Resources for Human Health from the Plant Kingdom: The Potential Role of the Flavonoid Apigenin in Cancer Counteraction
Abstract
:1. Introduction
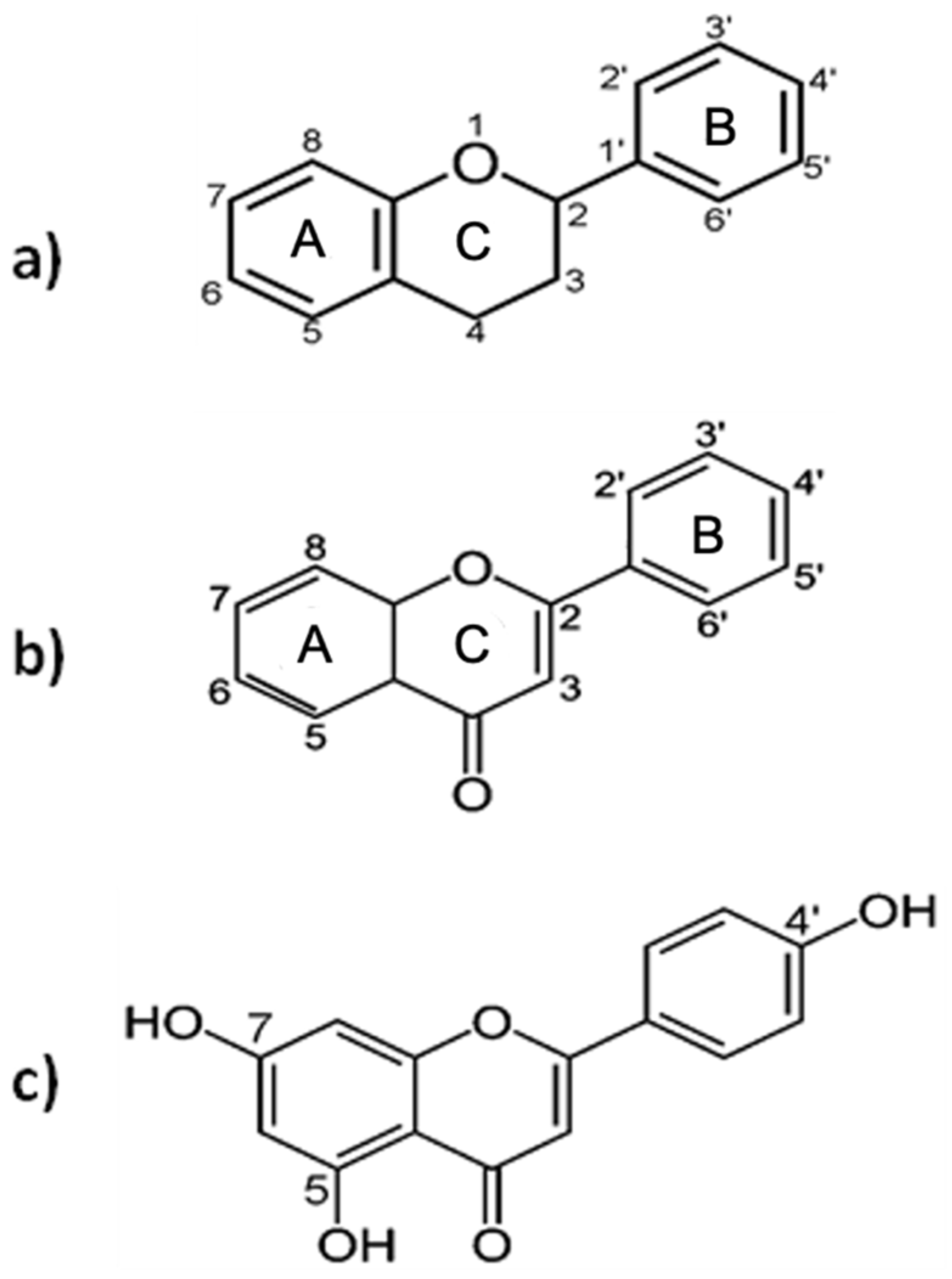
2. General Aspects of Apigenin
2.1. Natural Sources
2.2. Chemistry and Biological Activity
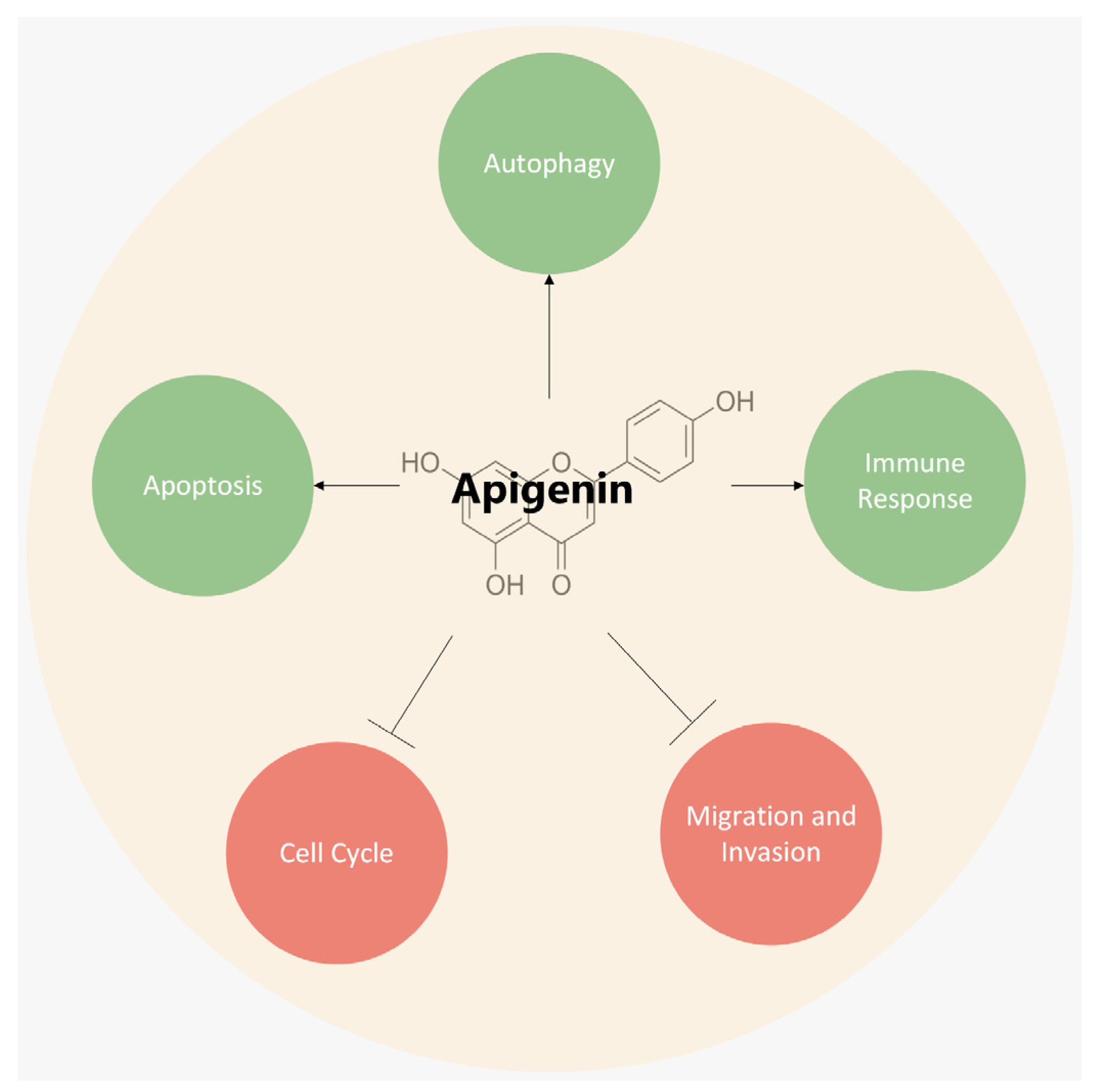
3. Mechanisms Underlying the Anticancer Role of Apigenin
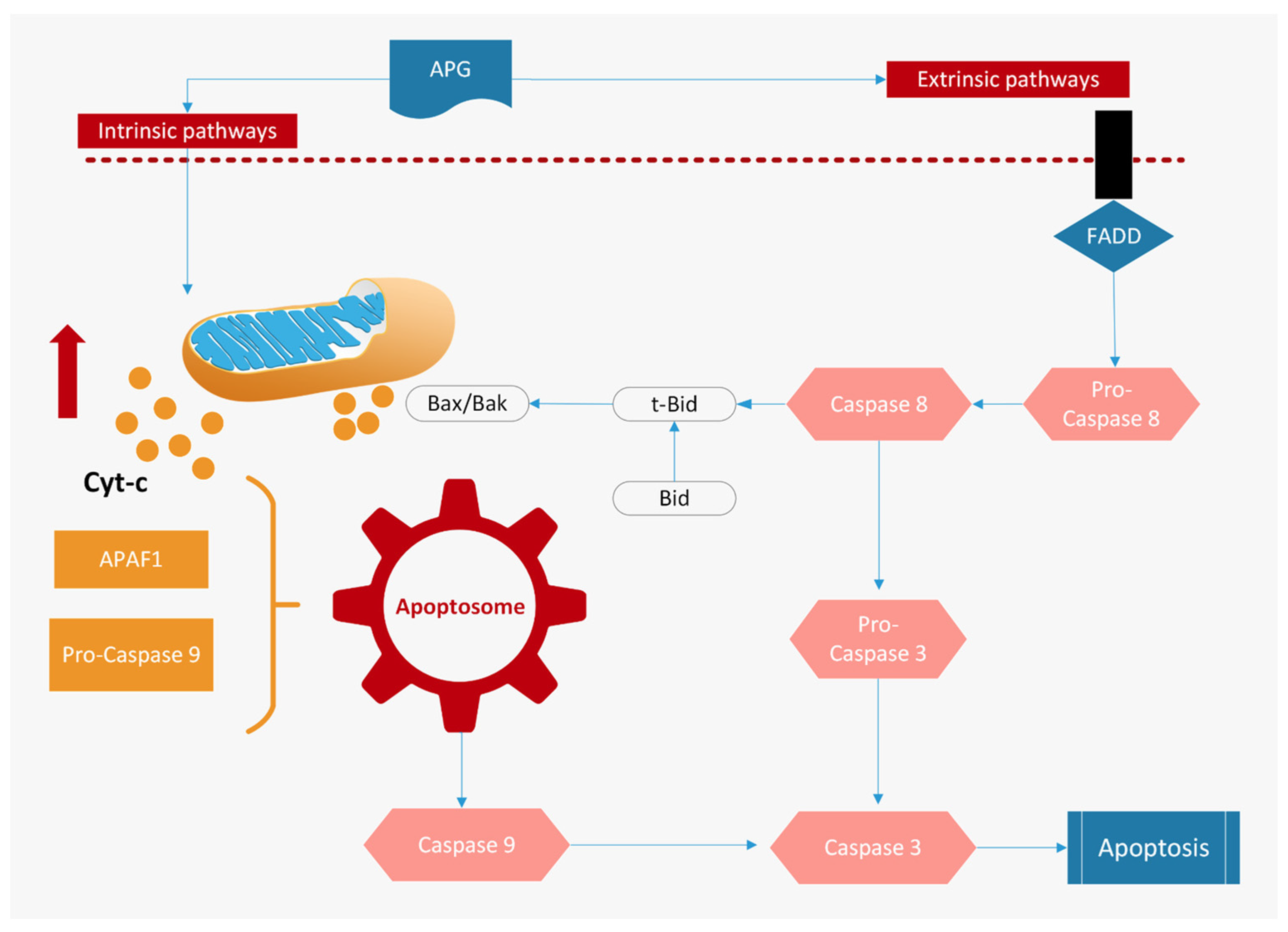
3.1. Induction of Apoptosis
