Bioactive Properties of Tagetes erecta Edible Flowers: Polyphenol and Antioxidant Characterization and Therapeutic Activity against Ovarian Tumoral Cells and Caenorhabditis elegans Tauopathy
Abstract
:1. Introduction
2. Results and Discussion
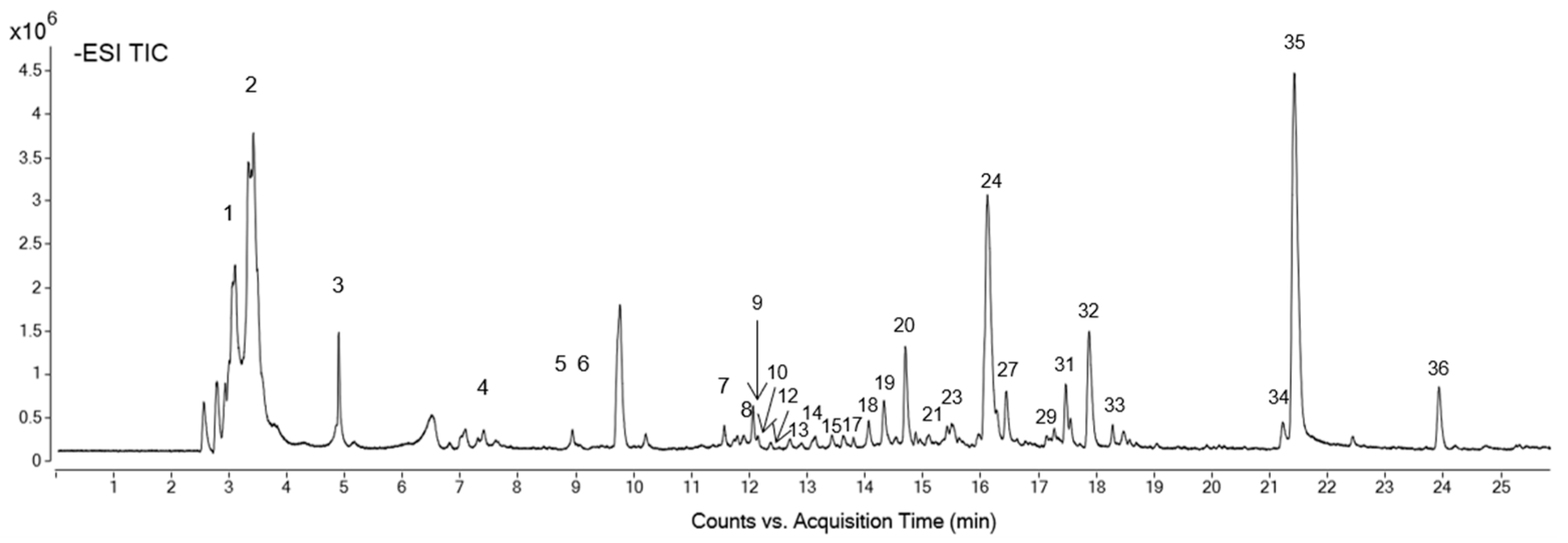
2.1. Petal Extract Characterization
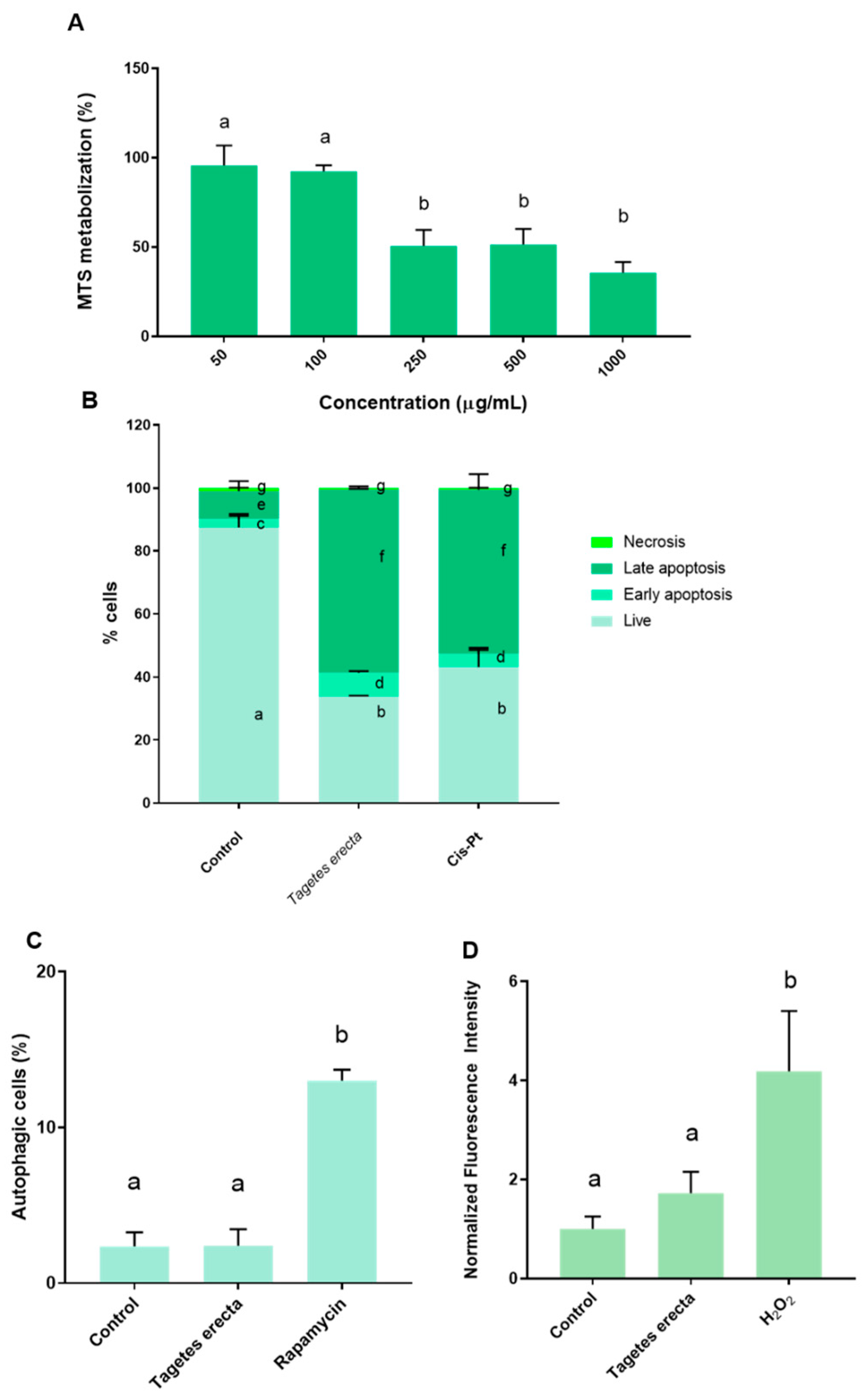
2.2. In Vitro Assays
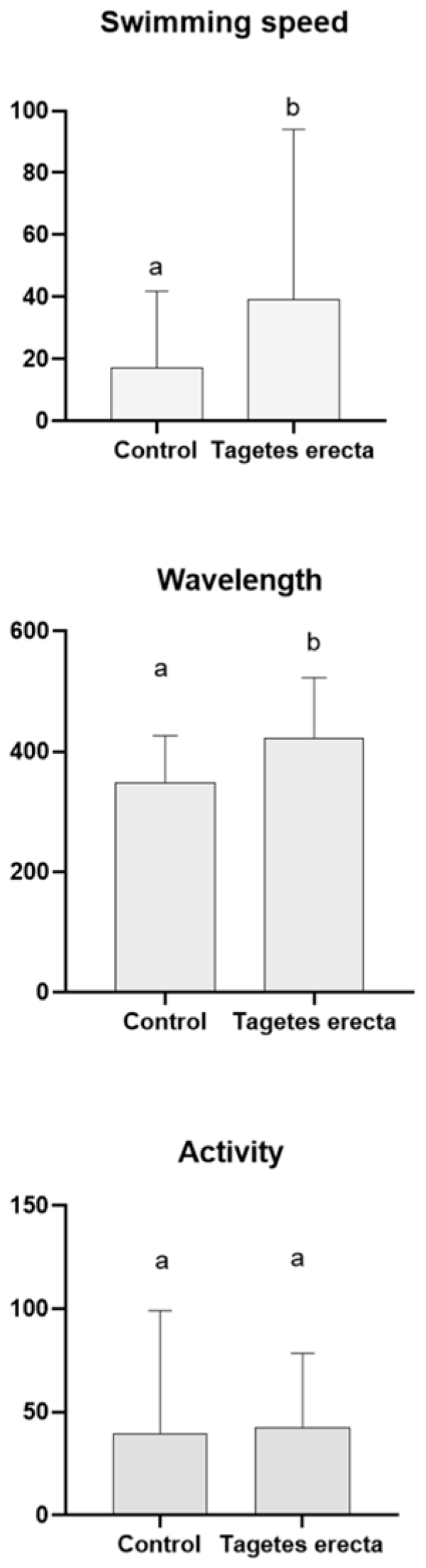
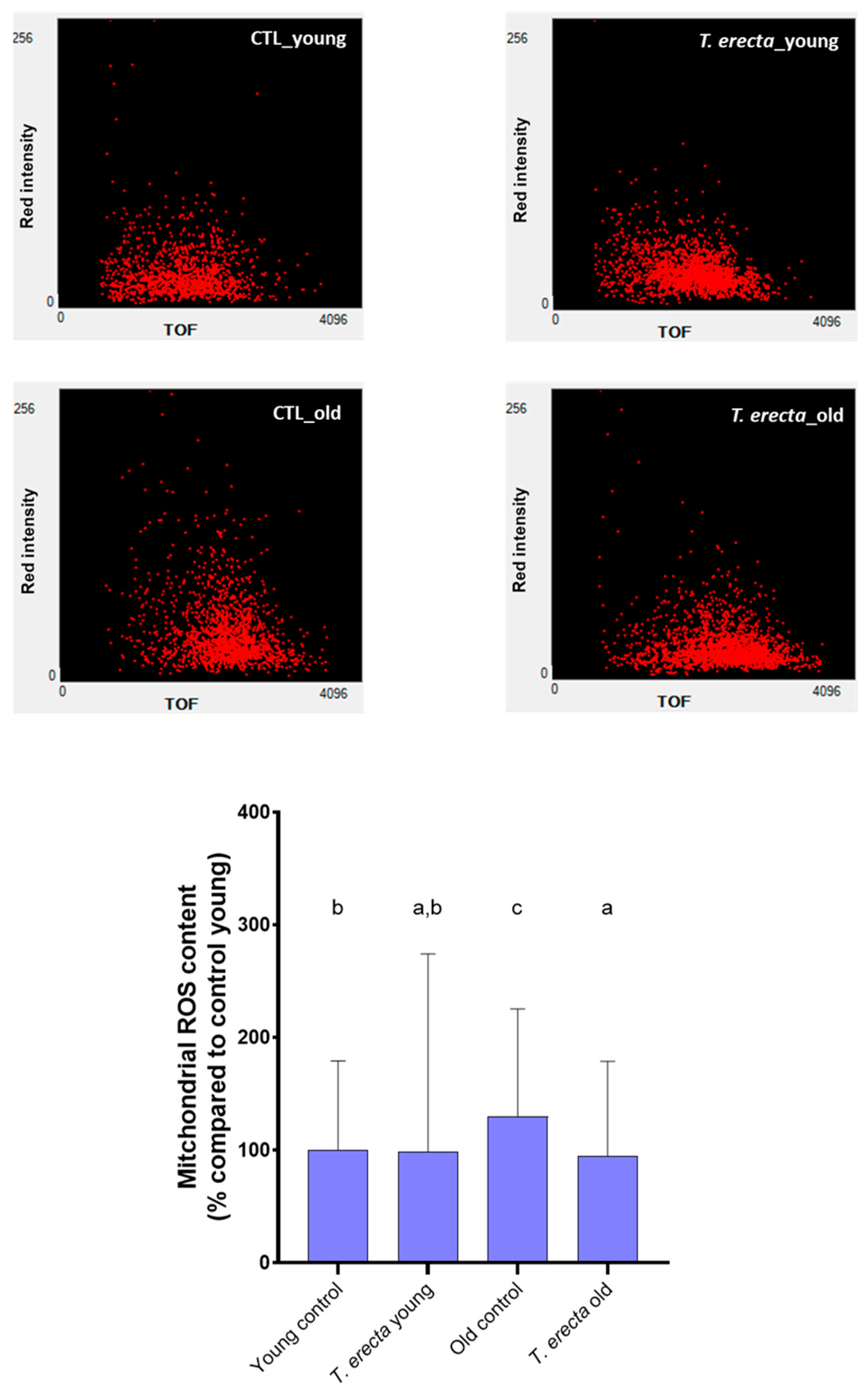
2.3. Caenorhabditis elegans Assays
3. Materials and Methods
3.1. Edible Flower
3.2. Extract Obtention Procedure
3.3. Extract Characterization
3.3.1. Total Phenolic Content
3.3.2. Total Flavonoid Content
3.3.3. Total Antioxidant Capacity
3.4. HPLC-QTOF-MS/MS
3.5. In Vitro Assays
3.5.1. Cell Conditions
3.5.2. Cell Viability Test
3.5.3. Cell Death Mechanism: Autophagy Induction
3.5.4. Cell Death Mechanism: Apoptosis and Necrosis
3.5.5. ROS Production
3.6. Caenorhabditis Elegans Assays
3.6.1. Maintenance of C. elegans
3.6.2. Tau Proteotoxicity Assessment
3.6.3. Intracellular ROS Content Determination
3.6.4. Mitochondrial ROS Content Determination
3.6.5. Lipofuscin Content Quantification
3.6.6. Acute Lethal Toxicity Assay
3.7. Figure Generation
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivas-García, L.; Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Forbes-Hernández, T.Y.; Varela-López, A.; Llopis, J.; Sánchez-González, C.; Quiles, J.L. Edible Flowers as a Health Promoter: An Evidence-Based Review. Trends Food Sci. Technol. 2021, 117, 46–59. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. An Overview on the Market of Edible Flowers. Food Rev. Int. 2020, 36, 258–275. [Google Scholar] [CrossRef]
- Pensamiento-Niño, C.A.; Castañeda-Ovando, A.; Añorve-Morga, J.; Hernández-Fuentes, A.D.; Aguilar-Arteaga, K.; Ojeda-Ramírez, D. Edible Flowers and Their Relationship with Human Health: Biological Activities. Food Rev. Int. 2023; in press. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Florença, S.G.; Villalobos Moya, K.; Anjos, O. Edible Flowers, Old Tradition or New Gastronomic Trend: A First Look at Consumption in Portugal versus Costa Rica. Foods 2020, 9, 977. [Google Scholar] [CrossRef] [PubMed]
- Chitrakar, B.; Zhang, M.; Bhandari, B. Edible Flowers with the Common Name “Marigold”: Their Therapeutic Values and Processing. Trends Food Sci. Technol. 2019, 89, 76–87. [Google Scholar] [CrossRef]
