The Potential of Congo Red Supplied Aggregates of Multitargeted Tyrosine Kinase Inhibitor (Sorafenib, BAY-43-9006) in Enhancing Therapeutic Impact on Bladder Cancer
Abstract
:1. Introduction
2. Results
2.1. Effect of Sorafenib (Alone) and in Aggregates with Congo Red on the Viability and Survival of Bladder Cancer Cells
2.2. Effect of Sorafenib (Alone) and in Aggregate with Congo Red (5:1) on Human Uroepithelial SV-HUC-1
2.3. BAY-43-9006 Inhibitor and Its Congo Red Aggregates Diminishes Migration and Invasion of Bladder Cancer Cells
2.4. Effect of Sorafenib (Alone) and in Aggregate with Congo Red on the Cytoskeleton and Nanomechanical Properties of Bladder Cancer Cells
2.5. Sorafenib and Its Congo Red Aggregates Affect the Phosphorylation Level of Akt and Erk1/2 Kinase in Bladder Cancer Cells
2.6. Analysis of Aggregates Formation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Treatments of the Cells
4.3. Cell Metabolic Viability Assays
- -
- Gi is the growth inhibition,
- -
- Ai is the average absorbance after incubation with the compounds,
- -
- A0 is the average absorbance of the control at the start of the experiment,
- -
- Ak is the average absorbance of the control after incubation without the compounds.
4.4. Cell Survival Analysis by Flow Cytometry
4.5. Caspase-3 Activity Assay
4.6. Cell Cytotoxicity by LDH Assay
4.7. Scratch Assay
4.8. Chemotaxis and Invasion through Matrigel
4.9. Cell Elasticity Measurements
4.10. Cytoskeleton Analysis
4.11. Western Blot Analysis
4.12. Sample Preparation
4.13. Dynamic Light Scattering (DLS)
4.14. UV-VIS
4.15. Electrophoresis
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobo, N.; Afferi, L.; Moschini, M.; Mostafid, H.; Porten, S.; Psutka, S.P.; Gupta, S.; Smith, A.B.; Williams, S.B.; Lotan, Y. Epidemiology, Screening, and Prevention of Bladder Cancer. Eur. Urol. Oncol. 2022, 5, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yu, W.; Yang, X.; Wu, C.; Cheng, F. Traditional Classification and Novel Subtyping Systems for Bladder Cancer. Front. Oncol. 2020, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K. Bladder cancer a review. J. Am. Med. Assoc. 