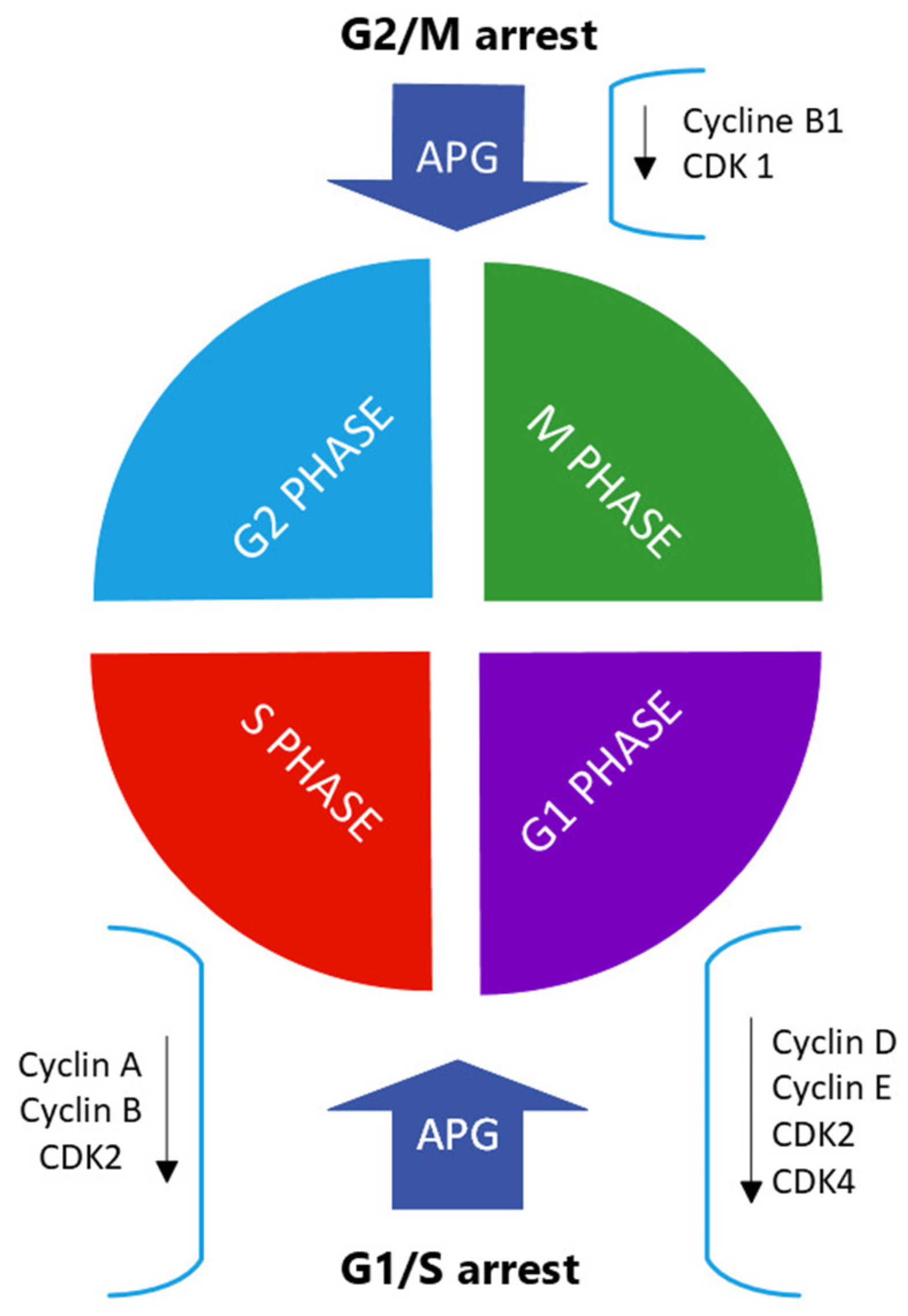
3.2. Inhibition of Cell-Cycle Progressions
3.3. Induction of Autophagy
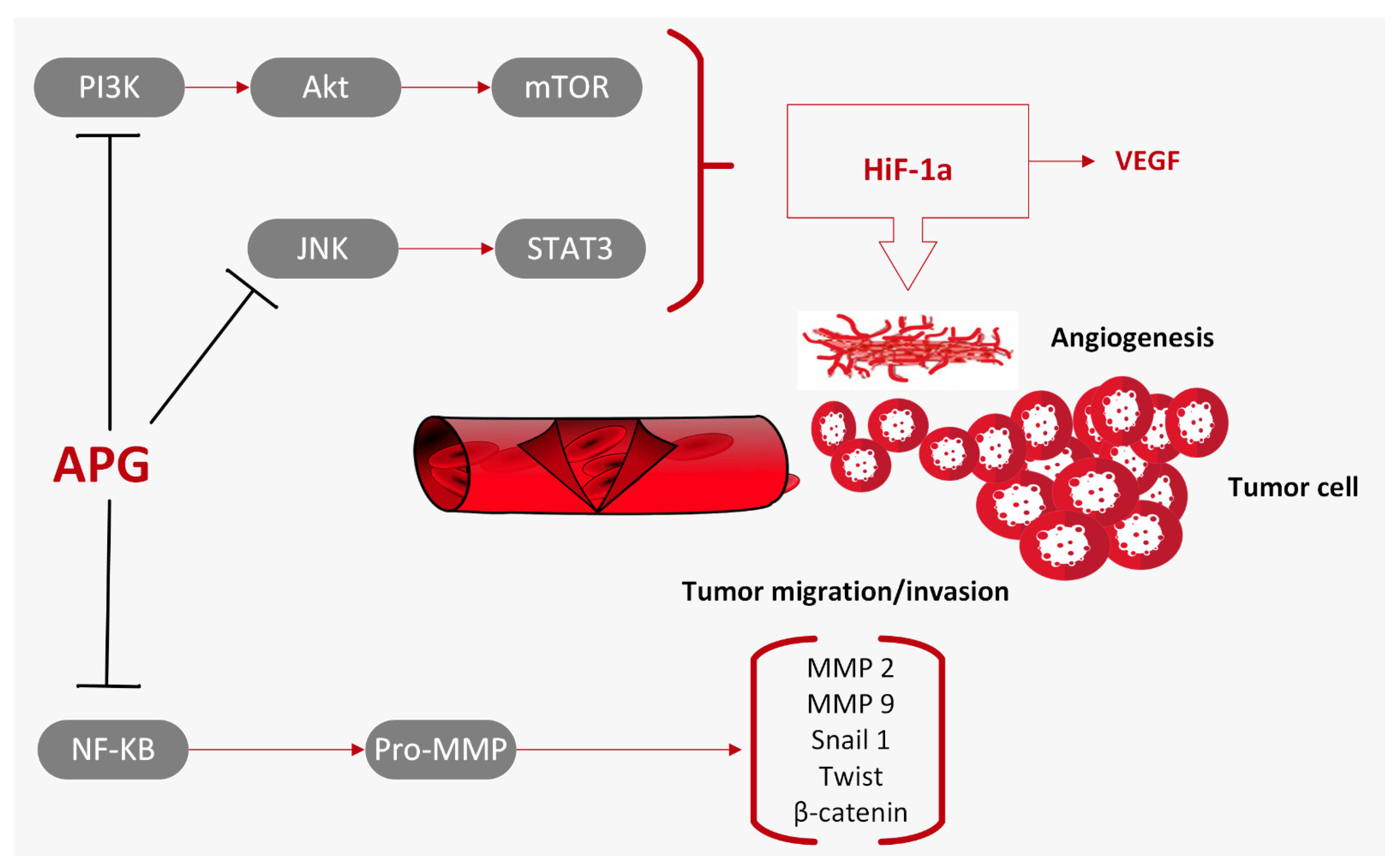
3.4. Inhibition of Cell Migration and Invasion
3.5. Intracellular Signal Pathways Modulated by Apigenin
3.5.1. Apigenin and the PI3K/Akt/mTOR Pathway
3.5.2. Apigenin and the MAPK/ERK Pathway
- In human melanoma cells, apigenin suppresses cell proliferation and cell migration along with the induction of apoptosis via decreasing the expression of phosphorylated (p)-ERK1/2 proteins [68];
- In colorectal cancer cells, apigenin enhances ABT-263-induced anti-tumor activity via the inhibition of Akt and ERK pathways [75];
- In an autochthonous mouse prostate cancer model, apigenin administration suppresses prostate cancer progression by decreasing IGF/IGFBP-3 and inhibiting p-Akt and p-ERK1/2 [50].
