Hexavalent Chromium Targets Securin to Drive Numerical Chromosome Instability in Human Lung Cells
Abstract
1. Introduction
2. Results
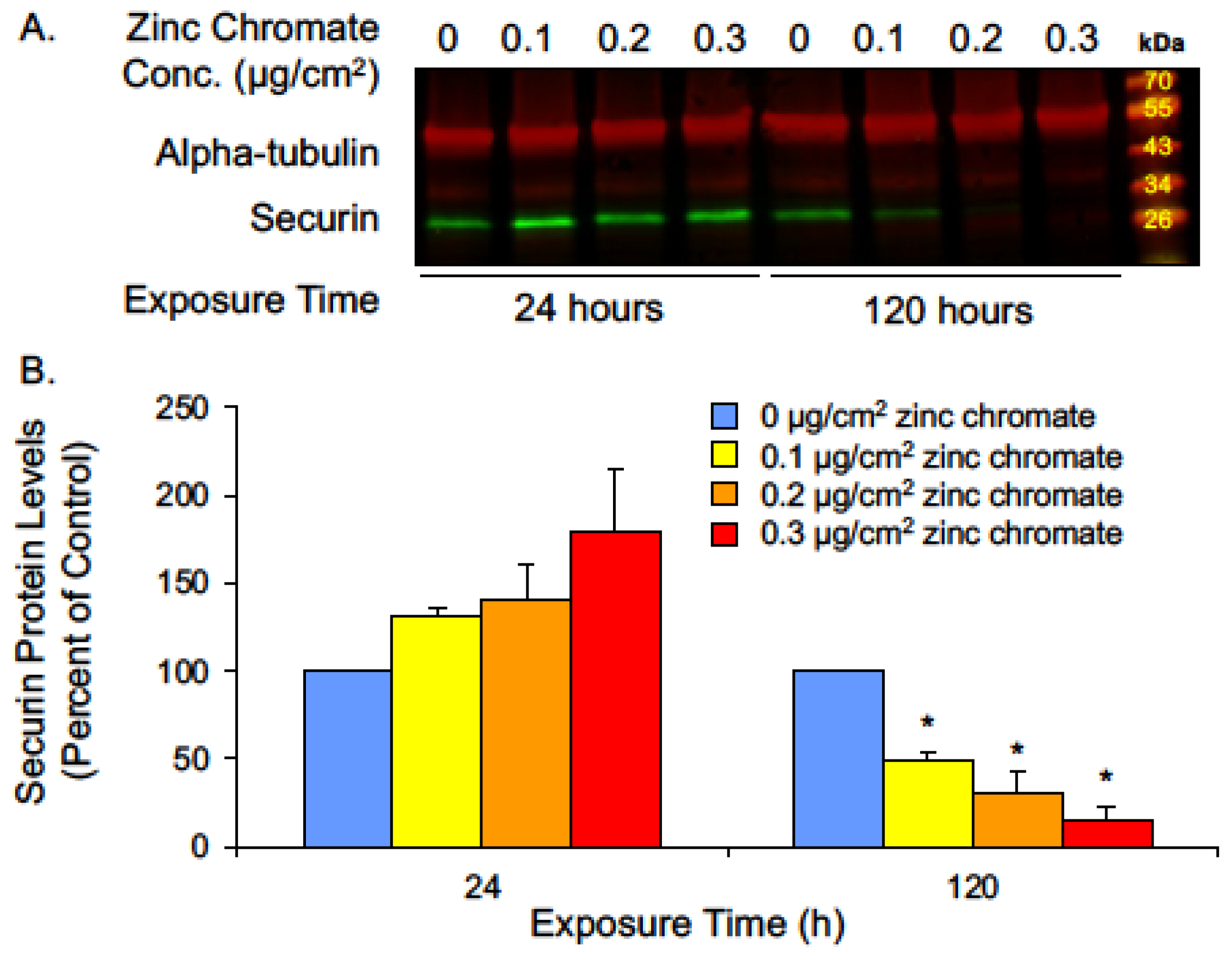
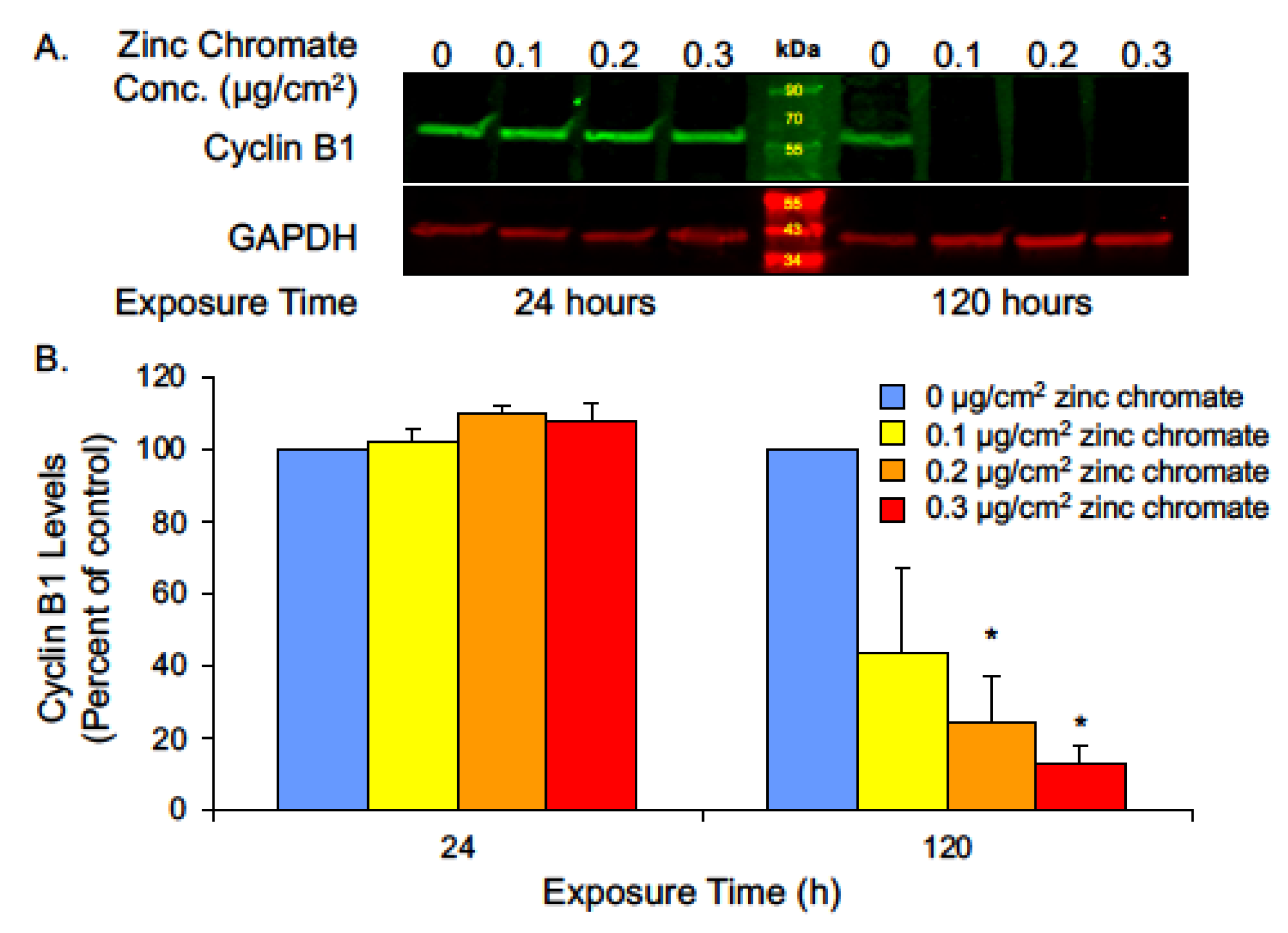
2.1. Prolonged Cr(VI) Exposure Causes Loss of Securin Protein
2.2. Prolonged Cr(VI) Exposure Targets Securin Transcription
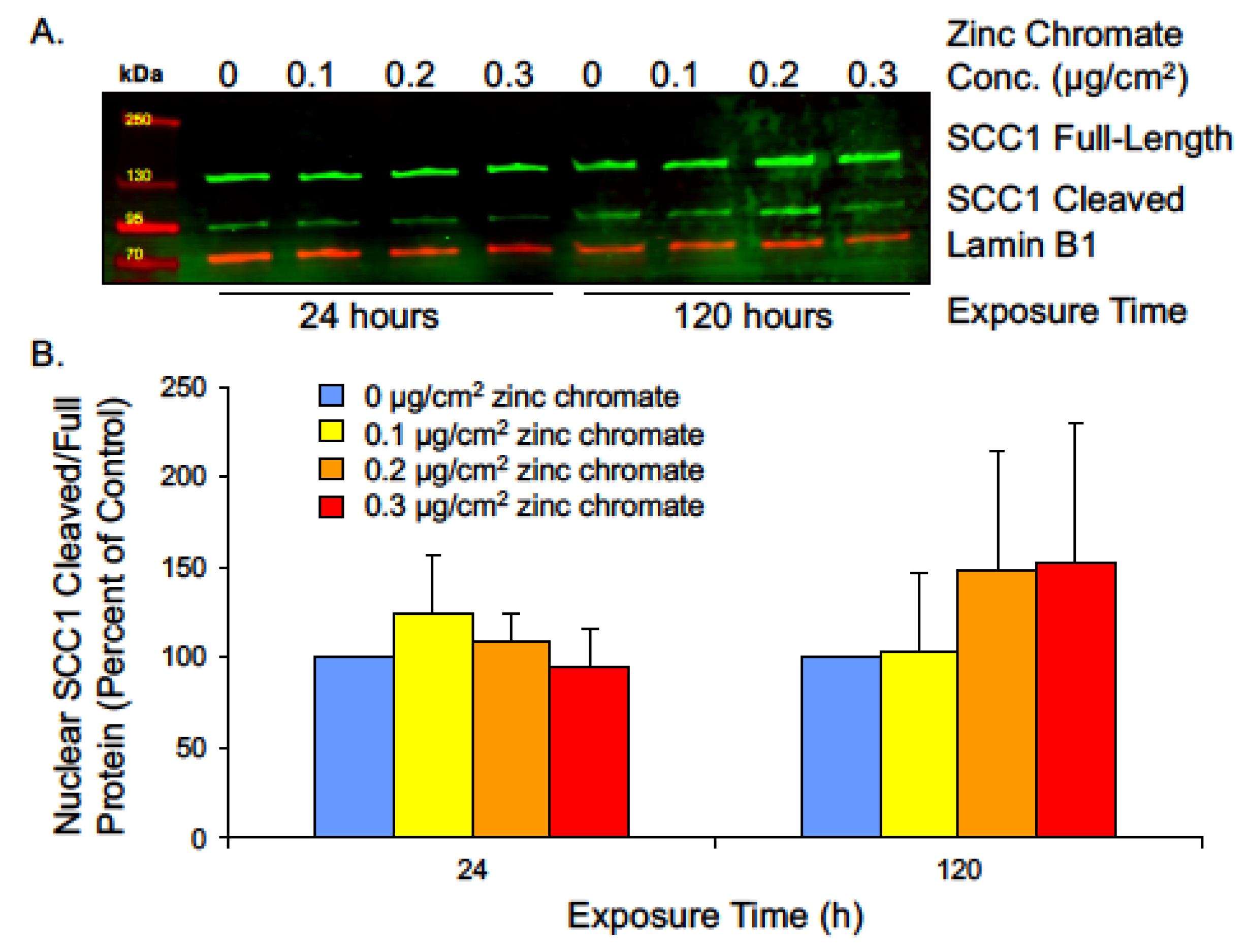
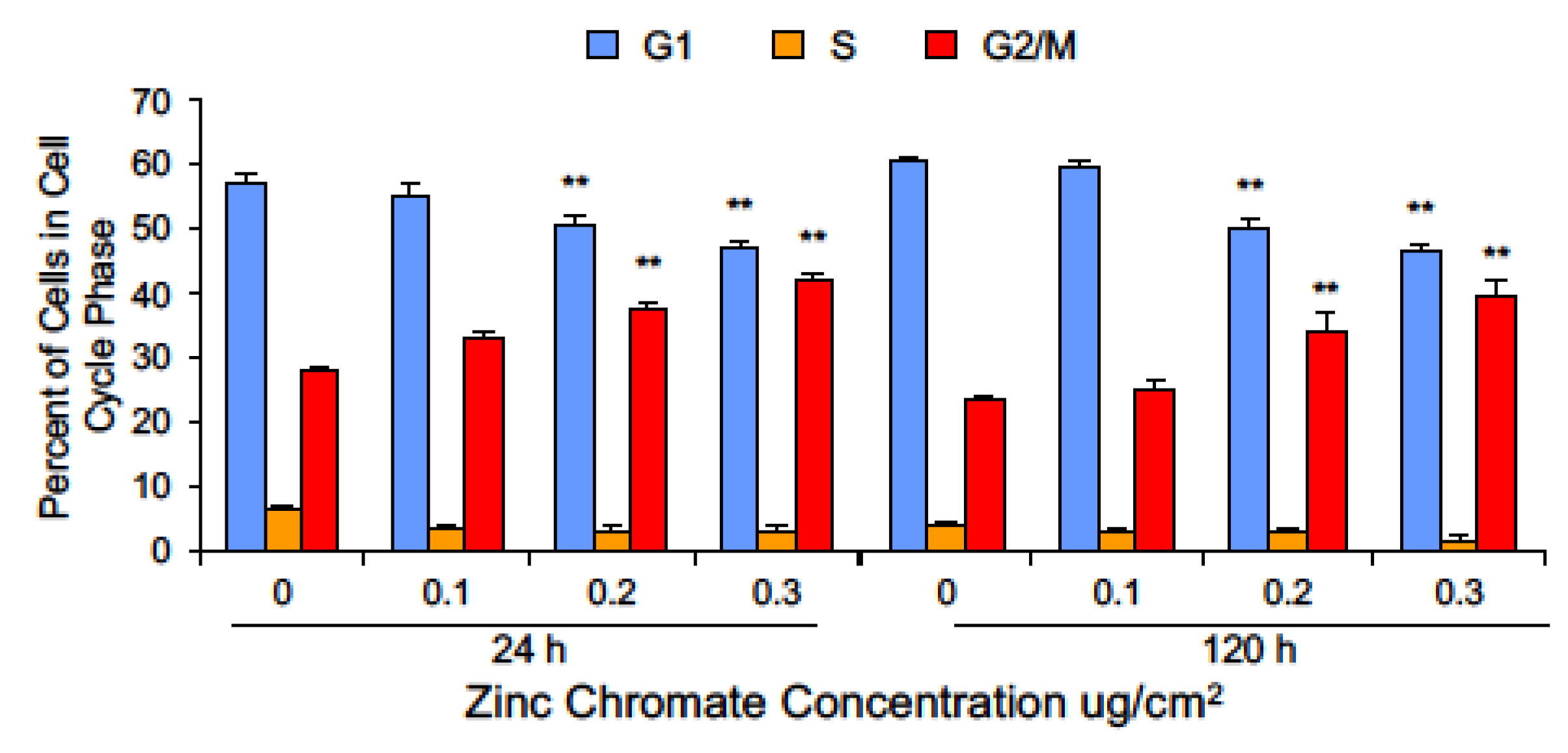
2.3. Prolonged Cr(VI) Exposure Causes Loss of Securin Function
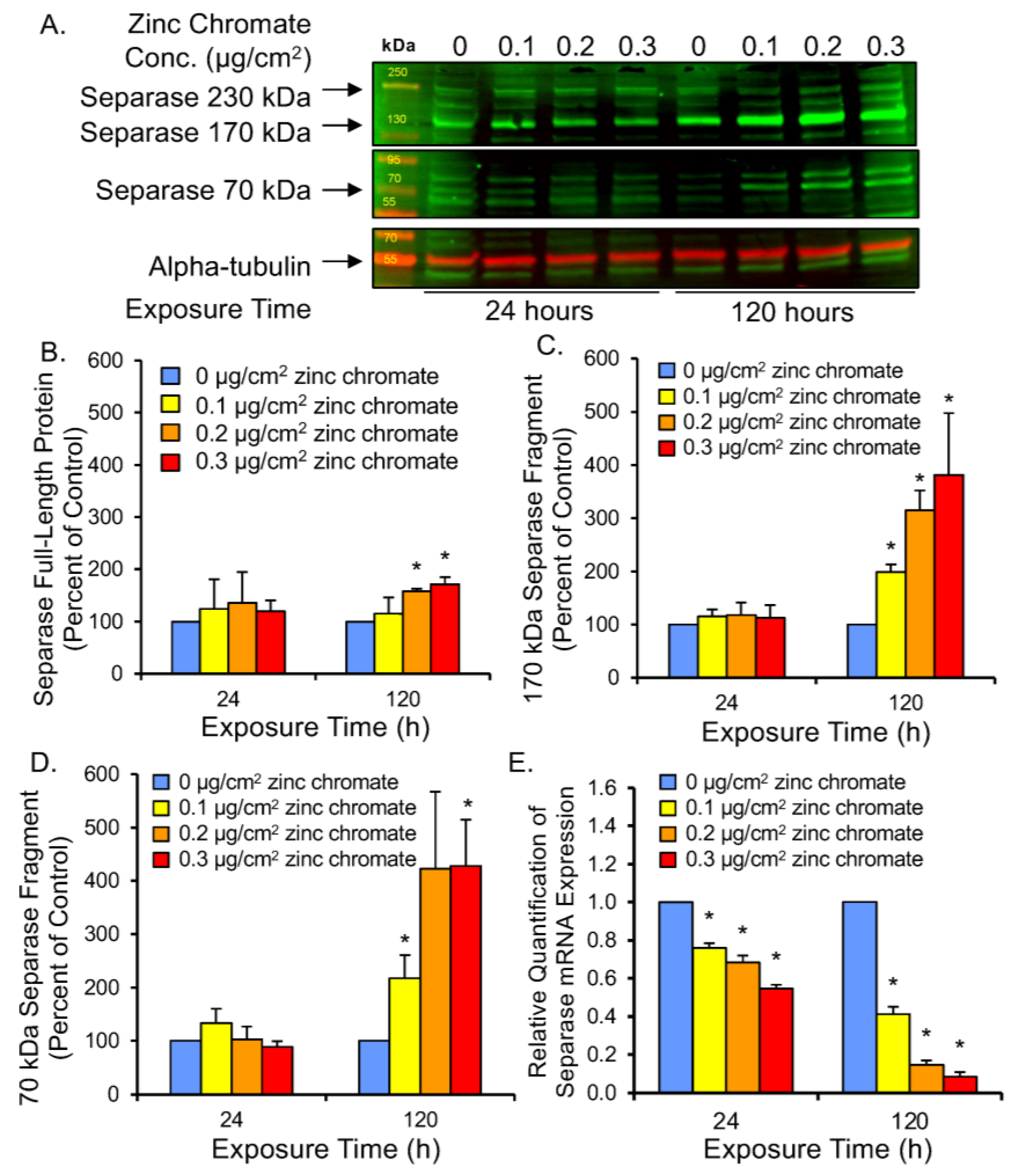
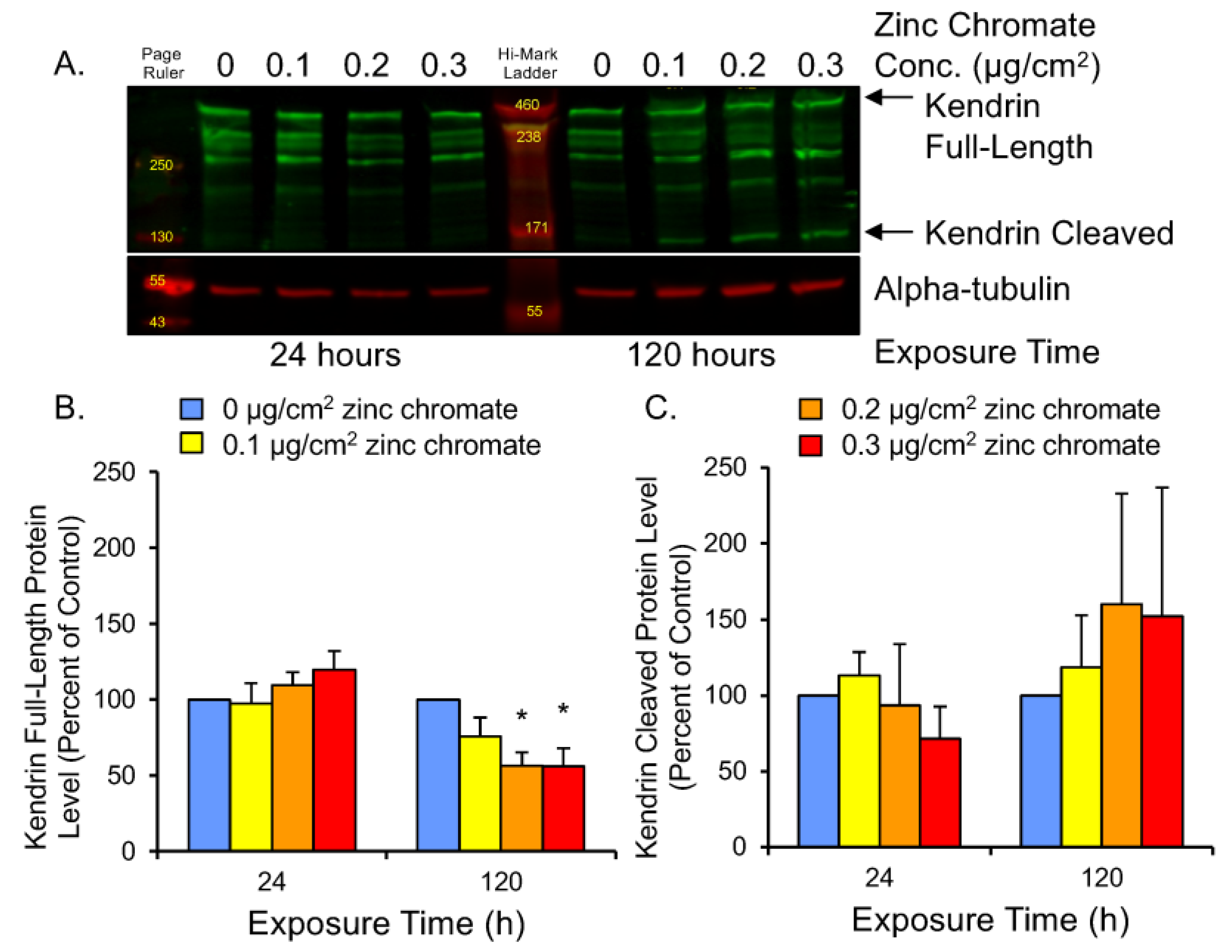
2.4. Prolonged Cr(VI) Exposure Compromises Compensatory Separase Regulators
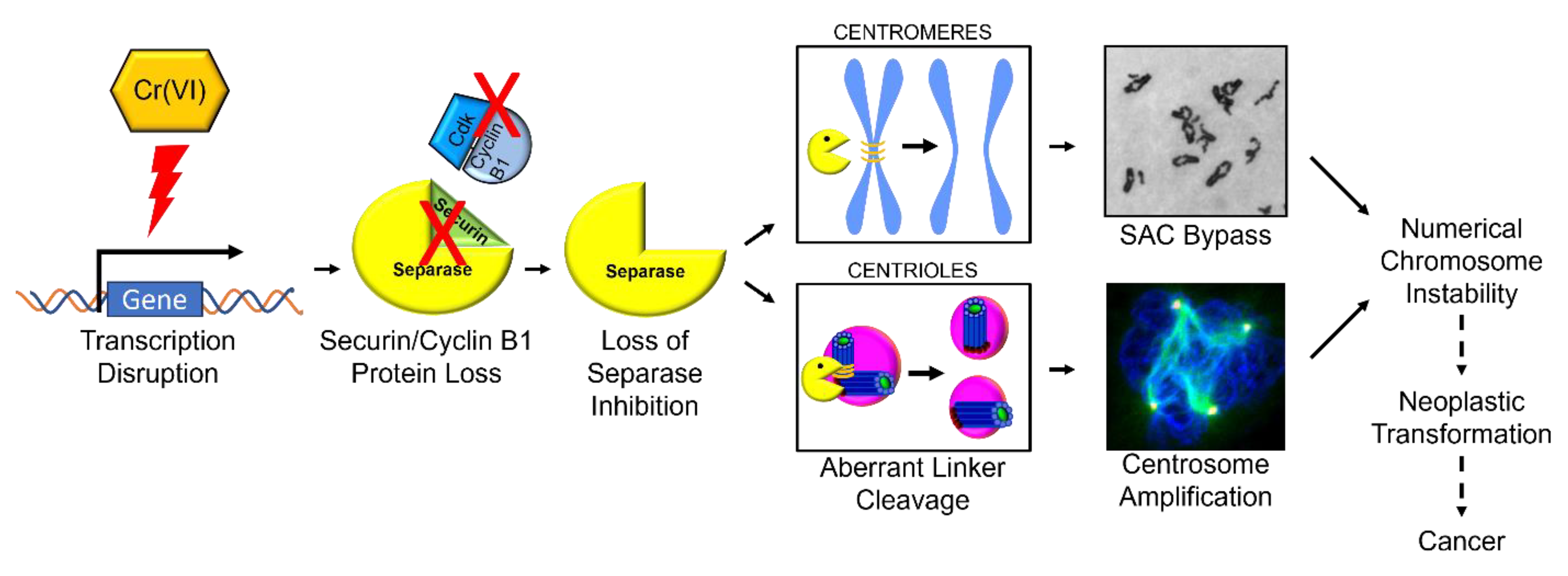
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Preparation of Zinc Chromate and Cell Treatments
4.4. Protein Analysis
4.5. Protein Half-Life Analysis
4.6. RNA Extraction, cDNA Synthesis, and RT-qPCR
4.7. Cell Cycle Analysis
4.8. Cell Authentication
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilbur, S.; Abadin, H.; Fay, M.; Yu, D.; Tencza, B.; Ingerman, L.; Klotzbach, J.; James, S. Toxicological Profile for Chromium; Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2012. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Arsenic, Metals, Fibres and Dusts; International Agency for Research on Cancer: Lyon, France, 2012; ISBN 978-92-832-0135-9. [Google Scholar]
- Langard, S. Role of Chemical Species and Exposure Characteristics in Cancer among Persons Occupationally Exposed to Chromium Compounds. Scand. J. Work. Environ. Health 1993, 19 (Suppl. 1), 81–89. [Google Scholar] [PubMed]
- Sorahan, T.; Burges, D.C.; Waterhouse, J.A. A Mortality Study of Nickel/Chromium Platers. Occup. Environ. Med. 1987, 44, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M.; Easton, D.F.; Bidstrup, P.L. Mortality from Respiratory Cancer and Other Causes in United Kingdom Chromate Production Workers. Occup. Environ. Med. 1991, 48, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Sunderman, F.W. Nasal Toxicity, Carcinogenicity, and Olfactory Uptake of Metals. Ann. Clin. Lab. Sci. 2001, 31, 3–24. [Google Scholar] [PubMed]
- Yatera, K.; Morimoto, Y.; Ueno, S.; Noguchi, S.; Kawaguchi, T.; Tanaka, F.; Suzuki, H.; Higashi, T. Cancer Risks of Hexavalent Chromium in the Respiratory Tract. J. UOEH 2018, 40, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Abe, S.; Kimura, K.; Tsuneta, Y.; Mikami, H.; Murao, M. Lung Cancer in Japanese Chromate Workers. Thorax 1978, 33, 372–374. [Google Scholar] [CrossRef]
- Langard, S.; Vigander, T. Occurrence of Lung Cancer in Workers Producing Chromium Pigments. Br. J. Ind. Med. 1983, 40, 71–74. [Google Scholar] [CrossRef]
- Luippold, R.S.; Mundt, K.A.; Austin, R.P.; Liebig, E.; Panko, J.; Crump, C.; Crump, K.; Proctor, D. Lung Cancer Mortality among Chromate Production Workers. Occup. Environ. Med. 2003, 60, 451–457. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Chromium; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012. [Google Scholar]
- Johnson, J.; Schewel, L.; Graedel, T.E. The Contemporary Anthropogenic Chromium Cycle. Environ. Sci. Technol. 2006, 40, 7060–7069. [Google Scholar] [CrossRef]
- Proctor, D.M.; Otani, J.M.; Finley, B.L.; Paustenbach, D.J.; Bland, J.A.; Speizer, N.; Sargent, E.V. Is Hexavalent Chromium Carcinogenic via Ingestion? A Weight-of-Evidence Review. J. Toxicol. Environ. Health A 2002, 65, 701–746. [Google Scholar] [CrossRef]