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Garg, M. Urothelial cancer stem cells and epithelial plasticity: Current concepts and therapeutic implications in bladder cancer. Cancer Metastasis Rev. 2015, 34, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Hinotsu, S.; Akaza, H.; Ohashi, Y.; Kotake, T. Intravesical chemotherapy for maximum prophylaxis of new early phase superficial bladder carcinoma treated by transurethral resection: A combined analysis of trials by the Japanese Urological Cancer Research Group using smoothed hazard function. Cancer 1999, 86, 1818–1826. [Google Scholar] [CrossRef]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Prim. 2017, 3, 17022. [Google Scholar] [CrossRef]
- Sylvester, R.J. Natural history, recurrence, and progression in superficial bladder cancer. Sci. World J. 2006, 6, 2617–2625. [Google Scholar] [CrossRef]
- Wong, V.K.; Ganeshan, D.; Jensen, C.T.; Devine, C.E. Imaging and management of bladder cancer. Cancers 2021, 13, 1396. [Google Scholar] [CrossRef]
- Griffiths, T.R.L. Current perspectives in bladder cancer management. Int. J. Clin. Pract. 2013, 67, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Upadhyay, T.K.; Seungjoon, M.; Park, M.N.; Kim, B. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed. Pharmacother. 2023, 161, 114491. [Google Scholar] [CrossRef] [PubMed]
- Bourn, J.; Cekanova, M. Cyclooxygenase inhibitors potentiate receptor tyrosine kinase therapies in bladder cancer cells in vitro. Drug Des. Devel. Ther. 2018, 12, 1727–1742. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Akbani, R.; Broom, B.M.; Wang, W.; Verhaak, R.G.W.; McConkey, D.; Lerner, S.; Morgan, M.; Creighton, C.J.; Smith, C.; et al. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Wong, Y.N.; Litwin, S.; Vaughn, D.; Cohen, S.; Plimack, E.R.; Lee, J.; Song, W.; Dabrow, M.; Brody, M.; Tuttle, H.; et al. Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J. Clin. Oncol. 2012, 30, 3545–3551. [Google Scholar] [CrossRef] [PubMed]
- Crew, J.P. Vascular endothelial growth factor: An important angiogenic mediator in bladder cancer. Eur. Urol. 1999, 35, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Huan, J.; Grivas, P.; Birch, J.; Hansel, D.E. Emerging Roles for Mammalian Target of Rapamycin (mTOR) Complexes in Bladder Cancer Progression and Therapy. Cancers 2022, 14, 1555. [Google Scholar] [CrossRef]