3.5.3. Apigenin and the JAK/STAT Pathway
3.5.4. Apigenin and the NF-κB Pathway
- Pro-survival genes (Bcl-2, Bcl-xL, survivin, XIAP);
- Cell-cycle-related genes (cyclin D1);
- Growth factor, inflammatory cytokines, and tumor metastasis genes (COX-2).
3.5.5. Apigenin and the Wnt/β-Catenin Pathway
4. Combination Therapy for Apigenin
5. Critical Aspects of Apigenin for Therapeutic Purposes
5.1. Bioavailability of Apigenin
- Water-in-oil-in-water (W/O/W) double emulsions loaded with apigenin. In vitro studies have confirmed the double emulsion’s capacity to transport bioactive compounds in an aqueous phase, minimizing degradation and potentially increasing in vivo bioavailability [101];
- Gold nanoparticles, widely used for their good biodistribution, stability, and low toxicity. Au3+ can be reduced by apigenin at a pH of 10 and at room temperature, forming highly stable and spherical apigenin-AuNPs. The apigenin-AuNPs are found to exhibit toxicity towards the A431 (epidermoid squamous cell carcinoma) cell line while being non-toxic towards normal epidermoid cells. This technique shows promise in the treatment of skin cancer [102];
- Phytosome, a phospholipid-based complex of apigenin, i.e., apigenin–phospholipid phytosome (APLC). Phytosome is highly compatible with human physiology and bioavailable thanks to its ability to cross the lipid bilayer membrane of enterocytes and reach systemic circulation. A study shows that APLC formulation demonstrated an over 36-fold higher aqueous solubility of apigenin, compared to that of pure apigenin [103];
- Self-microemulsifying drug-delivery systems (SMEDDSs). They are mixtures of oils, surfactants, solvents, and drug substances that form oil-in-water microemulsions with droplet sizes less than 100 nm when introduced into aqueous phases under gentle agitation or gastrointestinal motility [104]. A study shows that SMEDDSs could enhance the solubility and dissolution of apigenin and would be a potential carrier to improve the oral absorption of apigenin [105];
- Bioactive self-nanoemulsifying drug-delivery systems (BioSNEDDSs). They form a nanoemulsion with droplet sizes significantly smaller (by a factor of ten or similar) than droplets found in ordinary emulsions. The decreased droplet size increases the absorption rate and extent and prevents drug degradation in the gastrointestinal tract [106]. The BioSNEDDSs differ from conventional SNEDDSs for using bioactive lipid excipients such as black seed oil, Moringa oleifera seed oil, avocado oil, apricot oil, grape seed oil, safflower oil, and coconut oil fatty acid. A study shows that BioSNEDDSs formulated for apigenin provide collective advantages, such as a superior self-emulsification efficiency with an improved physical stability, high drug-loading capacity, antibacterial activity, and elevated apigenin bioavailability [107].
5.2. Absorption, Distribution, Metabolism, Excretion
5.2.1. Absorption
5.2.2. Distribution
5.2.3. Metabolism
- Direct systemic absorption;
- Excretion;
- Passage with the bile from the liver to the intestine, where they are hydrolyzed by bacterial beta-glucuronidases, returning to an absorbable form again (entero-hepatic circulation);
- Passage into the intestinal lumen, where they are subject to hydrolysis and subsequently reabsorbed (entero-enteric circulation and local enteric circulation) [117].
5.2.4. Excretion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC—WHO Italy Fact Sheet 380. Available online: https://gco.iarc.fr/today/data/factsheets/populations/380-italy-fact-sheets.pdf (accessed on 30 October 2023).
- Dixon, K.; Kopras, E. Genetic Alterations and DNA Repair in Human Carcinogenesis. Semin. Cancer Biol. 2004, 14, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Schottenfeld, D.; Beebe-Dimmer, J.L. Advances in Cancer Epidemiology: Understanding Causal Mechanisms and the Evidence for Implementing Interventions. Annu. Rev. Public Health 2005, 26, 37–60. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Senthakumaran, T.; Moen, A.; Tannæs, T.; Endres, A.; Brackmann, S.; Rounge, T.; Bemanian, V.; Tunsjø, H. Microbial Dynamics with CRC Progression: A Study of the Mucosal Microbiota at Multiple Sites in Cancers, Adenomatous Polyps, and Healthy Controls. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 305. [Google Scholar] [CrossRef]
- Chen, H.; Tong, T.; Lu, S.Y.; Ji, L.; Xuan, B.; Zhao, G.; Yan, Y.; Song, L.; Zhao, L.; Xie, Y.; et al. Urea Cycle Activation Triggered by Host-Microbiota Maladaptation Driving Colorectal Tumorigenesis. Cell Metab. 2023, 35, 651–666.e7. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer Chemoprevention with Dietary Phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Manson, M.M. Cancer Prevention—The Potential for Diet to Modulate Molecular Signalling. Trends Mol. Med. 2003, 9, 11–18. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of Polyphenols and Its Anticancer Properties in Biomedical Research: A Narrative Review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as Natural Phenolic Compounds and Their Role in Therapeutics: An Overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Almatrood, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydh, F.A.; Alsahl, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Kwah, M.X.Y.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.L.; Wang, L.; Ong, P.S.; et al. Resveratrol for Cancer Therapy: Challenges and Future Perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Boutas, I.; Kontogeorgi, A.; Dimitrakakis, C.; Kalantaridou, S.N. Soy Isoflavones and Breast Cancer Risk: A Meta-Analysis. In Vivo 2022, 36, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Goel, A. Curcumin and Colorectal Cancer: An Update and Current Perspective on This Natural Medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Firrman, J.; Liu, L.S.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A Review of the Dietary Flavonoid, Kaempferol on Human Health and Cancer Chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in Cancer Therapy: Anticancer Effects and Mechanisms of Action. Cell Biosci. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Lefort, É.C.; Blay, J. Apigenin and Its Impact on Gastrointestinal Cancers. Mol. Nutr. Food Res. 2013, 57, 126–144. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Khan, H.; D’onofrio, G.; Šamec, D.; Shirooie, S.; Dehpour, A.R.; Argüelles, S.; Habtemariam, S.; Sobarzo-Sanchez, E. Apigenin as Neuroprotective Agent: Of Mice and Men. Pharmacol. Res. 2018, 128, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective Effects of Apigenin against Inflammation, Neuronal Excitability and Apoptosis in an Induced Pluripotent Stem Cell Model of Alzheimer’s Disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.J.; Page, A.T. Herbal Medicine for Insomnia: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2015, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haytowitz, D.B.; Wu, X.; Bhagwat, S. USDA Database for the Flavonoid Content of Selected Foods Release 3.3 Prepared By. 2018. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 27 November 2023).