- Bucher, J.R. NTP Toxicity Studies of Sodium Dichromate Dihydrate (CAS No. 7789-12-0) Administered in Drinking Water to Male and Female F344/N Rats and B6C3F1 Mice and Male BALB/c and Am3-C57BL/6 Mice. Toxic Rep. Ser. 2007, 72, 1-G4. [Google Scholar]
- Linos, A.; Petralias, A.; Christophi, C.A.; Christoforidou, E.; Kouroutou, P.; Stoltidis, M.; Veloudaki, A.; Tzala, E.; Makris, K.C.; Karagas, M.R. Oral Ingestion of Hexavalent Chromium through Drinking Water and Cancer Mortality in an Industrial Area of Greece—an Ecological Study. Environ. Health 2011, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, J.J.; Sedman, R.M.; Reynolds, S.D.; Sherman, C.D.; Li, L.H.; Howd, R.A.; Sandy, M.S.; Zeise, L.; Alexeeff, G.V. Cancer Mortality in a Chinese Population Exposed to Hexavalent Chromium in Drinking Water. Epidemiology 2008, 19, 12–23. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S.; D’Agostini, F.; Balansky, R.; Micale, R.; Baluce, B.; Izzotti, A. Lack of Genotoxic Effects in Hematopoietic and Gastrointestinal Cells of Mice Receiving Chromium(VI) with the Drinking Water. Mutat. Res. 2008, 659, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. Toxicity and Carcinogenicity of Cr(VI) in Animal Models and Humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Wu, M.L.; Yang, C.C.; Ger, J.; Tsai, W.J.; Deng, J.F. Acute Severe Chromium Poisoning after Dermal Exposure to Hexavalent Chromium. J. Chin. Med. Assoc. 2009, 72, 219–221. [Google Scholar] [CrossRef]
- Paustenbach, D.J.; Finley, B.L.; Mowat, F.S.; Kerger, B.D. Human Health Risk and Exposure Assessment of Chromium (VI) in Tap Water. J. Toxicol. Environ. Health A 2003, 66, 1295–1339. [Google Scholar] [CrossRef]
- Stridsklev, I.C.; Schaller, K.H.; Langard, S. Monitoring of Chromium and Nickel in Biological Fluids of Stainless Steel Welders Using the Flux-Cored-Wire (FCW) Welding Method. Int. Arch. Occup. Environ. Health 2004, 77, 587–591. [Google Scholar] [CrossRef]
- Gao, M.; Levy, L.S.; Braithwaite, R.A.; Brown, S.S. Monitoring of Total Chromium in Rat Fluids and Lymphocytes Following Intratracheal Administration of Soluble Trivalent or Hexavalent Chromium Compounds. Hum. Exp. Toxicol. 1993, 12, 377–382. [Google Scholar] [CrossRef]
- Pan, C.H.; Jeng, H.A.; Lai, C.H. Biomarkers of Oxidative Stress in Electroplating Workers Exposed to Hexavalent Chromium. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 76–83. [Google Scholar] [CrossRef]
- Sutherland, J.E.; Zhitkovich, A.; Kluz, T.; Costa, M. Rats Retain Chromium in Tissues Following Chronic Ingestion of Drinking Water Containing Hexavalent Chromium. Biol. Trace Elem. Res. 2000, 74, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Langard, S.; Gundersen, N.; Tsalev, D.L.; Gylseth, B. Whole Blood Chromium Level and Chromium Excretion in the Rat after Zinc Chromate Inhalation. Acta Pharmacol. Toxicol. 1978, 42, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, M.; Tian, T.; Lin, S.; Xu, P.; Zhou, L.; Dai, C.; Hao, Q.; Wu, Y.; Zhai, Z.; et al. The Effect of Hexavalent Chromium on the Incidence and Mortality of Human Cancers: A Meta-Analysis Based on Published Epidemiological Cohort Studies. Front. Oncol. 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Hino, N.; Sasa, M.; Kamamura, Y.; Sakiyama, S.; Tsuyuguchi, M.; Hashimoto, M.; Uyama, T.; Monden, Y. Mutations of the P53 Gene in Human Lung Cancer from Chromate-Exposed Workers. Biochem. Biophys. Res. Commun. 1997, 239, 95–100. [Google Scholar] [CrossRef]