- Sathe, A.; Nawroth, R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1655, pp. 335–350. [Google Scholar]
- Houédé, N.; Pourquier, P. Targeting the genetic alterations of the PI3K-AKT-mTOR pathway: Its potential use in the treatment of bladder cancers. Pharmacol. Ther. 2015, 145, 1–18. [Google Scholar] [CrossRef]
- He, Y.; Luo, Y.; Huang, L.; Zhang, D.; Wang, X.; Ji, J.; Liang, S. New frontiers against sorafenib resistance in renal cell carcinoma: From molecular mechanisms to predictive biomarkers. Pharmacol. Res. 2021, 170, 105732. [Google Scholar] [CrossRef]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Z.; Zhang, W.; Cheng, Y.; Zhang, B.; Wu, F.; Wang, Q.; Wang, S.; Rong, D.; Reiter, F.P.; et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduct. Target. Ther. 2020, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Luo, Y.; Zhang, Q.; Zeng, F.; Xu, J.; Zhu, J. Sorafenib and radioiodine-refractory differentiated thyroid cancer (RR-DTC): A systematic review and meta-analysis. Endocrine 2020, 68, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Gupta-Abramson, V.; Troxel, A.B.; Nellore, A.; Puttaswamy, K.; Redlinger, M.; Ransone, K.; Mandel, S.J.; Flaherty, K.T.; Loevner, L.A.; O’Dwyer, P.J.; et al. Phase II trial of sorafenib in advanced thyroid cancer. J. Clin. Oncol. 2008, 26, 4714–4719. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef]
- Rose, A.; Grandoch, M.; vom Dorp, F.; Rübben, H.; Rosenkranz, A.; Fischer, J.; Weber, A. Stimulatory effects of the multi-kinase inhibitor sorafenib on human bladder cancer cells. Br. J. Pharmacol. 2010, 160, 1690–1698. [Google Scholar] [CrossRef]
- Amantini, C.; Morelli, M.B.; Santoni, M.; Soriani, A.; Cardinali, C.; Farfariello, V.; Eleuteri, A.M.; Bonfili, L.; Mozzicafreddo, M.; Nabissi, M.; et al. Sorafenib induces cathepsin B-mediated apoptosis of bladder cancer cells by regulating the Akt/PTEN pathway. The Akt inhibitor, perifosine, enhances the sorafenib-induced cytotoxicity against bladder cancer cells. Oncoscience 2015, 2, 395–409. [Google Scholar] [CrossRef]
