Investigation of the Potential of Selected Food-Derived Antioxidants to Bind and Stabilise the Bioactive Blue Protein C-Phycocyanin from Cyanobacteria Spirulina
Abstract
:1. Introduction
2. Results
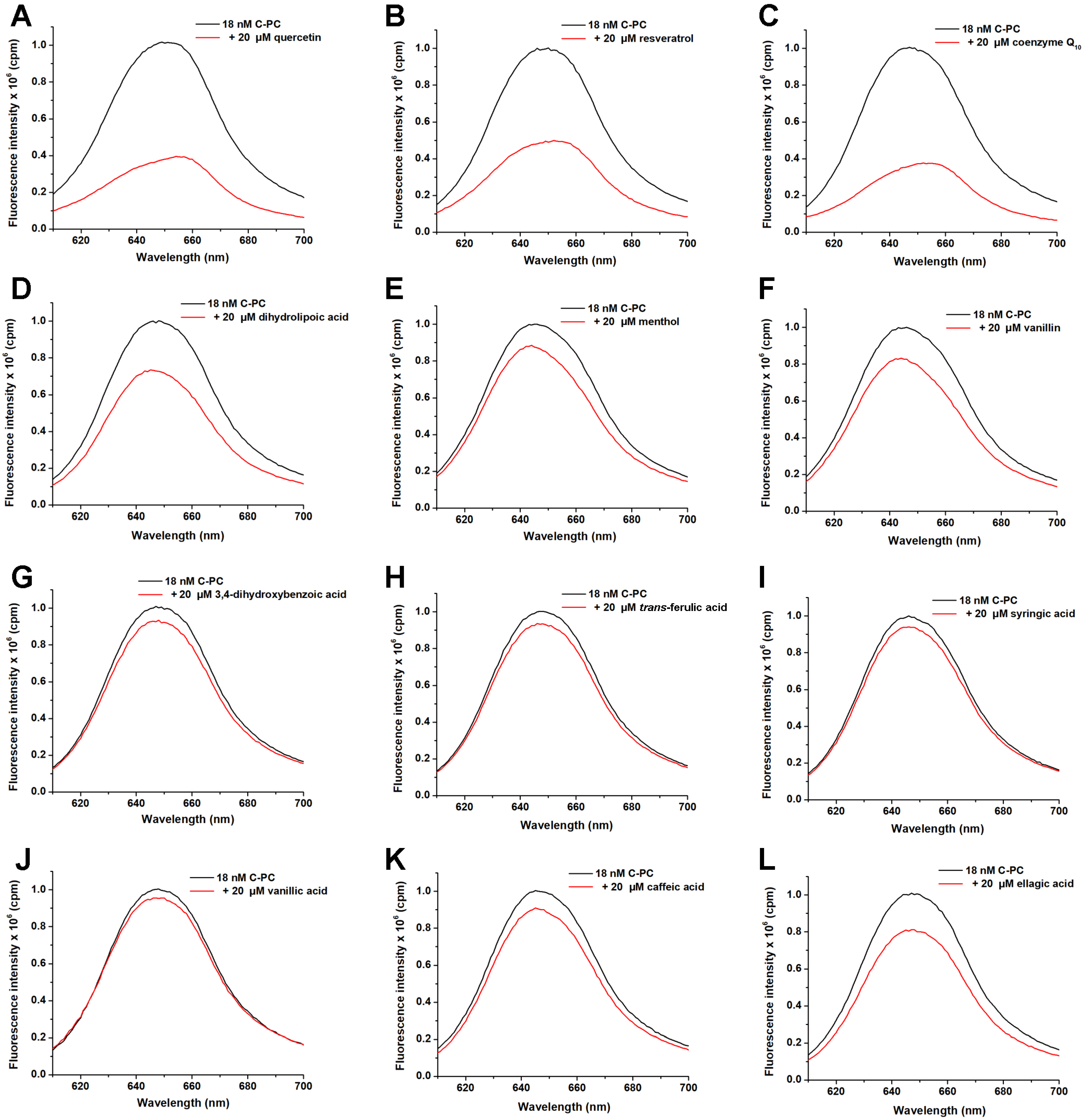
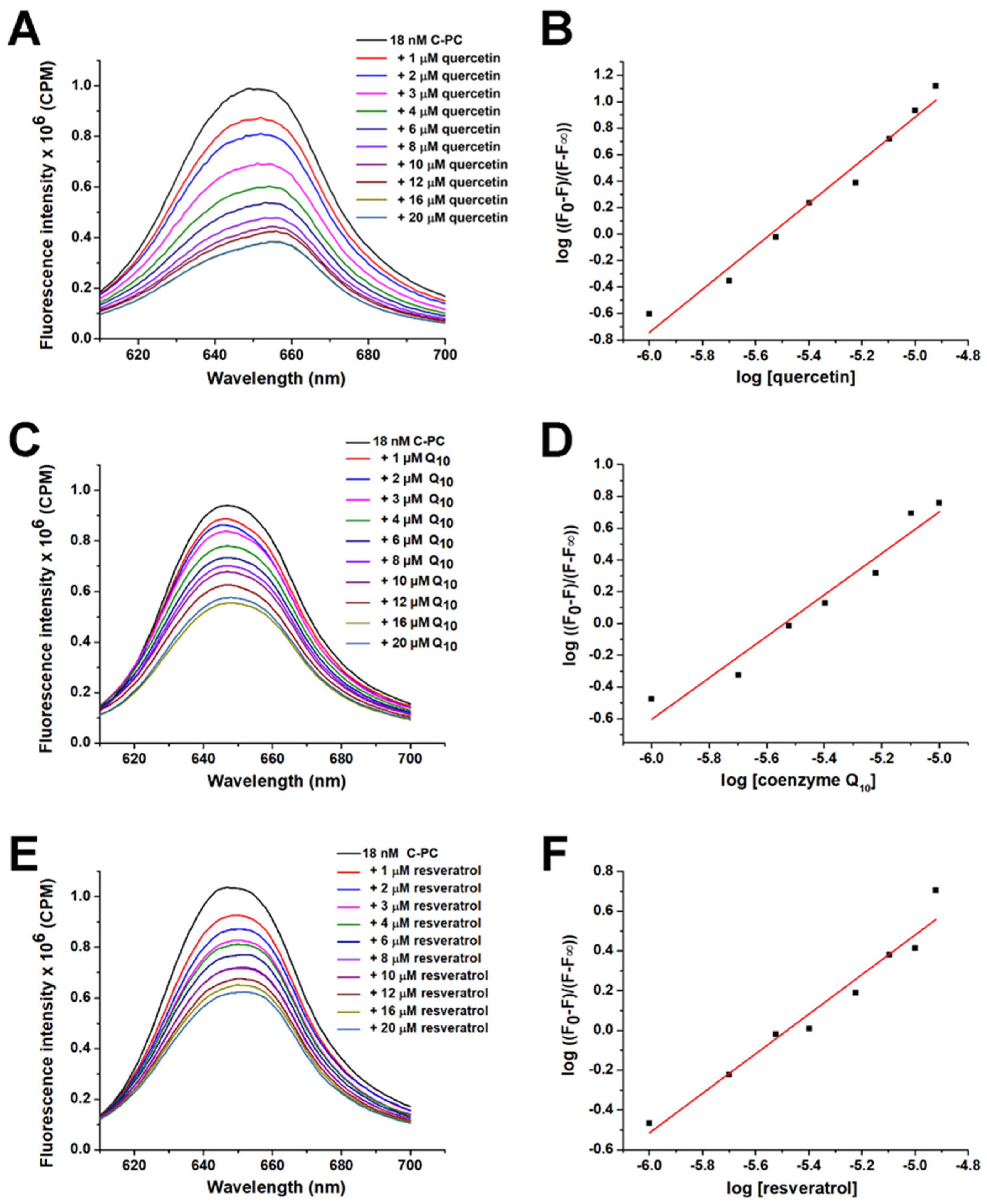
2.1. Screening of Suitable Ligands and Calculation of Affinity Constants to C-PC