- Hirose, T.; Kondo, K.; Takahashi, Y.; Ishikura, H.; Fujino, H.; Tsuyuguchi, M.; Hashimoto, M.; Yokose, T.; Mukai, K.; Kodama, T.; et al. Frequent Microsatellite Instability in Lung Cancer from Chromate-Exposed Workers. Mol. Carcinog. 2002, 33, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.L.; Wise, S.S.; Wise, J.P., Sr. Carcinogenicity of Hexavalent Chromium. Indian. J. Med. Res. 2008, 128, 353–372. [Google Scholar] [PubMed]
- Wise, S.S.; Wise, J.P. Aneuploidy as an Early Mechanistic Event in Metal Carcinogenesis. Biochem. Soc. Trans. 2010, 38, 1650–1654. [Google Scholar] [CrossRef]
- Proctor, D.M.; Suh, M.; Campleman, S.L.; Thompson, C.M. Assessment of the Mode of Action for Hexavalent Chromium-Induced Lung Cancer Following Inhalation Exposures. Toxicology 2014, 325, 160–179. [Google Scholar] [CrossRef]
- Holmes, A.L.; Wise, S.S.; Sandwick, S.J.; Lingle, W.L.; Negron, V.C.; Thompson, W.D.; Wise, J.P., Sr. Chronic Exposure to Lead Chromate Causes Centrosome Abnormalities and Aneuploidy in Human Lung Cells. Cancer Res. 2006, 66, 4041–4048. [Google Scholar] [CrossRef]
- Holmes, A.L.; Wise, S.S.; Pelsue, S.C.; Aboueissa, A.M.; Lingle, W.; Salisbury, J.; Gallagher, J.; Wise, J.P., Sr. Chronic Exposure to Zinc Chromate Induces Centrosome Amplification and Spindle Assembly Checkpoint Bypass in Human Lung Fibroblasts. Chem. Res. Toxicol. 2010, 23, 386–395. [Google Scholar] [CrossRef]
- Wise, S.S.; Aboueissa, A.E.-M.; Martino, J.; Wise, J.P. Hexavalent Chromium-Induced Chromosome Instability Drives Permanent and Heritable Numerical and Structural Changes and a DNA Repair-Deficient Phenotype. Cancer Res. 2018, 78, 4203–4214. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.I.; Hsu, S.H.; Tsou, T.C. Depletion of Securin Increases Arsenite-Induced Chromosome Instability and Apoptosis via a P53-Independent Pathway. Toxicol. Sci. 2006, 90, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Jallepalli, P.V.; Waizenegger, I.C.; Bunz, F.; Langer, S.; Speicher, M.R.; Peters, J.M.; Kinzler, K.W.; Vogelstein, B.; Lengauer, C. Securin Is Required for Chromosomal Stability in Human Cells. Cell 2001, 105, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; McGarry, T.J.; Bernal, T.; Kirschner, M.W. Identification of a Vertebrate Sister-Chromatid Separation Inhibitor Involved in Transformation and Tumorigenesis. Science 1999, 285, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Inanc, B.; Dodson, H.; Morrison, C.G. A Centrosome-Autonomous Signal That Involves Centriole Disengagement Permits Centrosome Duplication in G2 Phase after DNA Damage. Mol. Biol. Cell 2010, 21, 3866–3877. [Google Scholar] [CrossRef] [PubMed]
- Tsou, M.F.; Stearns, T. Controlling Centrosome Number: Licenses and Blocks. Curr. Opin. Cell Biol. 2006, 18, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Tsou, M.F.; Stearns, T. Mechanism Limiting Centrosome Duplication to Once per Cell Cycle. Nature 2006, 442, 947–951. [Google Scholar] [CrossRef]
- Agircan, F.G.; Schiebel, E.; Mardin, B.R. Separate to Operate: Control of Centrosome Positioning and Separation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 25047615. [Google Scholar] [CrossRef]
- Baum, P.; Yip, C.; Goetsch, L.; Byers, B. A Yeast Gene Essential for Regulation of Spindle Pole Duplication. Mol. Cell. Biol. 1988, 8, 5386–5397. [Google Scholar] [CrossRef]
- Fukasawa, K. Centrosome Amplification, Chromosome Instability and Cancer Development. Cancer Lett. 2005, 230, 6–19. [Google Scholar] [CrossRef]