- Duan, D.; Torosyan, H.; Elnatan, D.; McLaughlin, C.K.; Logie, J.; Shoichet, M.S.; Agard, D.A.; Shoichet, B.K. Internal structure and preferential protein binding of colloidal aggregates. ACS Chem. Biol. 2017, 12, 282–290. [Google Scholar] [CrossRef]
- Lak, P.; O’Donnell, H.; Du, X.; Jacobson, M.P.; Shoichet, B.K. A Crowding Barrier to Protein Inhibition in Colloidal Aggregates. J. Med. Chem. 2021, 64, 4109–4116. [Google Scholar] [CrossRef]
- McLaughlin, C.K.; Duan, D.; Ganesh, A.N.; Torosyan, H.; Shoichet, B.K.; Shoichet, M.S. Stable Colloidal Drug Aggregates Catch and Release Active Enzymes. ACS Chem. Biol. 2016, 11, 992–1000. [Google Scholar] [CrossRef]
- Owen, S.C.; Doak, A.K.; Wassam, P.; Shoichet, M.S.; Shoichet, B.K. Colloidal aggregation affects the efficacy of anticancer drugs in cell culture. ACS Chem. Biol. 2012, 7, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, M.; Stopa, B.; Konieczny, L.; Rybarska, J.; Piekarska, B.; Szneler, E.; Bakalarski, G.; Roterman, I. Self-assembly of Congo Red—A theoretical and experimental approach to identify its supramolecular organization in water and salt solutions. Biopolymers 1998, 46, 267–281. [Google Scholar] [CrossRef]
- Konieczny, L.; Piekarska, B.; Rybarska, J.; Stopa, B.; Krzykwa, B.; Noworolski, J.; Pawlicki, R.; Roterman, I. Bis azo dye liquid crystalline micelles as possible drug carriers in immunotargeting technique. J. Physiol. Pharmacol. 1994, 45, 441–454. [Google Scholar] [PubMed]
- Kwiecińska, K.; Stachowicz-Kuśnierz, A.; Korchowiec, B.; Roman, M.; Kwiatek, W.M.; Jagusiak, A.; Roterman, I.; Korchowiec, J. Congo Red as a Supramolecular Carrier System for Doxorubicin: An Approach to Understanding the Mechanism of Action. Int. J. Mol. Sci. 2022, 23, 8935. [Google Scholar] [CrossRef] [PubMed]
- Roterman, I.; Konieczny, L. Self-Assembled Molecules—New Kind of Protein Ligands: Supramolecular Ligands; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-31-965639-7. [Google Scholar]
- Lu, Y.; Wang, S.; Wang, Y.; Li, M.; Liu, Y.; Xue, D. Current Researches on Nanodrug Delivery Systems in Bladder Cancer Intravesical Chemotherapy. Front. Oncol. 2022, 12, 879828. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chang, I.H. A novel strategy for treatment of bladder cancer: Antibody-drug conjugates. Investig. Clin. Urol. 2022, 63, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Samarghandian, S.; Najafi, M. PTEN: What we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur. J. Pharmacol. 2020, 881, 173226. [Google Scholar] [CrossRef]