- Moliner, C.; Barros, L.; Dias, M.I.; López, V.; Langa, E.; Ferreira, I.C.F.R.; Gómez-Rincón, C. Edible Flowers of Tagetes erecta L. as Functional Ingredients: Phenolic Composition, Antioxidant and Protective Effects on Caenorhabditis elegans. Nutrients 2018, 10, 2002. [Google Scholar] [CrossRef] [PubMed]
- Meurer, M.; de Oliveira, B.M.M.; Cury, B.J.; Jerônimo, D.T.; Venzon, L.; França, T.C.S.; Mariott, M.; Silva-Nunes, R.; Santos, A.C.; Roman-Junior, W.A.; et al. Extract of Tagetes erecta L., a Medicinal Plant Rich in Lutein, Promotes Gastric Healing and Reduces Ulcer Recurrence in Rodents. J. Ethnopharmacol. 2022, 293, 115258. [Google Scholar] [CrossRef]
- Mir, R.A.; Irshad, S.; Argal, S.; Agarwal, R.M.; Khatoon, S. Quantitative Analysis of Polyphenolic Compounds in Two Different Cultivars of Marigold (Tagetes erecta L.) Using High-Performance Thin-Layer Chromatography. Front. Hortic. 2023, 2, 1120267. [Google Scholar] [CrossRef]
- Lin, M.T.; Flint Beal, M. The Oxidative Damage Theory of Aging. Clin. Neurosci. Res. 2003, 2, 305–315. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and Age-Related Diseases: From Mechanisms to Therapeutic Strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Rogoff, H.A. Implications of Reactive Oxygen Species on Cancer Formation and Its Treatment. Semin. Oncol. 2021, 48, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Hawk, M.; McCallister, C.; Schafer, Z. Antioxidant Activity during Tumor Progression: A Necessity for the Survival of Cancer Cells? Cancers 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yin, H. Role of Lipid Peroxidation Derived 4-Hydroxynonenal (4-HNE) in Cancer: Focusing on Mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef]
- Bhatt, S.; Puli, L.; Patil, C.R. Role of Reactive Oxygen Species in the Progression of Alzheimer’s Disease. Drug Discov. Today 2021, 26, 794–803. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways Towards and Away from Alzheimer’s Disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary Polyphenols in Chemoprevention and Synergistic Effect in Cancer: Clinical Evidences and Molecular Mechanisms of Action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, L.; Quiles, J.L.; Roma-Rodrigues, C.; Raposo, L.R.; Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Esteban-Muñoz, A.; Varela-López, A.; García, L.C.; Cianciosi, D.; et al. Rosa × Hybrida Extracts with Dual Actions: Antiproliferative Effects against Tumour Cells and Inhibitor of Alzheimer Disease. Food Chem. Toxicol. 