- Jusino, S.; Fernandez-Padin, F.M.; Saavedra, H.I. Centrosome Aberrations and Chromosome Instability Contribute to Tumorigenesis and Intra-Tumor Heterogeneity. J. Cancer Metastasis Treat. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Sluder, G.; Nordberg, J.J. The Good, the Bad and the Ugly: The Practical Consequences of Centrosome Amplification. Curr. Opin. Cell Biol. 2004, 16, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Guacci, V.; Koshland, D. Pds1p, an Inhibitor of Anaphase in Budding Yeast, Plays a Critical Role in the APC and Checkpoint Pathway(s). J. Cell Biol. 1996, 133, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Fix, O.; Peters, J.M.; Kirschner, M.W.; Koshland, D. Anaphase Initiation in Saccharomyces Cerevisiae Is Controlled by the APC-Dependent Degradation of the Anaphase Inhibitor Pds1p. Genes Dev. 1996, 10, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Funabiki, H.; Kumada, K.; Yanagida, M. Fission Yeast Cut1 and Cut2 Are Essential for Sister Chromatid Separation, Concentrate along the Metaphase Spindle and Form Large Complexes. EMBO J. 1996, 15, 6617–6628. [Google Scholar] [CrossRef] [PubMed]
- Waizenegger, I.; Gimenez-Abian, J.F.; Wernic, D.; Peters, J.M. Regulation of Human Separase by Securin Binding and Autocleavage. Curr. Biol. 2002, 12, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.; Holmes, A.L.; Xie, H.; Wise, S.S.; Wise, J.P., Sr. Chronic Exposure to Particulate Chromate Induces Premature Centrosome Separation and Centriole Disengagement in Human Lung Cells. Toxicol. Sci. 2015, 147, 490–499. [Google Scholar] [CrossRef][Green Version]
- Wise, S.S.; Holmes, A.L.; Xie, H.; Thompson, W.D.; Wise, J.P., Sr. Chronic Exposure to Particulate Chromate Induces Spindle Assembly Checkpoint Bypass in Human Lung Cells. Chem. Res. Toxicol. 2006, 19, 1492–1498. [Google Scholar] [CrossRef]
- Zhou, Y.; Mehta, K.R.; Choi, A.P.; Scolavino, S.; Zhang, X. DNA Damage-Induced Inhibition of Securin Expression Is Mediated by P53. J. Biol. Chem. 2003, 278, 462–470. [Google Scholar] [CrossRef]
- Kakar, S.S. Molecular Cloning, Genomic Organization, and Identification of the Promoter for the Human Pituitary Tumor Transforming Gene (PTTG). Gene 1999, 240, 317–324. [Google Scholar] [CrossRef]
- Hellmuth, S.; Pohlmann, C.; Brown, A.; Bottger, F.; Sprinzl, M.; Stemmann, O. Positive and Negative Regulation of Vertebrate Separase by Cdk1-Cyclin B1 May Explain Why Securin Is Dispensable. J. Biol. Chem. 2015, 290, 8002–8010. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Yanagida, M. Regulating Sister Chromatid Separation by Separase Phosphorylation. Dev. Cell 2002, 2, 2–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stemmann, O.; Zou, H.; Gerber, S.A.; Gygi, S.P.; Kirschner, M.W. Dual Inhibition of Sister Chromatid Separation at Metaphase. Cell 2001, 107, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Waizenegger, I.C.; Hauf, S.; Meinke, A.; Peters, J.M. Two Distinct Pathways Remove Mammalian Cohesin from Chromosome Arms in Prophase and from Centromeres in Anaphase. Cell 2000, 103, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Nishimura, T.; Hayakawa, A.; Ono, Y.; Takahashi, M. Involvement of a Centrosomal Protein Kendrin in the Maintenance of Centrosome Cohesion by Modulating Nek2A Kinase Activity. Biochem. Biophys. Res. Commun. 2010, 398, 217–223. [Google Scholar] [CrossRef]
- Matsuo, K.; Ohsumi, K.; Iwabuchi, M.; Kawamata, T.; Ono, Y.; Takahashi, M. Kendrin Is a Novel Substrate for Separase Involved in the Licensing of Centriole Duplication. Curr. Biol. 2012, 22, 915–921. [Google Scholar] [CrossRef]