- Casadei, C.; Dizman, N.; Schepisi, G.; Cursano, M.C.; Basso, U.; Santini, D.; Pal, S.K.; De Giorgi, U. Targeted therapies for advanced bladder cancer: New strategies with FGFR inhibitors. Ther. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef]
- Silay, M.S.; Miroglu, C. Sunitinib malate and sorafenib may be beneficial at the treatment of advanced bladder cancer due to their anti-angiogenic effects. Med. Hypotheses 2007, 69, 892–895. [Google Scholar] [CrossRef]
- Thomas, J.; Sonpavde, G. Molecularly Targeted Therapy towards Genetic Alterations in Advanced Bladder Cancer. Cancers 2022, 14, 1795. [Google Scholar] [CrossRef]
- Dreicer, R.; Li, H.; Stein, M.; DiPaola, R.; Eleff, M.; Roth, B.J.; Wilding, G. Phase 2 trial of sorafenib in patients with advanced urothelial cancer: A Trial of the Eastern Cooperative Oncology Group. Cancer 2009, 115, 4090–4095. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.S.; Winquist, E.; Eisen, A.; Hotte, S.J.; McWhirter, E.; Tannock, I.F.; Mukherjee, S.D.; Wang, L.; Blattler, C.; Wright, J.J.; et al. A phase II trial of sorafenib in first-line metastatic urothelial cancer: A study of the PMH Phase II Consortium. Investig. New Drugs 2011, 29, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, X.; Lin, T. Emerging strategies for the improvement of chemotherapy in bladder cancer: Current knowledge and future perspectives. J. Adv. Res. 2022, 39, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Bambury, R.M.; Rosenberg, J.E. Advanced urothelial carcinoma: Overcoming treatment resistance through novel treatment approaches. Front. Pharmacol. 2013, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Knievel, J.; Schulz, W.; Greife, A.; Hader, C.; Lübke, T.; Schmitz, I.; Albers, P.; Niegisch, G. Multiple Mechanisms Mediate Resistance to Sorafenib in Urothelial Cancer. Int. J. Mol. Sci. 2014, 15, 20500–20517. [Google Scholar] [CrossRef] [PubMed]