2021, 149, 112018. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, L.; Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Esteban-Muñoz, A.; Giampieri, F.; Sumalla-Cano, S.; Battino, M.; Quiles, J.L.; Llopis, J.; Sánchez-González, C. Unravelling Potential Biomedical Applications of the Edible Flower Tulbaghia violacea. Food Chem. 2022, 381, 132096. [Google Scholar] [CrossRef] [PubMed]
- Gostin, A.-I.; Waisundara, V.Y. Edible Flowers as Functional Food: A Review on Artichoke (Cynara cardunculus L.). Trends Food Sci. Technol. 2019, 86, 381–391. [Google Scholar] [CrossRef]
- Navarro-González, I.; González-Barrio, R.; García-Valverde, V.; Bautista-Ortín, A.B.; Periago, M.J. Nutritional Composition and Antioxidant Capacity in Edible Flowers: Characterisation of Phenolic Compounds by HPLC-DAD-ESI/MSn. Int. J. Mol. Sci. 2015, 16, 805–822. [Google Scholar] [CrossRef]
- Kaisoon, O.; Konczak, I.; Siriamornpun, S. Potential Health Enhancing Properties of Edible Flowers from Thailand. Food Res. Int. 2012, 46, 563–571. [Google Scholar] [CrossRef]
- Moliner, C.; Barros, L.; Dias, M.I.; Reigada, I.; Ferreira, I.C.F.R.; López, V.; Langa, E.; Rincón, C.G. Viola Cornuta and Viola × Wittrockiana: Phenolic Compounds, Antioxidant and Neuroprotective Activities on Caenorhabditis elegans. J. Food Drug Anal. 2019, 27, 849–859. [Google Scholar] [CrossRef]
- Burlec, A.F.; Pecio, Ł.; Kozachok, S.; Mircea, C.; Corciovă, A.; Vereștiuc, L.; Cioancă, O.; Oleszek, W.; Hăncianu, M. Phytochemical Profile, Antioxidant Activity, and Cytotoxicity Assessment of Tagetes erecta L. Flowers. Molecules 2021, 26, 1201. [Google Scholar] [CrossRef]
- Bashir, S.; Gilani, A.H. Studies on the Antioxidant and Analgesic Activities of Aztec Marigold (Tagetes erecta) Flowers. Phytother. Res. 2008, 22, 1692–1694. [Google Scholar] [CrossRef]
- Perisoara, A.; Marinas, I.C.; Geana, E.I.; Constantin, M.; Angheloiu, M.; Pirvu, L.; Cristea, S. Phytostimulation and Synergistic Antipathogenic Effect of Tagetes erecta Extract in Presence of Rhizobacteria. Horticulturae 2022, 8, 779. [Google Scholar] [CrossRef]
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of Extraction Solvent on the Phenolic Profile and Bioactivity of Two Achillea Species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef] [PubMed]
