Trophoblast Cell Invasion/Migration
3. Discussion
4. Materials and Methods
4.1. Clinical Sample Collection
4.2. Immunochemistry Staining
4.3. Cell Culture and Transfection
4.4. Western Blot
4.5. Phagocytosis Effect Detection
4.6. Flow Cytometry
4.7. Invasion/Migration Assay
4.8. Cytokine Array
4.9. ELISA
4.10. Real-Time PCR
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of preeclampsia. Annu. Rev. Pathol. 2010, 5, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Silasi, M.; Cohen, B.; Karumanchi, S.A.; Rana, S. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet. Gynecol. Clin. N. Am. 2010, 37, 239–253. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Women’s and Children’s Health. NICE Clinical Guidelines, No. 107. Hypertension in Pregnancy: The Management of Hypertensive Disorders during Pregnancy; RCOG Press: London, UK, 2010. [Google Scholar]
- WHO Guidelines Approved by the Guidelines Review Committee. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Collier, A.Y.; Smith, L.A.; Karumanchi, S.A. Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy. Hum. Immunol. 2021, 82, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Darling, N.J.; Toth, R.; Arthur, J.S.; Clark, K. Inhibition of SIK2 and SIK3 during differentiation enhances the anti-inflammatory phenotype of macrophages. Biochem. J. 2017, 474, 521–537. [Google Scholar] [CrossRef]
- Liang, Y.L.; Wu, C.H.; Kang, C.Y.; Lin, C.N.; Shih, N.Y.; Lin, S.H.; Chen, Y.C.; Hsu, K.F. Downregulated Salt-inducible Kinase 3 Expression Promotes Chemoresistance in Serous Ovarian Cancer via the ATP-binding Cassette Protein ABCG2. J. Cancer 2019, 10, 6025–6036. [Google Scholar] [CrossRef]
- Benyo, D.F.; Smarason, A.; Redman, C.W.; Sims, C.; Conrad, K.P. Expression of inflammatory cytokines in placentas from women with preeclampsia. J. Clin. Endocrinol. Metab. 2001, 86, 2505–2512. [Google Scholar] [CrossRef]
- Williams, P.J.; Bulmer, J.N.; Searle, R.F.; Innes, B.A.; Robson, S.C. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: A comparison with late normal pregnancy. Reproduction 2009, 138, 177–184. [Google Scholar] [CrossRef]
- Sanguansermsri, D.; Pongcharoen, S. Pregnancy immunology: Decidual immune cells. Asian Pac. J. Allergy Immunol. 2008, 26, 171–181. [Google Scholar]
- Solano, M.E. Decidual immune cells: Guardians of human pregnancies. Best. Pract. Res. Clin. Obstet. Gynaecol. 2019, 60, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lopez, G.E.; Vazquez, J.; Sun, Y.; Chavarria, M.; Lindner, P.N.; Fredrickson, S.; Karst, N.; Stanic, A.K. Decidual-Placental Immune Landscape during Syngeneic Murine Pregnancy. Front. Immunol. 2018, 9, 2087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dunk, C.; Croy, A.B.; Lye, S.J. To serve and to protect: The role of decidual innate immune cells on human pregnancy. Cell Tissue Res. 2016, 363, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.; Yan, X.; Zhang, H.; Zhou, C.; Zhang, C. Dysfunction of Decidual Macrophages Is a Potential Risk Factor in the Occurrence of Preeclampsia. Front. Immunol. 2021, 12, 655655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, H.; Chen, M.; Lu, Y.; Zheng, L.; Ma, L. CCL24/CCR3 axis plays a central role in angiotensin II-induced heart failure by stimulating M2 macrophage polarization and fibroblast activation. Cell Biol. Toxicol. 2023, 39, 1413–1431. [Google Scholar] [CrossRef] [PubMed]
- Lash, G.E.; Pitman, H.; Morgan, H.L.; Innes, B.A.; Agwu, C.N.; Bulmer, J.N. Decidual macrophages: Key regulators of vascular remodeling in human pregnancy. J. Leukoc. Biol. 2016, 100, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; LaMarca, B. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef]
- Schonkeren, D.; van der Hoorn, M.L.; Khedoe, P.; Swings, G.; van Beelen, E.; Claas, F.; van Kooten, C.; de Heer, E.; Scherjon, S. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am. J. Pathol. 2011, 178, 709–717. [Google Scholar] [CrossRef]