- Sarna, M.; Zadlo, A.; Hermanowicz, P.; Madeja, Z.; Burda, K.; Sarna, T. Cell elasticity is an important indicator of the metastatic phenotype of melanoma cells. Exp. Dermatol. 2014, 23, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Chighizola, M.; Schulte, C.; Bryniarska, N.; Wesolowska, J.; Pudelek, M.; Lasota, M.; Ryszawy, D.; Basta-Kaim, A.; Laidler, P.; et al. Stiffening of DU145 prostate cancer cells driven by actin filaments-microtubule crosstalk conferring resistance to microtubule-targeting drugs. Nanoscale 2021, 13, 6212–6226. [Google Scholar] [CrossRef] [PubMed]
- Lasota, M.; Bentke-Imiolek, A.; Skrzypek, K.; Bobrowska, J.; Jagusiak, A.; Bryniarska-Kubiak, N.; Zagajewski, J.; Kot, M.; Szydlak, R.; Lekka, M.; et al. Small-molecule inhibitor-tyrphostin AG1296 regulates proliferation, survival and migration of rhabdomyosarcoma cells. J. Physiol. Pharmacol. 2021, 72, 881–893. [Google Scholar] [CrossRef]
- Sarna, M.; Zadlo, A.; Czuba-Pelech, B.; Urbanska, K. Nanomechanical phenotype of melanoma cells depends solely on the amount of endogenous pigment in the cells. Int. J. Mol. Sci. 2018, 19, 607. [Google Scholar] [CrossRef]
- Sarna, M.; Wojcik, K.A.; Hermanowicz, P.; Wnuk, D.; Burda, K.; Sanak, M.; Czyż, J.; Michalik, M. Undifferentiated bronchial fibroblasts derived from asthmatic patients display higher elastic modulus than their non-asthmatic counterparts. PLoS ONE 2015, 10, e0116840. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Z.; Peng, K.; Gao, Y.; Wu, D.; Kim, J.; Mitragotri, S. Enhancement of Anticancer Efficacy and Tumor Penetration of Sorafenib by Ionic Liquids. Adv. Healthc. Mater. 2021, 10, 2001455. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Yin, H.S.; Li, H.S.; Yu, X.Q.; Han, X. Interleukin-27 augments the inhibitory effects of sorafenib on bladder cancer cells. Braz. J. Med. Biol. Res. 2017, 50, e6207. [Google Scholar] [CrossRef] [PubMed]
- Reker, D.; Rybakova, Y.; Kirtane, A.R.; Cao, R.; Yang, J.W.; Navamajiti, N.; Gardner, A.; Zhang, R.M.; Esfandiary, T.; L’Heureux, J.; et al. Computationally guided high-throughput design of self-assembling drug nanoparticles. Nat. Nanotechnol. 2021, 16, 725–733. [Google Scholar] [CrossRef]