- Loncarek, J.; Hergert, P.; Khodjakov, A. Centriole Reduplication during Prolonged Interphase Requires Procentriole Maturation Governed by Plk1. Curr. Biol. 2010, 20, 1277–1282. [Google Scholar] [CrossRef]
- Tsou, M.F.; Wang, W.J.; George, K.A.; Uryu, K.; Stearns, T.; Jallepalli, P.V. Polo Kinase and Separase Regulate the Mitotic Licensing of Centriole Duplication in Human Cells. Dev. Cell 2009, 17, 344–354. [Google Scholar] [CrossRef]
- Schockel, L.; Mockel, M.; Mayer, B.; Boos, D.; Stemmann, O. Cleavage of Cohesin Rings Coordinates the Separation of Centrioles and Chromatids. Nat. Cell Biol. 2011, 13, 966–972. [Google Scholar] [CrossRef]
- Agircan, F.G.; Schiebel, E. Sensors at Centrosomes Reveal Determinants of Local Separase Activity. PLoS Genet. 2014, 10, e1004672. [Google Scholar] [CrossRef]
- Mei, J.; Huang, X.; Zhang, P. Securin Is Not Required for Cellular Viability, but Is Required for Normal Growth of Mouse Embryonic Fibroblasts. Curr. Biol. 2001, 11, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Pfleghaar, K.; Heubes, S.; Cox, J.; Stemmann, O.; Speicher, M.R. Securin Is Not Required for Chromosomal Stability in Human Cells. PLoS Biol. 2005, 3, e416. [Google Scholar] [CrossRef] [PubMed]
- Gorr, I.H.; Boos, D.; Stemmann, O. Mutual Inhibition of Separase and Cdk1 by Two-Step Complex Formation. Mol. Cell 2005, 19, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.J.; Taylor, S.S. Cyclin-B1-Mediated Inhibition of Excess Separase Is Required for Timely Chromosome Disjunction. J. Cell Sci. 2006, 119, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.S.; Martin, P.A.; Bidstrup, P.L. Investigation of the Potential Carcinogenicity of a Range of Chromium Containing Materials on Rat Lung. Br. J. Ind. Med. 1986, 43, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kondo, K.; Ishikawa, S.; Uchihara, H.; Fujino, H.; Sawada, N.; Miyoshi, T.; Sakiyama, S.; Izumi, K.; Monden, Y. Microscopic Analysis of the Chromium Content in the Chromium-Induced Malignant and Premalignant Bronchial Lesions of the Rat. Environ. Res. 2005, 99, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Tsuneta, Y.; Ohsaki, Y.; Kimura, K.; Mikami, H.; Abe, S.; Murao, M. Chromium Content of Lungs of Chromate Workers with Lung Cancer. Thorax 1980, 35, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.P., Sr.; Wise, S.S.; Little, J.E. The Cytotoxicity and Genotoxicity of Particulate and Soluble Hexavalent Chromium in Human Lung Cells. Mutat. Res. 2002, 517, 221–229. [Google Scholar] [CrossRef]
- Xie, H.; Holmes, A.L.; Wise, S.S.; Huang, S.; Peng, C.; Wise, J.P., Sr. Neoplastic Transformation of Human Bronchial Cells by Lead Chromate Particles. Am. J. Respir. Cell Mol. Biol. 2007, 37, 544–552. [Google Scholar] [CrossRef]
- Suzuki, Y. Synergism of Ascorbic Acid and Glutathione in the Reduction of Hexavalent Chromium In Vitro. Ind. Health 1990, 28, 9–19. [Google Scholar] [CrossRef]