- Kandylis, P. Phytochemicals and Antioxidant Properties of Edible Flowers. Appl. Sci. 2022, 12, 9937. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Vedam, V.V.A.; Stanley Xavier, A.; Chellathai David, D. In-Vitro Evaluation of Antifungal and Anticancer Properties of Tagetes erecta Petal Extract. Biomed. Pharmacol. J. 2019, 12, 815–823. [Google Scholar] [CrossRef]
- Barhoi, D.; Upadhaya, P.; Barbhuiya, S.N.; Giri, A.; Giri, S. Extracts of Tagetes erecta Exhibit Potential Cytotoxic and Antitumor Activity That Could Be Employed as a Promising Therapeutic Agent against Cancer: A Study Involving In Vitro and In Vivo Approach. Phytomed. Plus 2022, 2, 100187. [Google Scholar] [CrossRef]
- Vallisuta, O.; Nukoolkarn, V.; Mitrevej, A.; Sarisuta, N.; Leelapornpisid, P.; Phrutivorapongkul, A.; Sinchaipanid, N. In Vitro Studies on the Cytotoxicity, and Elastase and Tyrosinase Inhibitory Activities of Marigold (Tagetes erecta L.) Flower Extracts. Exp. Ther. Med. 2014, 7, 246–250. [Google Scholar] [CrossRef]
- El-Maraghy, S.A.; Mehana, N.A. Modulatory Effects of L-Arginine and Soy Enriched Diet on Bone Homeostasis Abnormalities in Streptozotocin-Induced Diabetic Rats. Chem.-Biol. Interact. 2015, 229, 9–16. [Google Scholar] [CrossRef]
- Gupta, P.; Gupta, A.; Agarwal, K.; Tomar, P.; Satija, S. Antioxidant and Cytotoxic Potential of a New Thienyl Derivative from Tagetes erecta Roots. Pharm. Biol. 2012, 50, 1013–1018. [Google Scholar] [CrossRef]
- Gansukh, E.; Mya, K.K.; Jung, M.; Keum, Y.-S.; Kim, D.H.; Saini, R.K. Lutein Derived from Marigold (Tagetes erecta) Petals Triggers ROS Generation and Activates Bax and Caspase-3 Mediated Apoptosis of Human Cervical Carcinoma (HeLa) Cells. Food Chem. Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef]
- Yousuf, M.; Shamsi, A.; Khan, P.; Shahbaaz, M.; AlAjmi, M.F.; Hussain, A.; Hassan, G.M.; Islam, A.; Rizwanul Haque, Q.M.; Hassan, M.I. Ellagic Acid Controls Cell Proliferation and Induces Apoptosis in Breast Cancer Cells via Inhibition of Cyclin-Dependent Kinase 6. Int. J. Mol. Sci. 2020, 21, 3526. [Google Scholar] [CrossRef] [PubMed]