- Shamay, Y.; Shah, J.; Işlk, M.; Mizrachi, A.; Leibold, J.; Tschaharganeh, D.F.; Roxbury, D.; Budhathoki-Uprety, J.; Nawaly, K.; Sugarman, J.L.; et al. Quantitative self-assembly prediction yields targeted nanomedicines. Nat. Mater. 2018, 17, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.N.; Donders, E.N.; Shoichet, B.K.; Shoichet, M.S. Colloidal aggregation: From screening nuisance to formulation nuance. Nano Today 2018, 19, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, C.; Wang, J.; Chen, L.; Chen, J.; Chen, T.; Zeng, Q. Application of nanotechnology in the diagnosis and treatment of bladder cancer. J. Nanobiotechnol. 2021, 19, 393. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Karimi-Maleh, H.; Taheriazam, A.; Mirzaei, S.; Hashemi, M.; Hushmandi, K.; Makvandi, P.; Nazarzadeh Zare, E.; Sharifi, E.; et al. (Nano)platforms in bladder cancer therapy: Challenges and opportunities. Bioeng. Transl. Med. 2023, 8, e10353. [Google Scholar] [CrossRef]
- Owen, S.C.; Doak, A.K.; Ganesh, A.N.; Nedyalkova, L.; McLaughlin, C.K.; Shoichet, B.K.; Shoichet, M.S. Colloidal drug formulations can explain “bell-shaped” concentration-response curves. ACS Chem. Biol. 2014, 9, 777–784. [Google Scholar] [CrossRef]
- McCauley, J.; Zivanovic, A.; Skropeta, D. Bioassays for anticancer activities. Methods Mol. Biol. 2013, 1055, 191–205. [Google Scholar] [CrossRef]
- Lasota, M.; Klein, A.; Balwierz, W. Cytostatic and cytotoxic effects of tyrphostin AG1296 on RMS cells. Wspolczesna Onkol. 2012, 16, 1–5. [Google Scholar] [CrossRef]
- Bojko, A.; Cierniak, A.; Adamczyk, A.; Ligeza, J. Modulatory Effects of Curcumin and Tyrphostins (AG494 and AG1478) on Growth Regulation and Viability of LN229 Human Brain Cancer Cells. Nutr. Cancer 2015, 67, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Kusienicka, A.; Szewczyk, B.; Adamus, T.; Lukasiewicz, E.; Miekus, K.; Majka, M. Constitutive activation of MET signaling impairs myogenic differentiation of rhabdomyosarcoma and promotes its development and progression. Oncotarget 2015, 6, 31378–31398. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, P.; Sarna, M.; Burda, K.; Gabryś, H. AtomicJ: An open source software for analysis of force curves. Rev. Sci. Instrum. 2014, 85, 063703. [Google Scholar] [CrossRef] [PubMed]