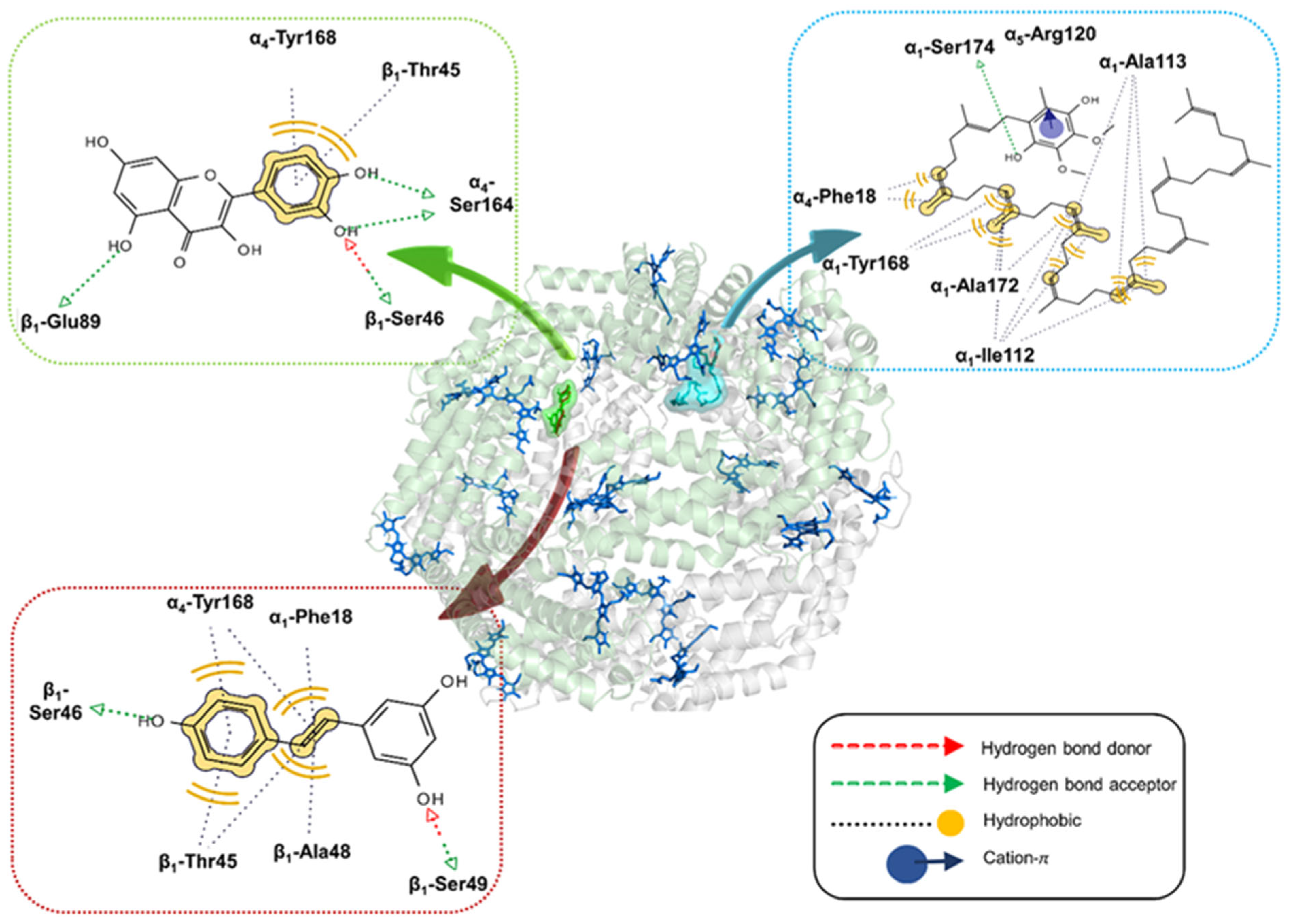
2.2. Molecular Docking Analysis
2.3. UV-VIS Spectroscopy Analysis
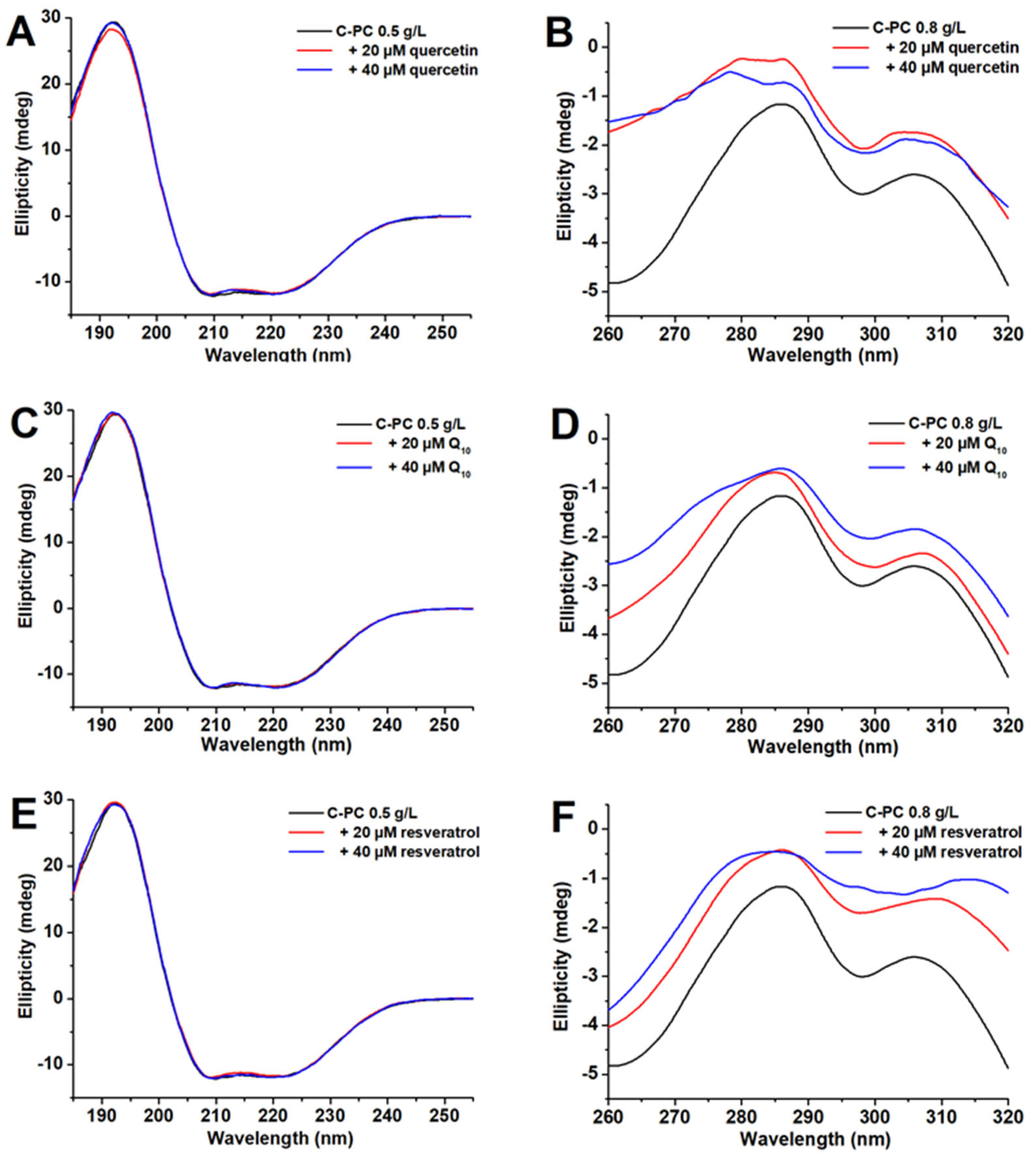
2.4. Structural Changes to C-PC Due to Binding of Ligands
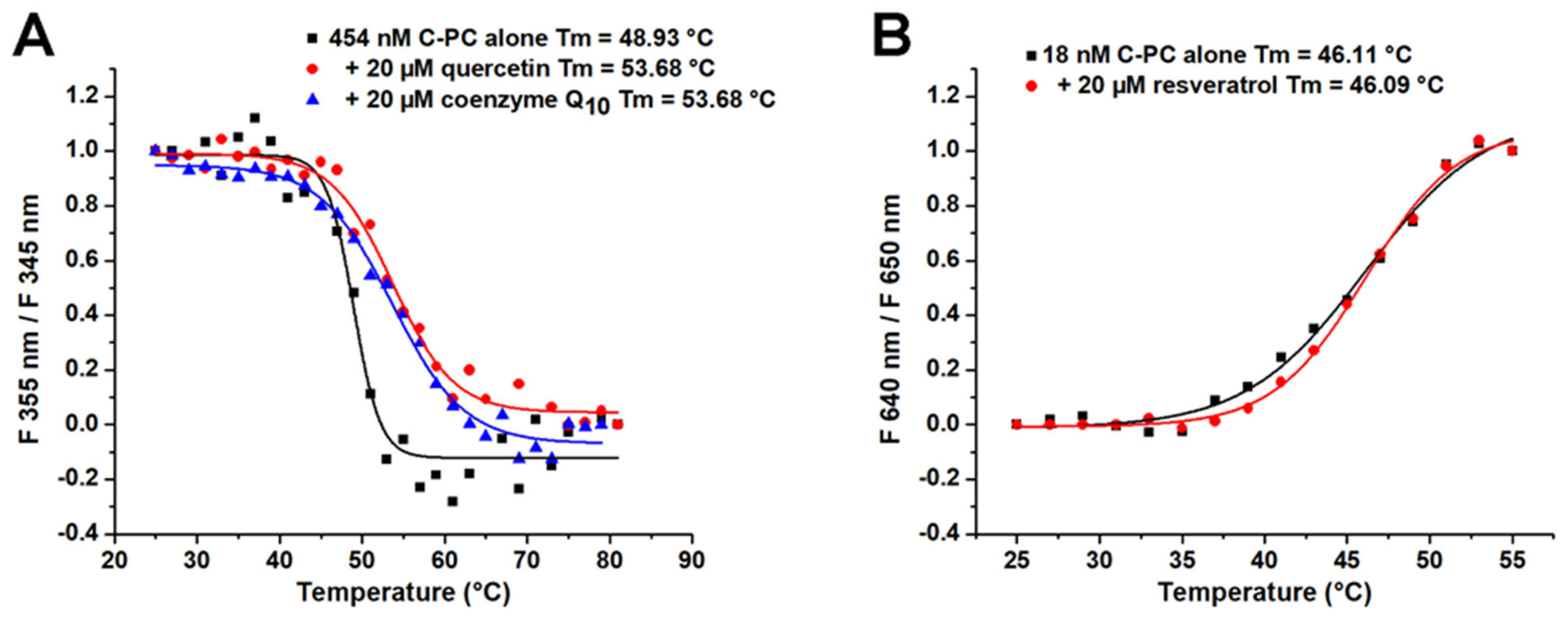
2.5. The Influence of Ligand Binding on Thermal Stability of C-PC
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Screening of Antioxidants Binding to C-PC
4.3. Calculation of Binding Affinity of Selected Ligands to C-PC
4.4. Molecular Docking
4.5. UV-VIS Absorption Spectroscopy
4.6. Circular Dichroism (CD) Spectroscopy
4.7. Thermal Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; Nimarshana, P.H.V.; Nagarajan, D.; Chang, J.-S.; Ariyadasa, T.U. Large-Scale Production of Spirulina-Based Proteins and c-Phycocyanin: A Biorefinery Approach. Biochem. Eng. J. 2022, 185, 108541. [Google Scholar] [CrossRef]
- Michael, A.; Kyewalyanga, M.S.; Lugomela, C.V. Biomass and Nutritive Value of Spirulina (Arthrospira Fusiformis) Cultivated in a Cost-Effective Medium. Ann. Microbiol. 2019, 69, 1387–1395. [Google Scholar] [CrossRef]
- Deshmukh, R. Phycocyanin Market Expected to Reach $409.8 Million by 2030-Allied Market Research. Available online: https://www.alliedmarketresearch.com/press-release/phycocyanin-market.html (accessed on 15 September 2023).