- Wetterhahn, K.E.; Hamilton, J.W. Molecular Basis of Hexavalent Chromium Carcinogenicity: Effect on Gene Expression. Sci. Total Environ. 1989, 86, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Wetterhahn, K.E.; Hamilton, J.W.; Aiyar, J.; Borges, K.M.; Floyd, R. Mechanism of Chromium(VI) Carcinogenesis. Reactive Intermediates and Effect on Gene Expression. Biol. Trace Elem. Res. 1989, 21, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Borges, K.M.; Wetterhahn, K.E. Chromium Cross-Links Glutathione and Cysteine to DNA. Carcinogenesis 1989, 10, 2165–2168. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Lockart, M.M.; Thomas, C.S.; Bowman, M.K.; Woski, S.A.; Vincent, J.B. Molecular Structure of Binary Chromium(III)-DNA Adducts. Chembiochem 2020, 21, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Hneihen, A.S.; Standeven, A.M.; Wetterhahn, K.E. Differential Binding of Chromium(VI) and Chromium(III) Complexes to Salmon Sperm Nuclei and Nuclear DNA and Isolated Calf Thymus DNA. Carcinogenesis 1993, 14, 1795–1803. [Google Scholar] [CrossRef]
- Peterson, R.L.; Banker, K.J.; Garcia, T.Y.; Works, C.F. Isolation of a Novel Chromium(III) Binding Protein from Bovine Liver Tissue after Chromium(VI) Exposure. J. Inorg. Biochem. 2008, 102, 833–841. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Funk, L.C.; Zasadil, L.M.; Weaver, B.A. Living in CIN: Mitotic Infidelity and Its Consequences for Tumor Promotion and Suppression. Dev. Cell 2016, 39, 638–652. [Google Scholar] [CrossRef]
- Palumbo, E.; Russo, A. Chromosome Imbalances in Cancer: Molecular Cytogenetics Meets Genomics. Cytogenet. Genome Res. 2016, 150, 176–184. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic Instability--an Evolving Hallmark of Cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Slade, P.G.; Hailer, M.K.; Martin, B.D.; Sugden, K.D. Guanine-Specific Oxidation of Double-Stranded DNA by Cr(VI) and Ascorbic Acid Forms Spiroiminodihydantoin and 8-Oxo-2′-Deoxyguanosine. Chem. Res. Toxicol. 2005, 18, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Messer, J.; Reynolds, M.; Stoddard, L.; Zhitkovich, A. Causes of DNA Single-Strand Breaks during Reduction of Chromate by Glutathione in Vitro and in Cells. Free Radic. Biol. Med. 2006, 40, 1981–1992. [Google Scholar] [CrossRef] [PubMed]
- Browning, C.L.; Qin, Q.; Kelly, D.F.; Prakash, R.; Vanoli, F.; Jasin, M.; Wise, J.P. Prolonged Particulate Hexavalent Chromium Exposure Suppresses Homologous Recombination Repair in Human Lung Cells. Toxicol. Sci. 2016, 153, 70–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ha, L.; Ceryak, S.; Patierno, S.R. Generation of S Phase-Dependent DNA Double-Strand Breaks by Cr(VI) Exposure: Involvement of ATM in Cr(VI) Induction of γ-H2AX. Carcinogenesis 2004, 25, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, S.F.; Silkworth, W.T.; Nardi, I.K.; Nicholson, J.M.; Compton, D.A.; Cimini, D. The Mitotic Origin of Chromosomal Instability. Curr. Biol. 2014, 24, R148–R149. [Google Scholar] [CrossRef] [PubMed]
- Cahill, D.P.; Lengauer, C.; Yu, J.; Riggins, G.J.; Willson, J.K.; Markowitz, S.D.; Kinzler, K.W.; Vogelstein, B. Mutations of Mitotic Checkpoint Genes in Human Cancers. Nature 1998, 392, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Cimini, D. Merotelic Kinetochore Orientation, Aneuploidy, and Cancer. Biochim. Biophys. Acta 2008, 1786, 32–40. [Google Scholar] [CrossRef]
- Duesberg, P.; Rausch, C.; Rasnick, D.; Hehlmann, R. Genetic Instability of Cancer Cells Is Proportional to Their Degree of Aneuploidy. Proc. Natl. Acad. Sci. USA 1998, 95, 13692–13697. [Google Scholar] [CrossRef]
- Uhlmann, F.; Lottspeich, F.; Nasmyth, K. Sister-Chromatid Separation at Anaphase Onset Is Promoted by Cleavage of the Cohesin Subunit Scc1. Nature 1999, 400, 37–42. [Google Scholar] [CrossRef]
- Lin, Z.; Luo, X.; Yu, H. Structural Basis of Cohesin Cleavage by Separase. Nature 2016, 532, 131–134. [Google Scholar] [CrossRef]