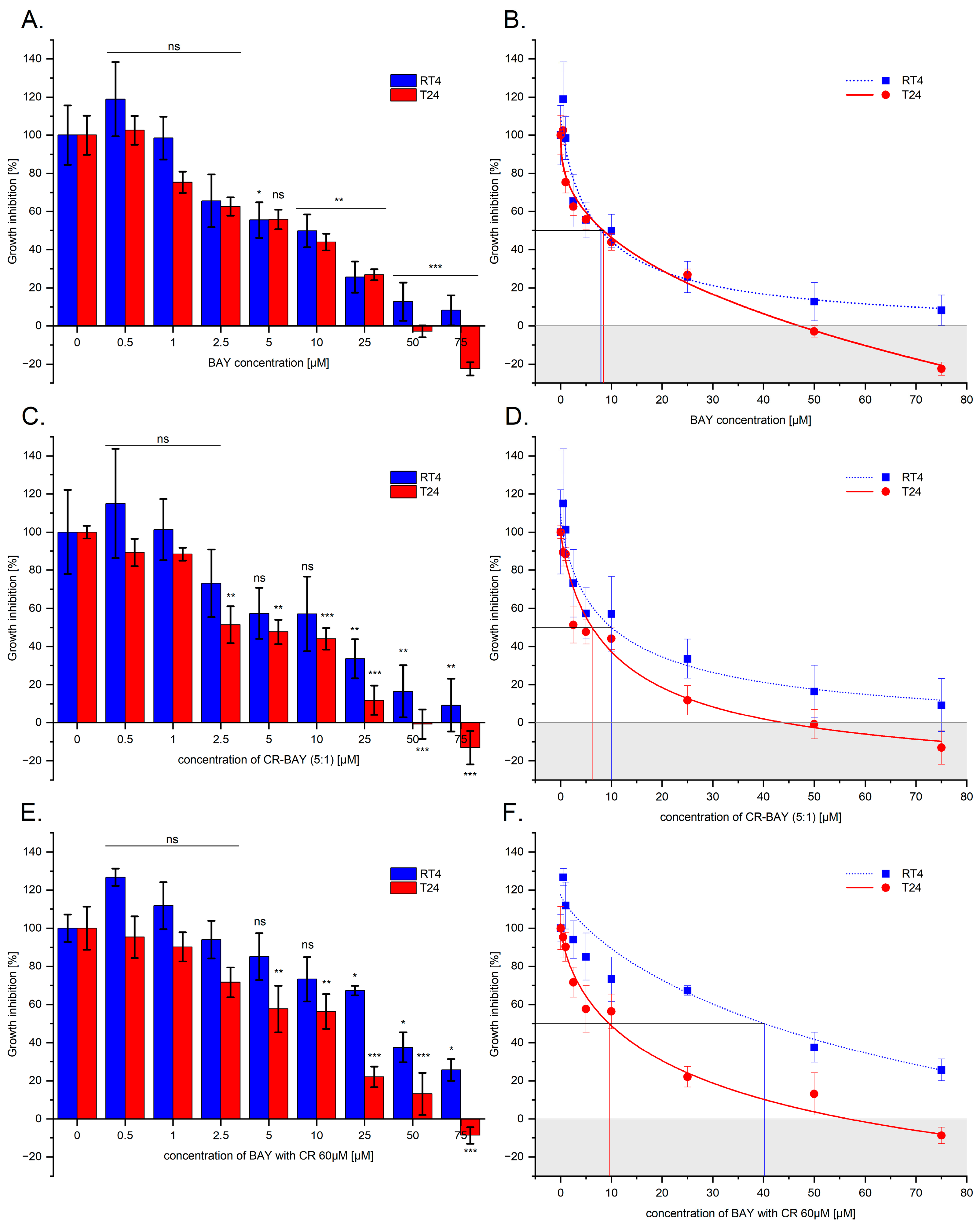
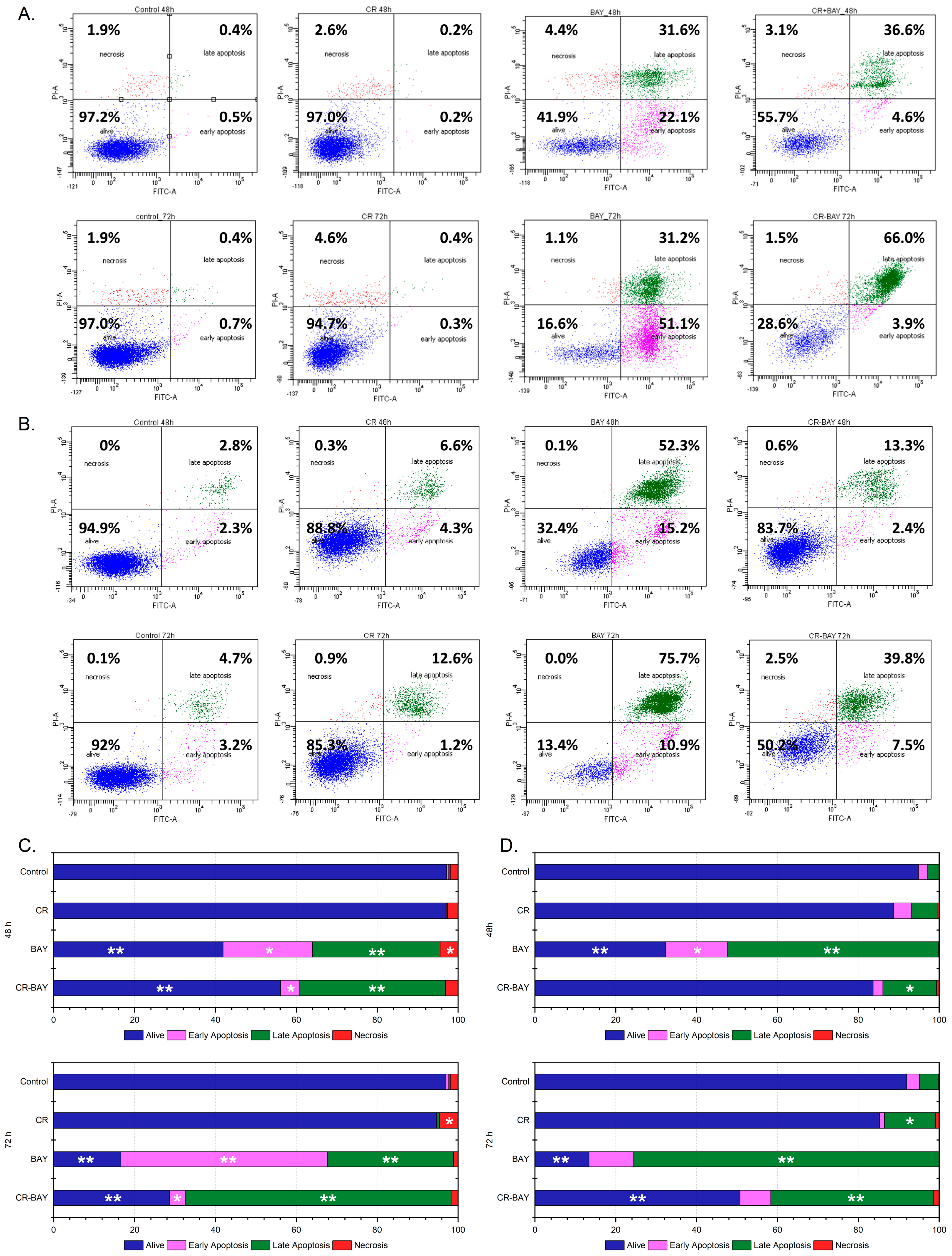






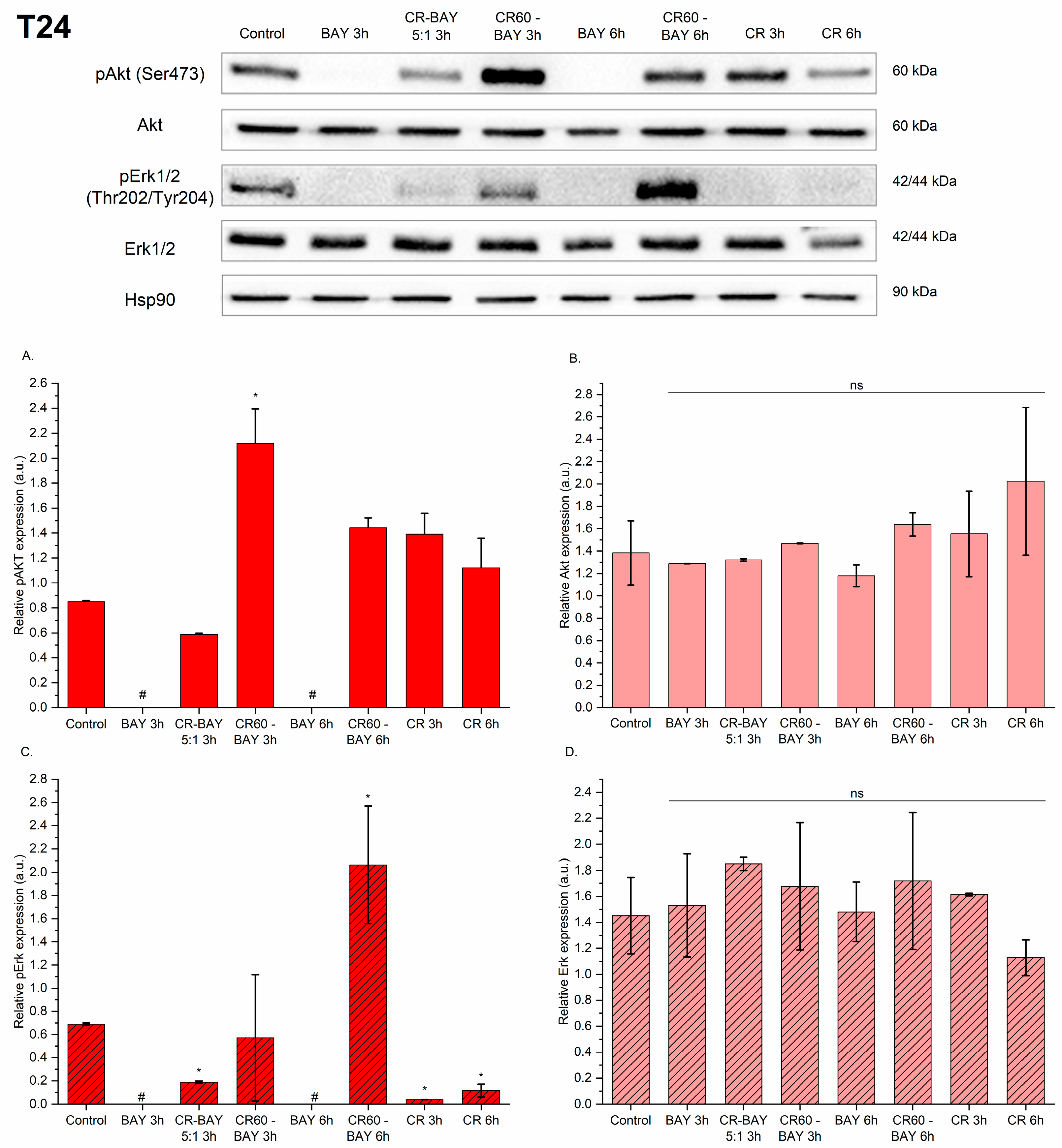
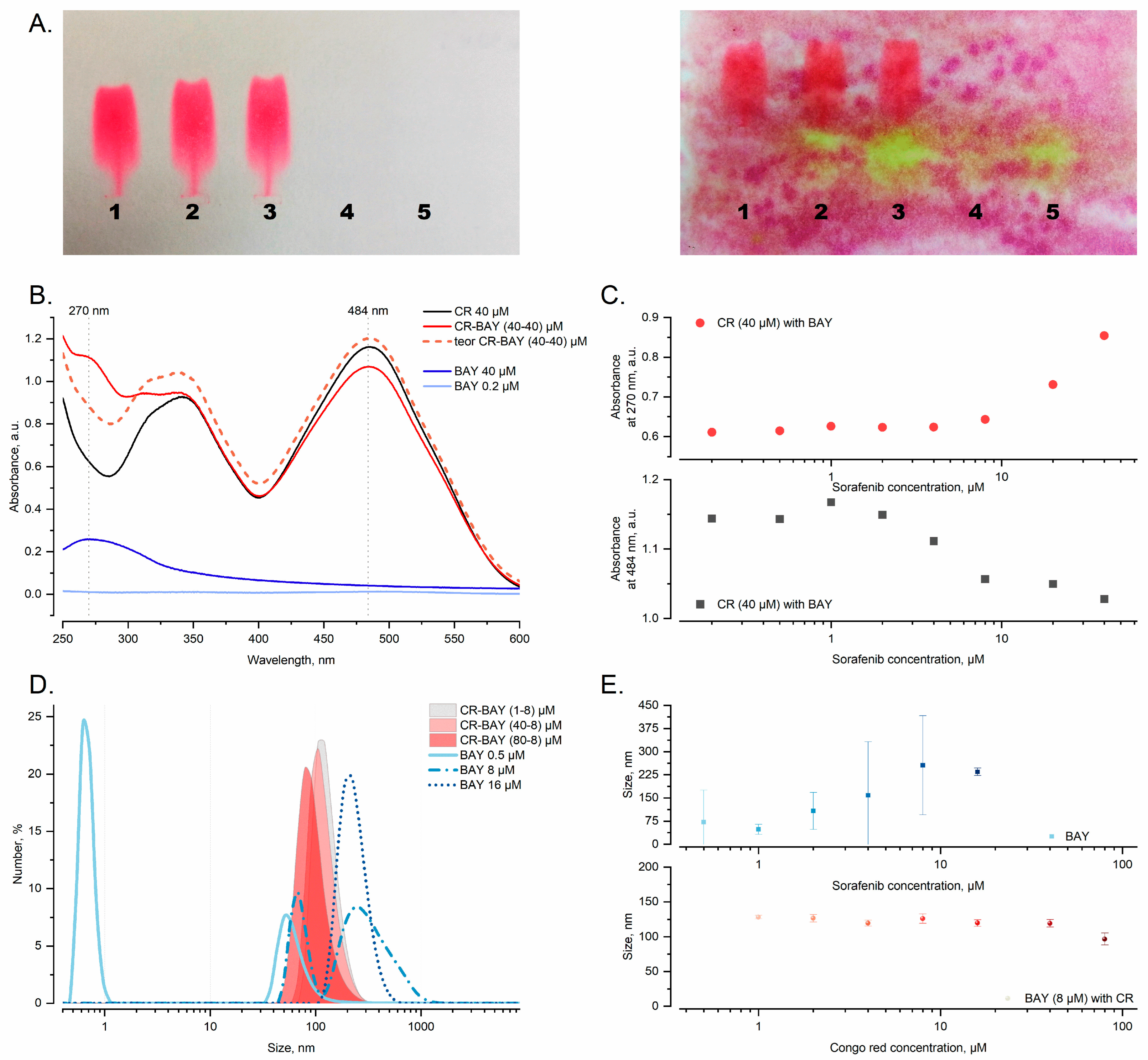
| Cell Line | RT4 | T24 | ||
|---|---|---|---|---|
| IC50 | IC90 | IC50 | IC90 | |
| BAY [µM] | 8.01 ± 0.05 | 68.8 ± 0.07 | 8.45 ± 0.05 | 36.15 ± 0.17 |
| CR-BAY (5:1) [µM] | 10.04 ± 0.04 | 62.70 ± 0.12 | 6.18 ± 0.05 | 28.00 ± 0.12 |
| CR60-BAY [µM] | 40.22 ± 0.06 | - | 9.56 ± 0.06 | 40.46 ± 0.15 |
| Primary Antibody | Material Number | Host Species | Dilution | Vendor |
|---|---|---|---|---|
| Akt | #9272 | rabbit | 1:1000 | Cell Signaling Technology Inc. (Danvers, MA, USA) |
| phospho-AKT (Ser473) | #4060 | rabbit | 1:1000 | |
| p44/42 MAPK (ERK1/2) | #4695 | rabbit | 1:1000 | |
| phospho-p44/42 MAPK (Thr202/Tyr204) (phospho-ERK1/2) | #9106 | mouse | 1:1000 | |
| Hsp90 | 610418 | mouse | 1:4000 | BD Transduction Laboratories |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasota, M.; Jankowski, D.; Wiśniewska, A.; Sarna, M.; Kaczor-Kamińska, M.; Misterka, A.; Szczepaniak, M.; Dulińska-Litewka, J.; Górecki, A. The Potential of Congo Red Supplied Aggregates of Multitargeted Tyrosine Kinase Inhibitor (Sorafenib, BAY-43-9006) in Enhancing Therapeutic Impact on Bladder Cancer. Int. J. Mol. Sci. 2024, 25, 269. https://doi.org/10.3390/ijms25010269
Lasota M, Jankowski D, Wiśniewska A, Sarna M, Kaczor-Kamińska M, Misterka A, Szczepaniak M, Dulińska-Litewka J, Górecki A. The Potential of Congo Red Supplied Aggregates of Multitargeted Tyrosine Kinase Inhibitor (Sorafenib, BAY-43-9006) in Enhancing Therapeutic Impact on Bladder Cancer. International Journal of Molecular Sciences. 2024; 25(1):269. https://doi.org/10.3390/ijms25010269
Chicago/Turabian StyleLasota, Małgorzata, Daniel Jankowski, Anna Wiśniewska, Michał Sarna, Marta Kaczor-Kamińska, Anna Misterka, Mateusz Szczepaniak, Joanna Dulińska-Litewka, and Andrzej Górecki. 2024. "The Potential of Congo Red Supplied Aggregates of Multitargeted Tyrosine Kinase Inhibitor (Sorafenib, BAY-43-9006) in Enhancing Therapeutic Impact on Bladder Cancer" International Journal of Molecular Sciences 25, no. 1: 269. https://doi.org/10.3390/ijms25010269