- Safaei, M.; Maleki, H.; Soleimanpour, H.; Norouzy, A.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Development of a Novel Method for the Purification of C-Phycocyanin Pigment from a Local Cyanobacterial Strain Limnothrix Sp. NS01 and Evaluation of Its Anticancer Properties. Sci. Rep. 2019, 9, 9474. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Schluchter, W.M.; Bryant, D.A. Biogenesis of Phycobiliproteins. J. Biol. Chem. 2008, 283, 7503–7512. [Google Scholar] [CrossRef] [PubMed]
- Jadaun, P.; Seniya, C.; Pal, S.K.; Kumar, S.; Kumar, P.; Nema, V.; Kulkarni, S.S.; Mukherjee, A. Elucidation of Antiviral and Antioxidant Potential of C-Phycocyanin against HIV-1 Infection through In Silico and In Vitro Approaches. Antioxidants 2022, 11, 1942. [Google Scholar] [CrossRef] [PubMed]
- Blas-Valdivia, V.; Moran-Dorantes, D.N.; Rojas-Franco, P.; Franco-Colin, M.; Mirhosseini, N.; Davarnejad, R.; Halajisani, A.; Tavakoli, O.; Cano-Europa, E. C-Phycocyanin Prevents Acute Myocardial Infarction-Induced Oxidative Stress, Inflammation and Cardiac Damage. Pharm. Biol. 2022, 60, 755–763. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, T.; Li, S.; Guan, F.; Zhang, J.; Liu, H. C-Phycocyanin Elicited Antitumor Efficacy via Cell-Cycle Arrest, Apoptosis Induction, and Invasion Inhibition in Esophageal Squamous Cell Carcinoma. J. Recept. Signal Transduct. 2019, 39, 114–121. [Google Scholar] [CrossRef]
- Liu, R.-Z.; Li, W.-J.; Zhang, J.-J.; Liu, Z.-Y.; Li, Y.; Liu, C.; Qin, S. The Inhibitory Effect of Phycocyanin Peptide on Pulmonary Fibrosis In Vitro. Mar. Drugs 2022, 20, 696. [Google Scholar] [CrossRef]
- Minic, S.L.; Stanic-Vucinic, D.; Mihailovic, J.; Krstic, M.; Nikolic, M.R.; Cirkovic Velickovic, T. Digestion by Pepsin Releases Biologically Active Chromopeptides from C-Phycocyanin, a Blue-Colored Biliprotein of Microalga Spirulina. J. Proteom. 2016, 147, 132–139. [Google Scholar] [CrossRef]
- Eriksen, N.T. Production of Phycocyanin-a Pigment with Applications in Biology, Biotechnology, Foods and Medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef]
- Pez Jaeschke, D.; Rocha Teixeira, I.; Damasceno Ferreira Marczak, L.; Domeneghini Mercali, G. Phycocyanin from Spirulina: A Review of Extraction Methods and Stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef]
- Su, C.-H.; Liu, C.-S.; Yang, P.-C.; Syu, K.-S.; Chiuh, C.-C. Solid–Liquid Extraction of Phycocyanin from Spirulina Platensis: Kinetic Modeling of Influential Factors. Sep. Purif. Technol. 2014, 123, 64–68. [Google Scholar] [CrossRef]
- Adjali, A.; Clarot, I.; Chen, Z.; Marchioni, E.; Boudier, A. Physicochemical Degradation of Phycocyanin and Means to Improve Its Stability: A Short Review. J. Pharm. Anal. 2022, 12, 406–414. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Z.; Shan, H.; Dashnyam, B.; Xu, X.; McClements, D.J.; Zhang, B.; Tan, M.; Wang, Z.; Cao, C. A Review of Recent Strategies to Improve the Physical Stability of Phycocyanin. Curr. Res. Food Sci. 2022, 5, 2329–2337. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Abbaspourrad, A. Improvement of the Colloidal Stability of Phycocyanin in Acidified Conditions Using Whey Protein-Phycocyanin Interactions. Food Hydrocoll. 2020, 105, 105747. [Google Scholar] [CrossRef]
- Pradeep, H.N.; Nayak, C.A. Enhanced Stability of C-Phycocyanin Colorant by Extrusion Encapsulation. J. Food Sci. Technol. 2019, 56, 4526–4534. [Google Scholar] [CrossRef]
- Martelli, G.; Folli, C.; Visai, L.; Daglia, M.; Ferrari, D. Thermal Stability Improvement of Blue Colorant C-Phycocyanin from Spirulina Platensis for Food Industry Applications. Process Biochem. 2014, 49, 154–159. [Google Scholar] [CrossRef]
- Padyana, A.K.; Bhat, V.B.; Madyastha, K.M.; Rajashankar, K.R.; Ramakumar, S. Crystal Structure of a Light-Harvesting Protein C-Phycocyanin from Spirulina Platensis. Biochem. Biophys. Res. Commun. 2001, 282, 893–898. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, D.J.; Arroyo-Hernández, M.; Posada-Ayala, M.; Santos, C. The High Content of Quercetin and Catechin in Airen Grape Juice Supports Its Application in Functional Food Production. Foods 2021, 10, 1532. [Google Scholar] [CrossRef]