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Hanrahan, J.R.; Chebib, M.; Johnston, G.A.R. Flavonoid Modulation of GABA A Receptors. Br. J. Pharmacol. 2011, 163, 234–245. [Google Scholar] [CrossRef]
- Park, S.-H.; Sim, Y.-B.; Han, P.-L.; Lee, J.-K.; Suh, H.-W. Antidepressant-like Effect of Kaempferol and Quercitirin, Isolated from Opuntia Ficus-Indica Var. Saboten. Exp. Neurobiol. 2010, 19, 30. [Google Scholar] [CrossRef]
- Borgonetti, V.; Benatti, C.; Governa, P.; Isoldi, G.; Pellati, F.; Alboni, S.; Tascedda, F.; Montopoli, M.; Galeotti, N.; Manetti, F.; et al. Non-Psychotropic Cannabis Sativa L. Phytocomplex Modulates Microglial Inflammatory Response through CB2 Receptors-, Endocannabinoids-, and NF-ΚB-Mediated Signaling. Phyther. Res. 2022, 36, 2246–2263. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.M.A.K.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as Antidiabetic and Anti-Inflammatory Agents: A Review on Structural Activity Relationship-Based Studies and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12605. [Google Scholar] [CrossRef]
- Abid, R.; Ghazanfar, S.; Farid, A.; Sulaman, S.M.; Idrees, M.; Amen, R.A.; Muzammal, M.; Shahzad, M.K.; Mohamed, M.O.; Khaled, A.A.; et al. Pharmacological Properties of 4′, 5, 7-Trihydroxyflavone (Apigenin) and Its Impact on Cell Signaling Pathways. Molecules 2022, 27, 4304. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M.; et al. Matricaria Genus as a Source of Antimicrobial Agents: From Farm to Pharmacy and Food Applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; da Mesquita, D.S.; de Moraes Barriga, J.R.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological Activities and Therapeutic Potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics Classification and Intestinal Absorption Study of Apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Garg, V.K.; Buttar, H.S.; Setzer, W.N.; Sethi, G. Apigenin: A Natural Bioactive Flavone-Type Molecule with Promising Therapeutic Function. J. Funct. Foods 2018, 48, 457–471. [Google Scholar] [CrossRef]
- Wu, C.C.; Fang, C.Y.; Cheng, Y.J.; Hsu, H.Y.; Chou, S.P.; Huang, S.Y.; Tsai, C.H.; Chen, J.Y. Inhibition of Epstein-Barr Virus Reactivation by the Flavonoid Apigenin. J. Biomed. Sci. 2017, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ganapathi, R.; Kumar, R.R.; Thomas, K.C.; Rafi, M.; Reddiar, K.S.; George, P.S.; Ramadas, K. Epstein-Barr Virus Dynamics and Its Prognostic Impact on Nasopharyngeal Cancers in a Non-Endemic Region. Ecancermedicalscience 2022, 16, 1479. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Hohmann, J.; El-Shazly, M.; Chang, L.K.; Dankó, B.; Kúsz, N.; Hsieh, C.T.; Hunyadi, A.; Chang, F.R. Bioactive Constituents of Lindernia Crustacea and Its Anti-EBV Effect via Rta Expression Inhibition in the Viral Lytic Cycle. J. Ethnopharmacol. 2020, 250, 112493. [Google Scholar] [CrossRef]
- Vágvölgyi, M.; Girst, G.; Kúsz, N.; Ötvös, S.B.; Fülöp, F.; Hohmann, J.; Servais, J.Y.; Seguin-Devaux, C.; Chang, F.R.; Chen, M.S.; et al. Less Cytotoxic Protoflavones as Antiviral Agents: Protoapigenone 1′-O-Isopropyl Ether Shows Improved Selectivity against the Epstein–Barr Virus Lytic Cycle. Int. J. Mol. Sci. 2019, 20, 6269. [Google Scholar] [CrossRef]
- Seo, H.-S.; Sikder, M.A.; Lee, H.J.; Ryu, J.; Lee, C.J. Apigenin Inhibits Tumor Necrosis Factor-α-Induced Production and Gene Expression of Mucin through Regulating Nuclear Factor-Kappa B Signaling Pathway in Airway Epithelial Cells. Biomol. Ther. 2014, 22, 525–531. [Google Scholar] [CrossRef]
- Shukla, S.; Fu, P.; Gupta, S. Apigenin Induces Apoptosis by Targeting Inhibitor of Apoptosis Proteins and Ku70-Bax Interaction in Prostate Cancer. Apoptosis 2014, 19, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Pamunuwa, G.; Nedra Karunaratne, D.; Waisundara, V.Y. Antidiabetic Properties, Bioactive Constituents, and Other Therapeutic Effects of Scoparia Dulcis. Evid.-Based Complement. Altern. Med. 2016, 2016, 8243215. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic Properties and Drug Interactions of Apigenin, a Natural Flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of Carcinogenesis by Dietary Polyphenolic Compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Walker, B.; Tibbels, M.G.; Bresnick, E. Anti-Mutagenesis and Anti-Promotion by Apigenin, Robinetin and Indole-3-Carbinol. Carcinogenesis 1986, 7, 959–963. [Google Scholar] [CrossRef]
- Sung, B.; Chung, H.Y.; Kim, N.D. Role of Apigenin in Cancer Prevention via the Induction of Apoptosis and Autophagy. J. Cancer Prev. 2016, 21, 216–226. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. 5-Fluorouracil Combined with Apigenin Enhances Anticancer Activity through Induction of Apoptosis in Human Breast Cancer MDA-MB-453 Cells. Oncol. Rep. 2009, 22, 1533–1537. [Google Scholar] [CrossRef]
- Pandey, M.; Kaur, P.; Shukla, S.; Abbas, A.; Fu, P.; Gupta, S. Plant Flavone Apigenin Inhibits HDAC and Remodels Chromatin to Induce Growth Arrest and Apoptosis in Human Prostate Cancer Cells: In Vitro and in Vivo Study. Mol. Carcinog. 2012, 51, 952–962. [Google Scholar] [CrossRef]
- Maeda, Y.; Takahashi, H.; Nakai, N.; Yanagita, T.; Ando, N.; Okubo, T.; Saito, K.; Shiga, K.; Hirokawa, T.; Hara, M.; et al. Apigenin Induces Apoptosis by Suppressing Bcl-Xl and Mcl-1 Simultaneously via Signal Transducer and Activator of Transcription 3 Signaling in Colon Cancer. Int. J. Oncol. 2018, 52, 1661–1673. [Google Scholar] [CrossRef]
- Yang, C.; Song, J.; Hwang, S.; Choi, J.; Song, G.; Lim, W. Apigenin Enhances Apoptosis Induction by 5-Fluorouracil through Regulation of Thymidylate Synthase in Colorectal Cancer Cells. Redox Biol. 2021, 47, 102144. [Google Scholar] [CrossRef]