- Nebenfuehr, S.; Kollmann, K.; Sexl, V. The Role of CDK6 in Cancer. Int. J. Cancer 2020, 147, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Romero-Márquez, J.M.; Forbes-Hernández, T.Y.; Navarro-Hortal, M.D.; Quirantes-Piné, R.; Grosso, G.; Giampieri, F.; Lipari, V.; Sánchez-González, C.; Battino, M.; Quiles, J.L. Molecular Mechanisms of the Protective Effects of Olive Leaf Polyphenols against Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4353. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Garcia, L.; Navarro-Hortal, M.D.; Romero-Marquez, J.M.; Llopis, J.; Forbes-Hernández, T.Y.; Xiao, J.; Quiles, J.L.; Sanchez-Gonzalez, C. Valorization of Olea Europaea and Olive Oil Processing By-Products/Wastes. In Valorization of Wastes/By-Products in the Design of Functional Foods/Supplements; Academic Press: Cambridge, MA, USA, 2023; pp. 193–212. [Google Scholar]
- Hamaguchi, T.; Sato, K.; Vicente, C.S.L.; Hasegawa, K. Nematicidal Actions of the Marigold Exudate α-Terthienyl: Oxidative Stress-Inducing Compound Penetrates Nematode Hypodermis. Biol. Open 2019, 8, bio038646. [Google Scholar] [CrossRef] [PubMed]
- Augusti, P.R.; Brasil, A.V.S.; Souto, C.; Göethel, G.; De Oliveira Rios, A.; Emanuelli, T.; Bürger, M.E.; Garcia, S.C. Microcystin-LR Exposure Induces Oxidative Damage in Caenorhabditis elegans: Protective Effect of Lutein Extracted from Marigold Flowers. Food Chem. Toxicol. 2017, 109, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, C.; Pir, G.J.; Biernat, J.; Koushika, S.P.; Mandelkow, E.; Mandelkow, E.-M.; Schmidt, E.; Baumeister, R. Inhibition of Tau Aggregation in a Novel Caenorhabditis elegans Model of Tauopathy Mitigates Proteotoxicity. Hum. Mol. Genet. 2012, 21, 3587–3603. [Google Scholar] [CrossRef] [PubMed]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Thirumalai, D.; Palaniappan, B. Role of Tau Protein in Alzheimer’s Disease: The Prime Pathological Player. Int. J. Biol. Macromol. 2020, 163, 1599–1617. [Google Scholar] [CrossRef]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The Role of Tau in Alzheimer’s Disease and Related Disorders: Role of Tau in AD and Related Disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Muñoz-Ollero, P.; Jiménez-Trigo, V.; Esteban-Muñoz, A.; Tutusaus, K.; Giampieri, F.; Battino, M.; Sánchez-González, C.; Rivas-García, L.; et al. Amyloid β-but Not Tau-Induced Neurotoxicity Is Suppressed by Manuka Honey via HSP-16.2 and SKN-1/Nrf2 Pathways in an In Vivo Model of Alzheimer’s Disease. Food Funct. 2022, 13, 11185–11199. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Orantes, F.J.; Esteban-Muñoz, A.; Pérez-Oleaga, C.M.; Battino, M.; Sánchez-González, C.; Rivas-García, L.; Giampieri, F.; Quiles, J.L.; et al. In Vivo Anti-Alzheimer and Antioxidant Properties of Avocado (Persea americana Mill.) Honey from Southern Spain. Antioxidants 2023, 12, 404. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Muñoz-Ollero, P.; Forbes-Hernández, T.Y.; Esteban-Muñoz, A.; Giampieri, F.; Delgado Noya, I.; Bullón, P.; Vera-Ramírez, L.; et al. An Olive-Derived Extract 20% Rich in Hydroxytyrosol Prevents β-Amyloid Aggregation and Oxidative Stress, Two Features of Alzheimer Disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis elegans. Antioxidants 2022, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and Aging-Related Diseases: From Molecular Mechanisms to Interventions and Treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Chaban, Y.; Boekema, E.J.; Dudkina, N.V. Structures of Mitochondrial Oxidative Phosphorylation Supercomplexes and Mechanisms for Their Stabilisation. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The Role of Mitochondrial Dysfunction in Alzheimer’s Disease Pathogenesis. Alzheimer’s Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, X.; He, W.-H.; Xu, H.-G.; Yuan, F.; Gao, Y.-X. Investigation into the Antioxidant Activity and Chemical Composition of Alcoholic Extracts from Defatted Marigold (Tagetes erecta L.) Residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans Methods: Synchronization and Observation. J. Vis. Exp. 2012, 64, e4019. [Google Scholar] [CrossRef]


| Parameter | Mean ± SEM |
|---|---|
| Total phenolic content (mg GAE/g extr) | 81.1 ± 10.6 |
| Total flavonoid content (mg catechin/g extr) | 23.17 ± 4.26 |
| FRAP (μM trolox/g extr) | 1475.3 ± 122.5 |
| DPPH (μM trolox/g extr) | 1950.3 ± 211.1 |
| ABTS (μM trolox/g extr) | 977.7 ± 62.4 |
| Formula | RT (min) | [M-H]−, m/z | Mass | Proposed Compound | Concentration (mg Standard eq/g Extract ± SD) | Peak in Figure 1 |
|---|---|---|---|---|---|---|
| C7H12O6 | 3.42 | 191.0569 | 192.0641 | Quinic acid a | 5.49 ± 0.02 | 1 |
| C13H22O11 | 3.55 | 707.2265 | 354.1168 | Quinic acid hexoside isomer 1 a | 0.20 ± 0.01 | 2 |
| C13H22 O11 | 4.9 | 707.2267 | 354.1176 | Quinic acid hexoside isomer 2 a | 0.16 ± 0.01 | 3 |
| C14H16O10 | 7.47 | 343.0676 | 344.0749 | Theogallin isomer 1 b | 0.3 ± 0.002 | 4 |
| C14H16O10 | 9.06 | 687.1427 | 344.0749 | Theogallin isomer 2 b | 0.22 ± 0.01 | 5 |
| C12H22O9 | 9.94 | 309.1201 | 310.1273 | Deoxy-fructofuranosyl deoxy-glucopyranoside/fructofuranosyl dideoxy-xylo-hexopyranoside c | 76.0 ± 3.00 | 6 |