- Abril, M.; Negueruela, A.; Perez, C.; Juan, T.; Estopanan, G. Preliminary Study of Resveratrol Content in Aragón Red and Rosé Wines. Food Chem. 2005, 92, 729–736. [Google Scholar] [CrossRef]
- Pravst, I.; Žmitek, K.; Žmitek, J. Coenzyme Q10 Contents in Foods and Fortification Strategies. Crit. Rev. Food Sci. Nutr. 2010, 50, 269–280. [Google Scholar] [CrossRef]
- Scheer, H.; Kufer, W. Conformational Studies on C-Phycocyanin from Spirulina Platensis. Z. Naturforschung C 1977, 32, 513–519. [Google Scholar] [CrossRef]
- Arciero, D.M.; Bryant, D.A.; Glazer, A.N. In Vitro Attachment of Bilins to Apophycocyanin. I. Specific Covalent Adduct Formation at Cysteinyl Residues Involved in Phycocyanobilin Binding in C-Phycocyanin. J. Biol. Chem. 1988, 263, 18343–18349. [Google Scholar] [CrossRef]
- Liu, W.; Guo, R. Interaction between Flavonoid, Quercetin and Surfactant Aggregates with Different Charges. J. Colloid Interface Sci. 2006, 302, 625–632. [Google Scholar] [CrossRef]
- Zsila, F.; Bikádi, Z.; Simonyi, M. Probing the Binding of the Flavonoid, Quercetin to Human Serum Albumin by Circular Dichroism, Electronic Absorption Spectroscopy and Molecular Modelling Methods. Biochem. Pharmacol. 2003, 65, 447–456. [Google Scholar] [CrossRef]
- Nair, M.S. Spectroscopic Study on the Interaction of Resveratrol and Pterostilbene with Human Serum Albumin. J. Photochem. Photobiol. B Biol. 2015, 149, 58–67. [Google Scholar] [CrossRef]
- Vaneková, Z.; Hubčík, L.; Toca-Herrera, J.L.; Furtműller, P.G.; Mučaji, P.; Nagy, M. Analysis of Binding Interactions of Ramipril and Quercetin on Human Serum Albumin: A Novel Method in Affinity Evaluation. Molecules 2020, 25, 547. [Google Scholar] [CrossRef]
- Pina, F.; Basílio, N.; Parola, A.J.; Melo, M.J.; Oliveira, J.; de Freitas, V. The Triumph of the Blue in Nature and in Anthropocene. Dye. Pigment. 2023, 210, 110925. [Google Scholar] [CrossRef]
- Fukui, K.; Saito, T.; Noguchi, Y.; Kodera, Y.; Matsushima, A.; Nishimura, H.; Inada, Y. Relationship between Color Development and Protein Conformation in the Phycocyanin Molecule. Dye. Pigment. 2004, 63, 89–94. [Google Scholar] [CrossRef]
- Manirafasha, E.; Murwanashyaka, T.; Ndikubwimana, T.; Yue, Q.; Zeng, X.; Lu, Y.; Jing, K. Ammonium Chloride: A Novel Effective and Inexpensive Salt Solution for Phycocyanin Extraction from Arthrospira (Spirulina) Platensis. J. Appl. Phycol. 2017, 29, 1261–1270. [Google Scholar] [CrossRef]
- Edwards, M.R.; Hauer, C.; Stack, R.F.; Eisele, L.E.; MacColl, R. Thermophilic C-Phycocyanin: Effect of Temperature, Monomer Stability, and Structure. Biochim. Biophys. Acta Bioenerg. 1997, 1321, 157–164. [Google Scholar] [CrossRef]
- Ferraro, G.; Imbimbo, P.; Marseglia, A.; Illiano, A.; Fontanarosa, C.; Amoresano, A.; Olivieri, G.; Pollio, A.; Monti, D.M.; Merlino, A. A Thermophilic C-Phycocyanin with Unprecedented Biophysical and Biochemical Properties. Int. J. Biol. Macromol. 2020, 150, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Hasan, S.; Kumar, J.; Raj, I.; Pathan, A.; Parmar, A.; Shakil, S.; Gourinath, S.; Madamwar, D. Crystal Structure and Interaction of Phycocyanin with β-Secretase: A Putative Therapy for Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2014, 13, 691–698. [Google Scholar] [CrossRef]
- Liu, Y.; Jovcevski, B.; Pukala, T.L. C-Phycocyanin from Spirulina Inhibits α-Synuclein and Amyloid-β Fibril Formation but Not Amorphous Aggregation. J. Nat. Prod. 2019, 82, 66–73. [Google Scholar] [CrossRef]
- Luo, Y.-C.; Jing, P. Molecular Interaction of Protein-Pigment C-Phycocyanin with Bovine Serum Albumin in a Gomphosis Structure Inhibiting Amyloid Formation. Int. J. Mol. Sci. 2020, 21, 8207. [Google Scholar] [CrossRef]