- Hussain, A.R.; Khan, A.S.; Ahmed, S.O.; Ahmed, M.; Platanias, L.C.; Al-Kuraya, K.S.; Uddin, S. Apigenin Induces Apoptosis via Downregulation of S-Phase Kinase-Associated Protein 2-Mediated Induction of P27Kip1 in Primary Effusion Lymphoma Cells. Cell Prolif. 2010, 43, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.H.; Chien, M.H.; Lin, W.L.; Wen, Y.C.; Chow, J.M.; Chen, C.K.; Kuo, T.C.; Lee, W.J. Inhibition of MDA-MB-231 Breast Cancer Cell Proliferation and Tumor Growth by Apigenin through Induction of G2/M Arrest and Histone H3 Acetylation-Mediated P21WAF1/CIP1 Expression. Environ. Toxicol. 2017, 32, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhu, Y.; Li, J.F.; Wang, X.; Liang, Z.; Li, S.Q.; Xu, X.; Chen, H.; Liu, B.; Zheng, X.Y.; et al. Apigenin Inhibits Renal Cell Carcinoma Cell Proliferation. Oncotarget 2017, 8, 19834–19842. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sung, B.; Kang, Y.J.; Kim, D.H.; Jang, J.Y.; Hwang, S.Y.; Kim, M.; Lim, H.S.; Yoon, J.H.; Chung, H.Y.; et al. Apigenin-Induced Apoptosis Is Enhanced by Inhibition of Autophagy Formation in HCT116 Human Colon Cancer Cells. Int. J. Oncol. 2014, 44, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, D.; Garavello, W.; Rigolio, R.; Pignataro, L.; Gaini, R.; Nicolini, G. Apigenin Impairs Oral Squamous Cell Carcinoma Growth in Vitro Inducing Cell Cycle Arrest and Apoptosis. Int. J. Oncol. 2013, 43, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Parama, D.; Daimari, E.; Girisa, S.; Banik, K.; Harsha, C.; Dutta, U.; Kunnumakkara, A.B. Rationalizing the Therapeutic Potential of Apigenin against Cancer. Life Sci. 2021, 267, 118814. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Bakhoda, M.R.; Bahmanpour, Z.; Ilkhani, K.; Zarrabi, A.; Makvandi, P.; Khan, H.; Mazaheri, S.; Darvish, M.; Mirzaei, H. Apigenin as Tumor Suppressor in Cancers: Biotherapeutic Activity, Nanodelivery, and Mechanisms with Emphasis on Pancreatic Cancer. Front. Chem. 2020, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Ruela-De-Sousa, R.R.; Fuhler, G.M.; Blom, N.; Ferreira, C.V.; Aoyama, H.; Peppelenbosch, M.P. Cytotoxicity of Apigenin on Leukemia Cell Lines: Implications for Prevention and Therapy. Cell Death Dis. 2010, 1, e19. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Ma, B. Autophagy and Autophagy-Related Proteins in Cancer. Mol. Cancer 2020, 19, 1–16. [Google Scholar] [CrossRef]
- Ferro, F.; Servais, S.; Besson, P.; Roger, S.; Dumas, J.F.; Brisson, L. Autophagy and Mitophagy in Cancer Metabolic Remodelling. Semin. Cell Dev. Biol. 2020, 98, 129–138. [Google Scholar] [CrossRef]
- Li, Y.J.; Lei, Y.H.; Yao, N.; Wang, C.R.; Hu, N.; Ye, W.C.; Zhang, D.M.; Chen, Z.S. Autophagy and Multidrug Resistance in Cancer. Chin. J. Cancer 2017, 36, 52. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, B.; Cao, W.; Zhang, W.; Zhang, F.; Zhao, H.; Meng, R.; Zhang, L.; Niu, R.; Hao, X.; et al. Autophagy Inhibition Enhances Apigenin-Induced Apoptosis in Human Breast Cancer Cells. Chin. J. Cancer Res. 2013, 25, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/MTOR Pathway by Apigenin Induces Apoptosis and Autophagy in Hepatocellular Carcinoma Cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Amos, S.E.; Choi, Y.S. The Cancer Microenvironment: Mechanical Challenges of the Metastatic Cascade. Front. Bioeng. Biotechnol. 2021, 9, 625859. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, J.; Li, S.; Wang, X.; Liang, Z.; Xu, X.; Xu, X.; Hu, Z.; Lin, Y.; Chen, H.; et al. Apigenin Inhibits Migration and Invasion via Modulation of Epithelial Mesenchymal Transition in Prostate Cancer. Mol. Med. Rep. 2015, 11, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Han, X.; Cheng, W.; Ni, J.; Zhang, Y.; Lin, J.; Song, Z. Apigenin Inhibits Proliferation and Invasion, and Induces Apoptosis and Cell Cycle Arrest in Human Melanoma Cells. Oncol. Rep. 2017, 37, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Gilardini Montani, M.S.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Apigenin, by Activating P53 and Inhibiting STAT3, Modulates the Balance between pro-Apoptotic and pro-Survival Pathways to Induce PEL Cell Death. J. Exp. Clin. Cancer Res. 2017, 36, 1–9. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/MTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- Tong, X.; Pelling, J. Targeting the PI3K/Akt/MTOR Axis by Apigenin for Cancer Prevention. Anticancer Agents Med. Chem. 2013, 13, 971–978. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, C.Y.; Lee, K.R.; Lin, H.J.; Chen, T.H.; Wan, L. Flavones Inhibit Breast Cancer Proliferation through the Akt/FOXO3a Signaling Pathway. BMC Cancer 2015, 15, 958. [Google Scholar] [CrossRef]
- Huang, S.; Yu, M.; Shi, N.; Zhou, Y.; Li, F.; Li, X.; Huang, X.; Jin, J. Apigenin and Abivertinib, a Novel BTK Inhibitor Synergize to Inhibit Diffuse Large B-Cell Lymphoma in Vivo and Vitro. J. Cancer 2020, 11, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Park, S.; Bazer, F.W.; Song, G. Apigenin Reduces Survival of Choriocarcinoma Cells by Inducing Apoptosis via the PI3K/AKT and ERK1/2 MAPK Pathways. J. Cell. Physiol. 2016, 231, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Jing, K.; Mahmoud, E.; Huang, H.; Fang, X.; Yu, C. Apigenin Sensitizes Colon Cancer Cells to Antitumor Activity of Abt-263. Mol. Cancer Ther. 2013, 12, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Chu, J.H.; Kwan, H.Y.; Su, T.; Yu, H.; Cheng, C.Y.; Fu, X.Q.; Guo, H.; Li, T.; Tse, A.K.W.; et al. Inhibition of the STAT3 Signaling Pathway Contributes to Apigenin-Mediated Anti-Metastatic Effect in Melanoma. Sci. Rep. 2016, 6, 21731. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Shankar, E.; Fu, P.; MacLennan, G.T.; Gupta, S. Suppression of NF-KB and NF-KB-Regulated Gene Expression by Apigenin through IkBα and IKK Pathway in TRAMP Mice. PLoS ONE 2015, 10, e0138710. [Google Scholar] [CrossRef] [PubMed]