| C26H30O19 | 11.69 | 645.1322 | 646.1393 | Digalloyl-dihexoside b | 0.51 ± 0.01 | 7 |
| C21H20O14 | 11.95 | 495.0793 | 496.0864 | Digalloylquinic acid b | 0.29 ± 0.003 | 8 |
| C20H20O14 | 12.09 | 483.0785 | 484.0857 | Digalloyl-hexoside b | 0.08 ± 0.001 | 9 |
| C19H26O12 | 12.12 | 445.1359 | 446.1428 | [(Xylopyranosyl-glucopyranosyl)oxy]benzeneacetic acid c | 0.92 ± 0.01 | 10 |
| C33H34O23 | 12.2 | 797.1436 | 798.1506 | Trigalloyl-dihexoside b | 0.53 ± 0.01 | 11 |
| C28H32O18 | 12.31 | 655.1525 | 656.1596 | Patuletin gentiobioside d | 0.63 ± 0.02 | 12 |
| C16H18O9 | 12.9 | 353.0884 | 354.0956 | Caffeoylquinic acid a | 0.26 ± 0.004 | 13 |
| C24H28O15 | 13.15 | 555.1363 | 556.1435 | Syringic acid-(dihydroxydimethoxybenzoic acid)-hexoside isomer 1 e | 1.8 ± 0.1 | 14 |
| C24H28O15 | 13.39 | 555.1366 | 556.1438 | Syringic acid-(dihydroxydimethoxybenzoic acid)-hexoside isomer 2 e | 4.0 ± 0.1 | 15 |
| 1C20H28O12 | 13.66 | 459.1514 | 460.1586 | Apiopaeonoside/paeonolide c | 1.35 ± 0.04 | 16 |
| C20H16O13 | 13.77 | 463.0522 | 464.0594 | Ellagic acid-hexoside b | 0.07 ± 0.002 | 17 |
| C15H14O10 | 14.59 | 353.0524 | 354.0596 | Coumaroylhydroxycitric acid f | 2.5 ± 0.1 | 18 |
| C28H32O18 | 14.72 | 655.1519 | 656.1591 | Patuletin 3-gentiobioside d | 0.55 ± 0.01 | 19 |
| C21H20O13 | 14.88 | 959.1753 | 480.0919 | Quercetagetin-3-O-hexoside d | 4.0 ± 0.04 | 20 |
| C28H24O17 | 15.13 | 631.0946 | 632.1017 | Quercetagetin-7-O-(galloyl-hexoside) d | 0.5 ± 0.01 | 21 |
| C24H28O14 | 15.32 | 539.1412 | 540.1484 | Di-syringic acid hexoside isomer 1 e | 2.25 ± 0.04 | 22 |
| C24H28O14 | 15.66 | 539.1409 | 540.1481 | Di-syringic acid hexoside isomer 2 e | 3.3 ± 0.1 | 23 |
| C22H22O13 | 16.24 | 987.2069 | 494.1079 | Patulitrin isomer 1 g | 76.0 ± 1.0 | 24 |
| C14H6O8 | 16.36 | 300.9996 | 302.0068 | Ellagic acid b | 0.12 ± 0.004 | 25 |
| C29H26O17 | 16.45 | 645.1109 | 646.1180 | Caffeoyl-digalloyl—glucopyranose b | 0.298 ± 0.005 | 26 |
| C16H16O10 | 16.71 | 367.0682 | 368.0754 | Methoxy-oxo-benzopyranyl glucopyranosiduronic acid c | 12.4 ± 0.3 | 27 |
| C29H26O16 | 16.95 | 629.1151 | 630.1223 | Coumaroyl-digalloylglucose/coumaroyl-digalloyl-glucopyranoside f | 0.336 ± 0.1 | 28 |
| C22H22O13 | 17.45 | 493.0994 | 494.1066 | Patulitrin isomer 2 g | 4.3 ± 0.1 | 29 |
| C24H22O14 | 17.51 | 533.0941 | 534.1013 | Luteolin (malonylglucoside)/apigenin (malonylglucoside)/kaempferol (malonyl-glucoside) h | 1.6 ± 0.1 | 30 |