- Luo, S.; Tong, L. Structure and Function of the Separase-Securin Complex. Subcell. Biochem. 2021, 96, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Hagting, A.; Den Elzen, N.; Vodermaier, H.C.; Waizenegger, I.C.; Peters, J.M.; Pines, J. Human Securin Proteolysis Is Controlled by the Spindle Checkpoint and Reveals When the APC/C Switches from Activation by Cdc20 to Cdh1. J. Cell Biol. 2002, 157, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Tong, L. Structural Biology of the Separase-Securin Complex with Crucial Roles in Chromosome Segregation. Curr. Opin. Struct. Biol. 2018, 49, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Raia, P.; Ghent, C.M.; Raisch, T.; Sadian, Y.; Cavadini, S.; Sabale, P.M.; Barford, D.; Raunser, S.; Morgan, D.O.; et al. Structural Basis of Human Separase Regulation by Securin and CDK1-Cyclin B1. Nature 2021, 596, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Wang, Y.; Yao, C.; Jin, L.; Wang, X.; Xiao, Y.; Wu, N.; Song, P.; Song, Y.; Tan, Y.; et al. Role of DNA Methylation in Cell Cycle Arrest Induced by Cr (VI) in Two Cell Lines. PLoS ONE 2013, 8, e71031. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Santos, C.; Bastos, V.; Oliveira, H. Cr(VI)-Induced Genotoxicity and Cell Cycle Arrest in Human Osteoblast Cell Line MG-63. J. Appl. Toxicol. 2019, 39, 1057–1065. [Google Scholar] [CrossRef]
- Xiao, F.; Feng, X.; Zeng, M.; Guan, L.; Hu, Q.; Zhong, C. Hexavalent Chromium Induces Energy Metabolism Disturbance and P53-Dependent Cell Cycle Arrest via Reactive Oxygen Species in L-02 Hepatocytes. Mol. Cell. Biochem. 2012, 371, 65–76. [Google Scholar] [CrossRef]
- Xie, H.; Holmes, A.L.; Wise, S.S.; Young, J.L.; Wise, J.T.; Wise, J.P., Sr. Human Skin Cells Are More Sensitive than Human Lung Cells to the Cytotoxic and Cell Cycle Arresting Impacts of Particulate and Soluble Hexavalent Chromium. Biol. Trace Elem. Res. 2015, 166, 49–56. [Google Scholar] [CrossRef][Green Version]
- Kaltreider, R.C.; Pesce, C.A.; Ihnat, M.A.; Lariviere, J.P.; Hamilton, J.W. Differential Effects of Arsenic(III) and Chromium(VI) on Nuclear Transcription Factor Binding. Mol. Carcinog. 1999, 25, 219–229. [Google Scholar] [CrossRef]
- Raja, N.S.; Nair, B.U. Chromium(III) Complexes Inhibit Transcription Factors Binding to DNA and Associated Gene Expression. Toxicology 2008, 251, 61–65. [Google Scholar] [CrossRef]
- Clem, A.L.; Hamid, T.; Kakar, S.S. Characterization of the Role of Sp1 and NF-Y in Differential Regulation of PTTG/Securin Expression in Tumor Cells. Gene 2003, 322, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.E.; Ghiassi-Nejad, Z.; Paris, A.J.; Yea, S.; Narla, G.; Walsh, M.; Friedman, S.L. Tumor Suppressor Activity of KLF6 Mediated by Downregulation of the PTTG1 Oncogene. FEBS Lett. 2010, 584, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.K.; Kondo, K.; Namura, T.; Senba, Y.; Takizawa, H.; Nakagawa, Y.; Toba, H.; Kenzaki, K.; Sakiyama, S.; Tangoku, A. Aberrant DNA Methylation of Some Tumor Suppressor Genes in Lung Cancers from Workers with Chromate Exposure: DNA methylation of tumor suppressor genes. Mol. Carcinog. 2011, 50, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Takahashi, Y.; Hirose, Y.; Nagao, T.; Tsuyuguchi, M.; Hashimoto, M.; Ochiai, A.; Monden, Y.; Tangoku, A. The Reduced Expression and Aberrant Methylation of p16INK4a in Chromate Workers with Lung Cancer. Lung Cancer 2006, 53, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, P.; Li, Y.; Wang, T.; Gao, X.; Zhang, W.; Jia, G. Methylation Levels of P16 and TP53 That Are Involved in DNA Strand Breakage of 16HBE Cells Treated by Hexavalent Chromium. Toxicol. Lett. 2016, 249, 15–21. [Google Scholar] [CrossRef] [PubMed]