- Adham, A.N.; Abdelfatah, S.; Naqishbandi, A.M.; Mahmoud, N.; Efferth, T. Cytotoxicity of Apigenin toward Multiple Myeloma Cell Lines and Suppression of INOS and COX-2 Expression in STAT1-Transfected HEK293 Cells. Phytomedicine 2021, 80, 153371. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, S.; Song, Y.; Yao, J.; Huang, K.; Zhu, X. Apigenin Suppresses Colorectal Cancer Cell Proliferation, Migration and Invasion via Inhibition of the Wnt/β-Catenin Signaling Pathway. Oncol. Lett. 2016, 11, 3075–3080. [Google Scholar] [CrossRef]
- Zhong, Z.; Virshup, D.M. Wnt Signaling and Drug Resistance in Cancer. Mol. Pharmacol. 2020, 97, 72–89. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Lv, L.; Chen, D.; Shen, L.; Xie, Z. Apigenin Inhibits the Proliferation and Invasion of Osteosarcoma Cells by Suppressing the Wnt/β-Catenin Signaling Pathway. Oncol. Rep. 2015, 34, 1035–1041. [Google Scholar] [CrossRef]
- Lin, C.M.; Chen, H.H.; Lin, C.A.; Wu, H.C.; Sheu, J.J.C.; Chen, H.J. Apigenin-Induced Lysosomal Degradation of β-Catenin in Wnt/β-Catenin Signaling. Sci. Rep. 2017, 7, 372. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Alsahli, M.A.; Khan, M.A.; Khan, A.A.; Rahmani, A.H. Natural Products: Implication in Cancer Prevention and Treatment through Modulating Various Biological Activities. Anticancer Agents Med. Chem. 2020, 20, 2025–2040. [Google Scholar] [CrossRef] [PubMed]
- Papachristou, F.; Anninou, N.; Koukoulis, G.; Paraskakis, S.; Sertaridou, E.; Tsalikidis, C.; Pitiakoudis, M.; Simopoulos, C.; Tsaroucha, A. Differential Effects of Cisplatin Combined with the Flavonoid Apigenin on HepG2, Hep3B, and Huh7 Liver Cancer Cell Lines. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2021, 866, 503352. [Google Scholar] [CrossRef] [PubMed]
- Nozhat, Z.; Heydarzadeh, S.; Memariani, Z.; Ahmadi, A. Chemoprotective and Chemosensitizing Effects of Apigenin on Cancer Therapy. Cancer Cell Int. 2021, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.P.; Chou, T.H.; Ding, H.Y.; Chen, P.R.; Chiang, F.Y.; Kuo, P.L.; Liang, C.H. Apigenin Induces Apoptosis via Tumor Necrosis Factor Receptor- and Bcl-2-Mediated Pathway and Enhances Susceptibility of Head and Neck Squamous Cell Carcinoma to 5-Fluorouracil and Cisplatin. Biochim. Biophys. Acta-Gen. Subj. 2012, 1820, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, A.; Le Maitre, C.; Haywood-Small, S.; Cross, N.; Jordan-Mahy, N. Polyphenols Act Synergistically with Doxorubicin and Etoposide in Leukaemia Cell Lines. Cell Death Discov. 2015, 1, 15043. [Google Scholar] [CrossRef]
- Berköz, M.; Yalın, S.; Özkan-Yılmaz, F.; Özlüer-Hunt, A.; Krośniak, M.; Francik, R.; Yunusoğlu, O.; Adıyaman, A.; Gezici, H.; Yiğit, A.; et al. Protective Effect of Myricetin, Apigenin, and Hesperidin Pretreatments on Cyclophosphamide-Induced Immunosuppression. Immunopharmacol. Immunotoxicol. 2021, 43, 353–369. [Google Scholar] [CrossRef]
- Chen, R.Q.; Liu, F.; Qiu, X.Y.; Chen, X.Q. The Prognostic and Therapeutic Value of PD-L1 in Glioma. Front. Pharmacol. 2019, 9, 1503. [Google Scholar] [CrossRef]
- Xu, Y.; Xin, Y.; Diao, Y.; Lu, C.; Fu, J.; Luo, L.; Yin, Z. Synergistic Effects of Apigenin and Paclitaxel on Apoptosis of Cancer Cells. PLoS ONE 2011, 6, e29169. [Google Scholar] [CrossRef]
- Strouch, M.J.; Milam, B.M.; Melstrom, L.G.; McGill, J.J.; Salabat, M.R.; Ujiki, M.B.; Ding, X.Z.; Bentrem, D.J. The Flavonoid Apigenin Potentiates the Growth Inhibitory Effects of Gemcitabine and Abrogates Gemcitabine Resistance in Human Pancreatic Cancer Cells. Pancreas 2009, 38, 409–415. [Google Scholar] [CrossRef]
- Sahindokuyucu-Kocasari, F.; Akyol, Y.; Ozmen, O.; Erdemli-Kose, S.B.; Garli, S. Apigenin Alleviates Methotrexate-Induced Liver and Kidney Injury in Mice. Hum. Exp. Toxicol. 2021, 40, 1721–1731. [Google Scholar] [CrossRef]
- Yu, W.; Sun, H.; Zha, W.; Cui, W.; Xu, L.; Min, Q.; Wu, J. Apigenin Attenuates Adriamycin-Induced Cardiomyocyte Apoptosis via the PI3K/AKT/MTOR Pathway. Evid. Based. Complement. Alternat. Med. 2017, 2017, 2590676. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.F.R.; Rakhshan, K.; Aboutaleb, N.; Nikbakht, F.; Naderi, N.; Bakhshesh, M.; Azizi, Y. Apigenin Attenuates Doxorubicin Induced Cardiotoxicity via Reducing Oxidative Stress and Apoptosis in Male Rats. Life Sci. 2019, 232, 116623. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Khalaf, M.M.; Sadek, S.A.; Abo-Youssef, A.M. Protective Effects of Apigenin and Myricetin against Cisplatin-Induced Nephrotoxicity in Mice. Pharm. Biol. 2017, 55, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.; Habtemariam, S.; Daglia, M.; Nabavi, S. Apigenin and Breast Cancers: From Chemistry to Medicine. Anticancer Agents Med. Chem. 2015, 15, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of Apigenin as a Potent Antioxidant with Emphasis on Bioavailability, Metabolism, Action Mechanism and in Vitro and in Vivo Studies: A Review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Blaut, M. Anaerobic Degradation of Flavonoids by Eubacterium Ramulus. Arch. Microbiol. 2000, 173, 71–75. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef]
- Kim, B.K.; Cho, A.R.; Park, D.J. Enhancing Oral Bioavailability Using Preparations of Apigenin-Loaded W/O/W Emulsions: In Vitro and in Vivo Evaluations. Food Chem. 2016, 206, 85–91. [Google Scholar] [CrossRef]