- Faieta, M.; Neri, L.; Sacchetti, G.; Di Michele, A.; Pittia, P. Role of Saccharides on Thermal Stability of Phycocyanin in Aqueous Solutions. Food Res. Int. 2020, 132, 109093. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Chen, W.; Zou, M.; Ma, X.; Lv, R.; Ding, T.; Liu, D. Co-Encapsulation of EGCG and Quercetin in Liposomes for Optimum Antioxidant Activity. J. Food Sci. 2019, 84, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Gligorijević, N.; Radomirović, M.; Rajković, A.; Nedić, O.; Ćirković Veličković, T. Fibrinogen Increases Resveratrol Solubility and Prevents It from Oxidation. Foods 2020, 9, 780. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Tajmir-Riahi, H.A.; Subirade, M. Interaction of β-Lactoglobulin with Resveratrol and Its Biological Implications. Biomacromolecules 2008, 9, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Inada, A.; Oue, T.; Yamashita, S.; Yamasaki, M.; Oshima, T.; Matsuyama, H. Development of Highly Water-Dispersible Complexes between Coenzyme Q10 and Protein Hydrolysates. Eur. J. Pharm. Sci. 2019, 136, 104936. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; McClements, D.J.; Zhu, Y.; Chen, Y.; Zou, L.; Liu, W.; Cheng, C.; Fu, D.; Liu, C. Enhancement of the Solubility, Stability and Bioaccessibility of Quercetin Using Protein-Based Excipient Emulsions. Food Res. Int. 2018, 114, 30–37. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Chen, F. A Simple Method for Efficient Separation and Purification of C-Phycocyanin and Allophycocyanin from Spirulina Platensis. Biotechnol. Tech. 1999, 13, 601–603. [Google Scholar] [CrossRef]
- Nikolic, M.R.; Minic, S.; Macvanin, M.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Analytical Protocols in Phycobiliproteins Analysis. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M., Zepka, L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 179–201. [Google Scholar] [CrossRef]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical p K a Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A. MMFF VI. MMFF94s Option for Energy Minimization Studies. J. Comput. Chem. 1999, 20, 720–729. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC: A Semiempirical Molecular Orbital Program. J. Comput. Aided. Mol. Des. 1990, 4, 1–103. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA Suite of Programs: An Versatile Platform for Cheminformatics and Drug Design Projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef]
- Wolber, G.; Langer, T. LigandScout: 3-D Pharmacophores Derived from Protein-Bound Ligands and Their Use as Virtual Screening Filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]


| Ligand | Ka (M−1) |
|---|---|
| Quercetin | 3.7 × 105 |
| Coenzyme Q10 | 3.4 × 105 |
| Resveratrol | 2.2 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gligorijević, N.; Jovanović, Z.; Cvijetić, I.; Šunderić, M.; Veličković, L.; Katrlík, J.; Holazová, A.; Nikolić, M.; Minić, S. Investigation of the Potential of Selected Food-Derived Antioxidants to Bind and Stabilise the Bioactive Blue Protein C-Phycocyanin from Cyanobacteria Spirulina. Int. J. Mol. Sci. 2024, 25, 229. https://doi.org/10.3390/ijms25010229
Gligorijević N, Jovanović Z, Cvijetić I, Šunderić M, Veličković L, Katrlík J, Holazová A, Nikolić M, Minić S. Investigation of the Potential of Selected Food-Derived Antioxidants to Bind and Stabilise the Bioactive Blue Protein C-Phycocyanin from Cyanobacteria Spirulina. International Journal of Molecular Sciences. 2024; 25(1):229. https://doi.org/10.3390/ijms25010229
Chicago/Turabian StyleGligorijević, Nikola, Zorana Jovanović, Ilija Cvijetić, Miloš Šunderić, Luka Veličković, Jaroslav Katrlík, Alena Holazová, Milan Nikolić, and Simeon Minić. 2024. "Investigation of the Potential of Selected Food-Derived Antioxidants to Bind and Stabilise the Bioactive Blue Protein C-Phycocyanin from Cyanobacteria Spirulina" International Journal of Molecular Sciences 25, no. 1: 229. https://doi.org/10.3390/ijms25010229