- VonHandorf, A.; Sanchez-Martin, F.J.; Biesiada, J.; Zhang, H.; Zhang, X.; Medvedovic, M.; Puga, A. Chromium Disrupts Chromatin Organization and CTCF Access to Its Cognate Sites in Promoters of Differentially Expressed Genes. Epigenetics 2018, 13, 363–375. [Google Scholar] [CrossRef] [PubMed]
- VonHandorf, A.; Zablon, H.A.; Puga, A. Hexavalent Chromium Disrupts Chromatin Architecture. Semin. Cancer Biol. 2021, 76, 54–60. [Google Scholar] [CrossRef]
- Speer, R.M.; Meaza, I.; Toyoda, J.H.; Lu, Y.; Xu, Q.; Walter, R.B.; Kong, M.; Lu, H.; Kouokam, J.C.; Wise, J.P., Sr. Particulate Hexavalent Chromium Alters microRNAs in Human Lung Cells That Target Key Carcinogenic Pathways. Toxicol. Appl. Pharmacol. 2022, 438, 115890. [Google Scholar] [CrossRef]
- Wise, S.S.; Holmes, A.L.; Ketterer, M.E.; Hartsock, W.J.; Fomchenko, E.; Katsifis, S.; Thompson, W.D.; Wise, J.P., Sr. Chromium Is the Proximate Clastogenic Species for Lead Chromate-Induced Clastogenicity in Human Bronchial Cells. Mutat. Res. 2004, 560, 79–89. [Google Scholar] [CrossRef]
- Kondo, K.; Takahashi, Y.; Ishikawa, S.; Uchihara, H.; Hirose, Y.; Yoshizawa, K.; Tsuyuguchi, M.; Takizawa, H.; Miyoshi, T.; Sakiyama, S.; et al. Microscopic Analysis of Chromium Accumulation in the Bronchi and Lung of Chromate Workers. Cancer 2003, 98, 2420–2429. [Google Scholar] [CrossRef]
- Xie, H.; Holmes, A.L.; Young, J.L.; Qin, Q.; Joyce, K.; Pelsue, S.C.; Peng, C.; Wise, S.S.; Jeevarajan, A.S.; Wallace, W.T.; et al. Zinc Chromate Induces Chromosome Instability and DNA Double Strand Breaks in Human Lung Cells. Toxicol. Appl. Pharmacol. 2009, 234, 293–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Speer, R.M.; Toyoda, J.H.; Croom-Perez, T.J.; Liu, K.J.; Wise, J.P. Particulate Hexavalent Chromium Inhibits E2F1 Leading to Reduced RAD51 Nuclear Foci Formation in Human Lung Cells. Toxicol. Sci. 2021, 181, 35–46. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyoda, J.H.; Martino, J.; Speer, R.M.; Meaza, I.; Lu, H.; Williams, A.R.; Bolt, A.M.; Kouokam, J.C.; Aboueissa, A.E.-M.; Wise, J.P., Sr. Hexavalent Chromium Targets Securin to Drive Numerical Chromosome Instability in Human Lung Cells. Int. J. Mol. Sci. 2024, 25, 256. https://doi.org/10.3390/ijms25010256
Toyoda JH, Martino J, Speer RM, Meaza I, Lu H, Williams AR, Bolt AM, Kouokam JC, Aboueissa AE-M, Wise JP Sr. Hexavalent Chromium Targets Securin to Drive Numerical Chromosome Instability in Human Lung Cells. International Journal of Molecular Sciences. 2024; 25(1):256. https://doi.org/10.3390/ijms25010256
Chicago/Turabian StyleToyoda, Jennifer H., Julieta Martino, Rachel M. Speer, Idoia Meaza, Haiyan Lu, Aggie R. Williams, Alicia M. Bolt, Joseph Calvin Kouokam, Abou El-Makarim Aboueissa, and John Pierce Wise, Sr. 2024. "Hexavalent Chromium Targets Securin to Drive Numerical Chromosome Instability in Human Lung Cells" International Journal of Molecular Sciences 25, no. 1: 256. https://doi.org/10.3390/ijms25010256
APA StyleToyoda, J. H., Martino, J., Speer, R. M., Meaza, I., Lu, H., Williams, A. R., Bolt, A. M., Kouokam, J. C., Aboueissa, A. E.-M., & Wise, J. P., Sr. (2024). Hexavalent Chromium Targets Securin to Drive Numerical Chromosome Instability in Human Lung Cells. International Journal of Molecular Sciences, 25(1), 256. https://doi.org/10.3390/ijms25010256