- Rajendran, I.; Dhandapani, H.; Anantanarayanan, R.; Rajaram, R. Apigenin Mediated Gold Nanoparticle Synthesis and Their Anticancer Effect on Human Epidermoid Carcinoma (A431) Cells. RSC Adv. 2015, 5, 51055–51066. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and Characterization of an Apigenin-Phospholipid Phytosome (APLC) for Improved Solubility, in Vivo Bioavailability, and Antioxidant Potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, M.C.; Piazzini, V.; Bilia, A.R. Nanovettori per La Veicolazione Dei Flavonoidi: Un’opportunità per Aumentarne La Biodisponibilità Orale e l’efficacia. Erbor. Domani 2017, 395, 60–62. [Google Scholar]
- Zhao, L.; Zhang, L.; Meng, L.; Wang, J.; Zhai, G. Design and Evaluation of a Self-Microemulsifying Drug Delivery System for Apigenin. Drug Dev. Ind. Pharm. 2013, 39, 662–669. [Google Scholar] [CrossRef] [PubMed]
- DeRango-Adem, E.F.; Blay, J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Alhajri, A.; Alshehri, S.M.; Elzayat, E.M.; Al Meanazel, O.T.; Shakeel, F.; Noman, O.; Altamimi, M.A.; Alanazi, F.K. Enhancing Oral Bioavailability of Apigenin Using a Bioactive Self-Nanoemulsifying Drug Delivery System (Bio-SNEDDS): In Vitro, in Vivo and Stability Evaluations. Pharmaceutics 2020, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- Kariagina, A.; Doseff, A.I. Anti-Inflammatory Mechanisms of Dietary Flavones: Tapping into Nature to Control Chronic Inflammation in Obesity and Cancer. Int. J. Mol. Sci. 2022, 23, 15753. [Google Scholar] [CrossRef] [PubMed]
- Hanske, L.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. The Bioavailability of Apigenin-7-Glucoside Is Influenced by Human Intestinal Microbiota in Rats 1-3. J. Nutr. 2009, 139, 1095–1102. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity 1,2. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Wan, L.; Guo, C.; Yu, Q.; Li, Y.; Wang, X.; Wang, X.; Chen, C. Quantitative Determination of Apigenin and Its Metabolism in Rat Plasma after Intravenous Bolus Administration by HPLC Coupled with Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 855, 286–289. [Google Scholar] [CrossRef]
- Cai, H.; Boocock, D.J.; Steward, W.P.; Gescher, A.J. Tissue Distribution in Mice and Metabolism in Murine and Human Liver of Apigenin and Tricin, Flavones with Putative Cancer Chemopreventive Properties. Cancer Chemother. Pharmacol. 2007, 60, 257–266. [Google Scholar] [CrossRef]
- Lu, X.Y.; Sun, D.L.; Chen, Z.J.; Chen, T.; Li, L.P.; Xu, Z.H.; Jiang, H.D.; Zeng, S. Relative Contribution of Small and Large Intestine to Deglycosylation and Absorption of Flavonoids from Chrysanthemun Morifolium Extract. J. Agric. Food Chem. 2010, 58, 10661–10667. [Google Scholar] [CrossRef] [PubMed]
- Sandu, N.; Chilom, C.G.; Popescu, A.I. Structural and Molecular Aspects of Flavonoids as Ligands for Serum Transferrin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119600. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Fong, R.Y.; Ensunsa, J.L.; Kimball, J.; Medici, V.; Ottaviani, J.I.; Crozier, A. Absorption, Distribution, Metabolism and Excretion of Apigenin and Its Glycosides in Healthy Male Adults. Free Radic. Biol. Med. 2022, 185, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yi, T.; Lam, C.W.K. Inhibition of Human Efflux Transporter ABCC2 (MRP2) by Self-Emulsifying Drug Delivery System: Influences of Concentration and Combination of Excipients. J. Pharm. Pharm. Sci. 2014, 17, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhou, Q.; Zheng, Z.; Ye, L.; Hu, M.; Liu, Z. A Novel Local Recycling Mechanism That Enhances Enteric Bioavailability of Flavonoids and Prolongs Their Residence Time in the Gut. Mol. Pharm. 2012, 9, 3246–3258. [Google Scholar] [CrossRef] [PubMed]
- Gradolatto, A.; Basly, J.P.; Berges, R.; Teyssier, C.; Chagnon, M.C.; Siess, M.H.; Canivenc-Lavier, M.C. Pharmacokinetics and Metabolism of Apigenin in Female and Male Rats after a Single Oral Administration. Drug Metab. Dispos. 2005, 33, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Mangal, M.; Sagar, P.; Singh, H.; Raghava, G.P.S.; Agarwal, S.M. NPACT: Naturally Occurring Plant-Based Anticancer Compound-Activity-Target Database. Nucleic Acids Res. 2013, 41, D1124. [Google Scholar] [CrossRef]
- Thiery-Vuillemin, A.; Nguyen, T.; Pivot, X.; Spano, J.P.; Dufresnne, A.; Soria, J.C. Molecularly Targeted Agents: Their Promise as Cancer Chemopreventive Interventions. Eur. J. Cancer 2005, 41, 2003–2015. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F.; et al. Apigenin Suppresses PD-L1 Expression in Melanoma and Host Dendritic Cells to Elicit Synergistic Therapeutic Effects. J. Exp. Clin. Cancer Res. 2018, 37, 1–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fossatelli, L.; Maroccia, Z.; Fiorentini, C.; Bonucci, M. Resources for Human Health from the Plant Kingdom: The Potential Role of the Flavonoid Apigenin in Cancer Counteraction. Int. J. Mol. Sci. 2024, 25, 251. https://doi.org/10.3390/ijms25010251
Fossatelli L, Maroccia Z, Fiorentini C, Bonucci M. Resources for Human Health from the Plant Kingdom: The Potential Role of the Flavonoid Apigenin in Cancer Counteraction. International Journal of Molecular Sciences. 2024; 25(1):251. https://doi.org/10.3390/ijms25010251
Chicago/Turabian StyleFossatelli, Laura, Zaira Maroccia, Carla Fiorentini, and Massimo Bonucci. 2024. "Resources for Human Health from the Plant Kingdom: The Potential Role of the Flavonoid Apigenin in Cancer Counteraction" International Journal of Molecular Sciences 25, no. 1: 251. https://doi.org/10.3390/ijms25010251
APA StyleFossatelli, L., Maroccia, Z., Fiorentini, C., & Bonucci, M. (2024). Resources for Human Health from the Plant Kingdom: The Potential Role of the Flavonoid Apigenin in Cancer Counteraction. International Journal of Molecular Sciences, 25(1), 251. https://doi.org/10.3390/ijms25010251