- Faas, M.M.; Spaans, F.; De Vos, P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front. Immunol. 2014, 5, 298. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, Y.; Zhang, J.; Ruan, C.C.; Gao, P.J. Immune imbalance is associated with the development of preeclampsia. Medicine 2019, 98, e15080. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Y.H.; Li, M.Q.; Meng, Y.H.; Chen, X.; Shao, J.; Tang, C.L.; Du, M.R.; Jin, L.P.; Li, D.J. Trophoblasts-derived chemokine CCL24 promotes the proliferation, growth and apoptosis of decidual stromal cells in human early pregnancy. Int. J. Clin. Exp. Pathol. 2013, 6, 1028–1037. [Google Scholar] [PubMed]
- Chau, S.E.; Murthi, P.; Wong, M.H.; Whitley, G.S.; Brennecke, S.P.; Keogh, R.J. Control of extravillous trophoblast function by the eotaxins CCL11, CCL24 and CCL26. Hum. Reprod. 2013, 28, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, Y.H.; Shang, W.Q.; Liu, L.B.; Chen, X.; Yuan, M.M.; Jin, L.P.; Li, M.Q.; Li, D.J. Chemokine CCL24 promotes the growth and invasiveness of trophoblasts through ERK1/2 and PI3K signaling pathways in human early pregnancy. Reproduction 2015, 150, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Manousou, P.; Kolios, G.; Valatas, V.; Drygiannakis, I.; Bourikas, L.; Pyrovolaki, K.; Koutroubakis, I.; Papadaki, H.A.; Kouroumalis, E. Increased expression of chemokine receptor CCR3 and its ligands in ulcerative colitis: The role of colonic epithelial cells in in vitro studies. Clin. Exp. Immunol. 2010, 162, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Nath, M.C.; Cubro, H.; McCormick, D.J.; Milic, N.M.; Garovic, V.D. Preeclamptic Women Have Decreased Circulating IL-10 (Interleukin-10) Values at the Time of Preeclampsia Diagnosis: Systematic Review and Meta-Analysis. Hypertension 2020, 76, 1817–1827. [Google Scholar] [CrossRef]
- Cubro, H.; Kashyap, S.; Nath, M.C.; Ackerman, A.W.; Garovic, V.D. The Role of Interleukin-10 in the Pathophysiology of Preeclampsia. Curr. Hypertens. Rep. 2018, 20, 36. [Google Scholar] [CrossRef]
- Hennessy, A.; Pilmore, H.L.; Simmons, L.A.; Painter, D.M. A deficiency of the placental IL-10 in preeclampsia. J. Immunol. 1999, 163, 3491–3495. [Google Scholar] [CrossRef]




| GEO Dataset | Case Number | SIK3 Gene Expression (PE/Np) | p Value |
|---|---|---|---|
| GSE4707 | Non-PE = 4, PE = 9 | −0.41 | 0.0122 |
| GSE10588 | Non-PE = 26, PE = 5 | 1.1 | 0.2893 |
| GSE14722 | Non-PE = 11, PE = 12 | 0.98 | 0.4915 |
| GSE24129 | Non-PE = 8, PE = 8 | 0.98 | 0.0446 |
| GSE30186 | Non-PE = 6, PE = 6 | 0.73 | 0.0164 |
| GSE43942 | Non-PE = 7, PE = 5 | 1.2 | 0.3526 |
| GSE54618 | Non-PE = 11, PE = 10 | −1.17 | 0.0253 |
| Non-Preeclampsia, | Preeclampsia, | p Value | |
|---|---|---|---|
| N = 7 (%) | N = 7 (%) | ||
| Maternal age (years) | 34 ± 9 | 34 ± 4 | 0.28 |
| Gestational age (weeks) | 39.4 ± 0.7 | 36.2 ± 2.4 | 0.018 |
| Newborn’s weight (g) | 3129.3 ± 244.0 | 2257.7 ± 698.2 | 0.005 |
| SIK3 | 0.008 | ||
| High expression | 6 (85.7) | 1 (14.3) | |
| Low expression | 1 (14.3) | 6 (85.7) | |
| CD68 | 0.593 | ||
| High expression | 3 (42.8) | 4 (47.2) | |
| Low expression | 4 (57.2) | 3 (42.8) | |
| CD204 | 0.008 | ||
| High expression | 1 (14.3) | 6 (85.7) | |
| Low expression | 6 (85.7) | 1 (14.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-F.; Tseng, C.-F.; Liang, Y.-L.; Wu, P.-Y.; Huang, L.-Y.; Lin, Y.-H.; Lin, L.-H.; Lin, C.-N.; Hsu, K.-F. Downregulation of Salt-Inducible Kinase 3 Enhances CCL24 Activation in the Placental Environment with Preeclampsia. Int. J. Mol. Sci. 2024, 25, 222. https://doi.org/10.3390/ijms25010222
Tsai H-F, Tseng C-F, Liang Y-L, Wu P-Y, Huang L-Y, Lin Y-H, Lin L-H, Lin C-N, Hsu K-F. Downregulation of Salt-Inducible Kinase 3 Enhances CCL24 Activation in the Placental Environment with Preeclampsia. International Journal of Molecular Sciences. 2024; 25(1):222. https://doi.org/10.3390/ijms25010222
Chicago/Turabian StyleTsai, Hsing-Fen, Ching-Fen Tseng, Yu-Ling Liang, Pei-Ying Wu, Lan-Yin Huang, Yu-Han Lin, Li-Hsuan Lin, Chang-Ni Lin, and Keng-Fu Hsu. 2024. "Downregulation of Salt-Inducible Kinase 3 Enhances CCL24 Activation in the Placental Environment with Preeclampsia" International Journal of Molecular Sciences 25, no. 1: 222. https://doi.org/10.3390/ijms25010222