| C22H22O12 | 17.65 | 477.1048 | 478.1120 | Isorhamnetin hexoside h | 22.6 ± 0.2 | 31 |
| C15H10O8 | 18.08 | 317.0312 | 318.0384 | Quercetagetin d | 22.0 ± 1.0 | 32 |
| C22H22O13 | 18.1 | 493.0992 | 494.1064 | Patulitrin isomer 3 g | 4.2 ± 0.1 | 33 |
| C15H10O6 | 21.38 | 285.0415 | 286.0487 | Luteolin i | 0.45 ± 0.02 | 34 |
| C16H12O8 | 21.58 | 331.0469 | 332.0542 | Methoxyquercetin j | 47.0 ± 1.0 | 35 |
| C16H12O7 | 24.06 | 315.0519 | 316.0591 | Isorhamnetin i | 0.98 ± 0.04 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-García, L.; Crespo-Antolín, L.; Forbes-Hernández, T.Y.; Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Arredondo, M.; Llopis, J.; Quiles, J.L.; Sánchez-González, C. Bioactive Properties of Tagetes erecta Edible Flowers: Polyphenol and Antioxidant Characterization and Therapeutic Activity against Ovarian Tumoral Cells and Caenorhabditis elegans Tauopathy. Int. J. Mol. Sci. 2024, 25, 280. https://doi.org/10.3390/ijms25010280
Rivas-García L, Crespo-Antolín L, Forbes-Hernández TY, Romero-Márquez JM, Navarro-Hortal MD, Arredondo M, Llopis J, Quiles JL, Sánchez-González C. Bioactive Properties of Tagetes erecta Edible Flowers: Polyphenol and Antioxidant Characterization and Therapeutic Activity against Ovarian Tumoral Cells and Caenorhabditis elegans Tauopathy. International Journal of Molecular Sciences. 2024; 25(1):280. https://doi.org/10.3390/ijms25010280
Chicago/Turabian StyleRivas-García, Lorenzo, Lara Crespo-Antolín, Tamara Y. Forbes-Hernández, Jose M. Romero-Márquez, María D. Navarro-Hortal, Miguel Arredondo, Juan Llopis, José L. Quiles, and Cristina Sánchez-González. 2024. "Bioactive Properties of Tagetes erecta Edible Flowers: Polyphenol and Antioxidant Characterization and Therapeutic Activity against Ovarian Tumoral Cells and Caenorhabditis elegans Tauopathy" International Journal of Molecular Sciences 25, no. 1: 280. https://doi.org/10.3390/ijms25010280
APA StyleRivas-García, L., Crespo-Antolín, L., Forbes-Hernández, T. Y., Romero-Márquez, J. M., Navarro-Hortal, M. D., Arredondo, M., Llopis, J., Quiles, J. L., & Sánchez-González, C. (2024). Bioactive Properties of Tagetes erecta Edible Flowers: Polyphenol and Antioxidant Characterization and Therapeutic Activity against Ovarian Tumoral Cells and Caenorhabditis elegans Tauopathy. International Journal of Molecular Sciences, 25(1), 280. https://doi.org/10.3390/ijms25010280











